| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 19, September 2022, pages 143-153
Antidiabetic effect of Melicope glabra (Blume) T.G. Hartley (Rutaceae) in high-fat diet/streptozotocin-induced diabetic rats and its bioactive components
Alexandra Queka, Dai Chuan Tana, Pei Cee Limb, Nur Kartinee Kassima, c, *, Amin Ismaild, Khozirah Shaarie, Siti Nuraisyah Mohd Shuiba
aDepartment of Chemistry, Faculty of Science, Universiti Putra Malaysia, UPM Serdang 43400, Malaysia
bFaculty of Pharmacy, Mahsa University, Bandar Saujana Putra, 42610 Jenjarom, Selangor, Malaysia
cIntegrated Chemical BioPhysics Research, Faculty of Science, Universiti Putra Malaysia, UPM Serdang 43400, Malaysia
dDepartment of Nutrition and Dietetics, Faculty of Medicine and Health Sciences, Universiti Putra Ma-laysia, UPM Serdang 43400, Malaysia
eNatural Medicines & Products Research Laboratory, Institute of Bioscience, Universiti Putra Malaysia, UPM Serdang 43400, Malaysia
*Corresponding author: Nur Kartinee Kassim, Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, UPM Serdang 43400, Malaysia. Tel: +603-9769 6803; E-mail: kartinee@upm.edu.my
DOI: 10.31665/JFB.2022.18320
Received: July 6, 2022
Revised received & accepted: September 29, 2022
| Abstract | ▴Top |
Melicope glabra (Blume) T.G. Hartley is a plant of Rutaceae family. The present study aimed to evaluate the in vivo hypoglycemic effect of M. glabra leaves extract on diabetic rats and identify its bioactive components. The induction of diabetes in Sprague-Dawley rats was done by a combination of high-fat diet and low-dose streptozotocin injection. The M. glabra extracts and standard sitagliptin were orally administered once daily to the diabetic rats for 28 days. The diabetic rats showed drastic weight loss, hyperglycemia, poor glucose tolerance, hyperlipidemia, and disturbances in the liver and kidney functions. However, oral administration of M. glabra extracts to the diabetic rats significantly lowered the DPP-4 level and increased glucagon-like peptide-1 (GLP-1) level. Scopoletin, pachypodol, and stigmasterol were revealed as the active DPP-4 and α-amylase inhibitors. This study revealed novel findings which suggest the potential of M. glabra as alternative antidiabetic treatment and source of antidiabetic agents.
Keywords: Antidiabetic; DPP-4; α-amylase; GLP-1; Isolation; Rutaceae
| 1. Introduction | ▴Top |
Diabetes mellitus is characterized by high blood glucose resulting from insufficient insulin secretion and/or insulin action. With the current trajectory, it was estimated that 642 million people worldwide will be diabetic in 2040 (Ogurtsova et al., 2017). Disordered carbohydrate and lipid metabolism were important determinants for the development of type 2 diabetes. Obesity-induced insulin resistance was reported to quicken the exhaustion of pancreatic islet cells and consequently the onset of type 2 diabetes. Health complications such as diabetic neuropathy, diabetic nephropathy, and diabetic retinopathy due to long-term damage of various organs prevails as poor management of diabetes mellitus (Kleinert et al., 2018).
To combat diabetes, wide range of hypoglycemia drugs have been made available. α-Glucosidase and α-amylase inhibitors such as acarbose, voglibose, and miglitol as well as dipeptidyl peptidase-4 (DPP-4) inhibitors such as sitagliptin and linagliptin were developed to lower the postprandial hyperglycemia (Akhtar et al., 2018). α-Glucosidase and α-amylase inhibitors reduce the digestion of carbohydrates and hinder the absorption of glucose into the bloodstream. Meanwhile, DDP-4 inhibitors are used to prolong the half-life of incretin hormones, namely glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). GLP-1 and GIP aids the regulation of insulin and glucagon secretion. However, the current developed drugs were reported to cause adverse side effects to the patients (Akhtar et al., 2018). Therefore, managing diabetes effectively with minimum side effects remains a great challenge. Alternative medicines with good efficacy, cost-effective, and fewer side effects are considered by health practitioners.
Medicinal plants have been widely used in both developed and developing countries as complementary and alternative medicine. Increased consumption of medicinal plants is associated with a great surge in public acceptance and interest in natural therapies due to their high availability in the markets and food stores (Ekor, 2014). Studies found that the presence of various phytochemicals in the plants is the main contributor for the therapeutic effects of medicinal plants. Specifically, compounds like kaempferol, rutin, myricetin, anthocyanidins, and feramidin showed antidiabetic properties (Zhang et al., 2016).
Melicope glabra (Blume) T.G. Hartley, an edible plant belonging to Rutaceae, is widely distributed in Peninsular Malaysia, Singapore, and Indonesia. The local Malaysian often called this plant by the name of “tenggek burung” or “pepauh daun besar”. Scientific research showed that the plant is rich of coumarins and flavonoids and exhibited good antioxidant properties (Kassim et al., 2013; Saputri et al., 2018). The plant extract showed strong antioxidant activities in 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging and β-carotene bleaching assay owing to its high phenolic content (Kassim et al., 2013). Previous study has ascertained that chloro-form extract of M. glabra leaves inhibit α-amylase and DPP-4 enzymes (Quek et al., 2021). Yet, the glucose lowering effect and isolation of bioactive components of M. glabra have not been re-ported thus far.
Evidence shows that the incorporation of animal models in experimental research is essential to address various biological and biomedical questions due to their close phylogenetic relatedness to humans (Andersen and Winter, 2019). Diabetic animal model developed by the combination of high-fat diet (HFD) and low dose streptozotocin (STZ) injection to induce insulin resistance and partial dysfunction of β-cells has shown to closely resemble the natural development of type 2 diabetes (Shimokawa et al., 2019). Careful assessments on animal models enable better understanding on the pathogenesis of the disease and are important for the validation and optimization of therapeutic agents before clinical evaluation Therefore, the aim of the present study was to evaluate the oral acute toxicity and glucose lowering or antidiabetic potential of chloroform extract of M. glabra leaves in HFD and low dose STZ-induced type 2 diabetic rats as well as identification of bioactive compounds in the plant.
| 2. Materials and methods | ▴Top |
2.1. Plant collection and extract preparation
The leaves of M. glabra were collected from Pasir Putih, Kelantan, Malaysia in 2018. The plant material was identified by Dr. Mohd. Firdaus Ismail from Biodiversity Unit, Institute of Bioscience, Universiti Putra Malaysia (UPM), where a voucher specimen (no. SK3326/18) had been deposited. No permission is required for the plant collection. Shade-dried leaves (1.0 kg) of M. glabra were ground to a fine powder. The plant materials were macerated with chloroform (5 L) at room temperature for 72 h, followed by filtration of the extracts using filter paper Whatman No.1. The process was repeated for two times with fresh batch of chloroform. The filtrates collected were pooled and evaporated to dryness using a rotary evaporator at 40 °C (Buchi, Switzerland) to yield crude chloroform (CHCl3) extract of the leaves.
2.2. Animals
Healthy, adult male Sprague-Dawley rats (180–210 g; 8–10 weeks) were purchase from the Faculty of Veterinary Medicine, Universiti Putra Malaysia. The complete in vivo study was done in a laboratory animal house, Comparative Medicine and Technology Unit, UPM with established standardized conditions of temperature 22–25 °C, 45–50% relative humidity, and light and dark cycle of about 12 h each. Animals were individually housed in standard polypropylene cages with stainless steel covers. Sufficient sample size was determined through power analysis using one-way ANOVA with an alpha of 0.05, a power of 0.80, and an effect size of 0.60. The analysis indicated that a total of 6 rats per group were necessary. All the animals were acclimatized for one week and fed with standard rodent chow (Altromin GmbH, Large-Lippe, Germany) and water ad libitum before experiments. The procedures and animal handling protocols were authorized by the Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia (Ref: UPM/IACUC/AUP-R004/2020) and compliance with the guidelines of the IACUC, UPM.
2.3. Acute toxicity study of M. glabra leaves extract
After a week of acclimatization, the acute toxicity of M. glabra leaves extract was evaluated using the fixed-dose procedure as described by Shimokawa et al. (2019). For sighting study, starting doses of 300 (n = 1) and 2,000 mg/kg (n = 1) of extracts were select-ed to determine the dose for the main study. Both rats were monitored carefully for 24 h to detect any abnormal changes in the skin, fur, eyes, and mucous membrane as well as conditions like tremors, salivation, convulsions, and coma. Since no abnormalities and mortalities were observed in individual rats with respective doses of 300 and 2,000 mg/kg, additional four rats in group 2000MG were administered with dose of 2,000 mg/kg in the main study. A total of five rats including one rat from the sighting study were used in the main study and monitored for a period of 14 days to detect any abnormalities and mortalities. Body weight of the rats were monitored before administration and at the end of each week. Food and water consumption were recorded daily. At the end of the experiment, the rats were euthanized with overdose mixture of ketamine and xylazine anaesthesia. The blood samples were collected via cardiac puncture for biochemical analyses. During and after 14 days, the extract did not produce any physical signs and no mortality was observed in the rats. The extract remained safe and non-toxic with dose up to 2,000 mg/kg. Thus, according to Organization for Economic Co-operation and Development (OECD) guidelines, 200 mg/kg dose that is 1/10th of the maximum tested dose (2,000 mg/kg) was selected as the highest dose in the in vivo antidiabetic study (Nazir et al., 2018).
2.4. Antidiabetic study of M. glabra leaves extract
2.4.1. Induction of type 2 diabetes in animals
Induction of type 2 diabetes in the present study was performed according to the method formerly described by Ishak et al. (2013). Healthy Sprague-Dawley rats were initially randomly divided into two groups. For 4 weeks, one group was fed standard rodent chow while another group was fed high-fat diet (HFD) (50% standard rodent chow + 38% animal ghee + 8% full-cream milk powder and 4% sugar). After 4 weeks, the group that received HFD was subjected to induction of hyperglycemia via intraperitoneal injection of STZ (Sigma-Aldrich, St. Louis, USA) at a dose of 35 mg/kg prepared in 0.1 M cold disodium citrate buffer (pH 4.5) after an overnight fast. 20% glucose solution was given for 24 h to the rats injected with STZ to prevent mortality caused by drug-induced hypoglycemia. Blood samples were collected from the tail vein via tail puncture after 72 h of STZ administration and the blood glucose level was checked using a glucometer (Accu-check Performa, Rochi diagnostic, USA). Rats having fasting blood glucose level ≥ 11.1 mmol/L were considered diabetic and were included in the study (Ablat et al., 2019)
2.4.2. Treatment protocol
The experimental rats (n = 36) were divided into a total of six groups (n = 6 per group). The non-diabetic rats were administered orally with vehicle and served as the normal control group. The diabetic rats were randomly divided into the rest of the five groups (Diabetic control, 50MG, 100MG, 200MG, and SITA). The diabetic rats in diabetic control group were administered orally with vehicle. The diabetic rats in the group 50MG, 100MG, and 200MG were treated orally with 50, 100, and 200 mg/kg of M. glabra leaves extract respectively. The rats from SITA group were treated orally with 10 mg/kg of sitagliptin which served as the standard drug group. The researchers were kept blinded to the group allocations until the end of the study. M. glabra leaves extracts and standard sitagliptin were prepared by dissolving in 1.0% (w/v) CMC.
Each treatment was administered daily in a single dose through oral gavage for 28 consecutive days. The body weight, food and water intake and fasting blood glucose (FBG) of each rat was recorded on the 0, 14th, and 28th day of the experiment period. After 28 days of treatment, the rats were fasted overnight and euthanized with a single intraperitoneal injection of overdose ketamine and xylazine anesthesia mixture. Blood samples were collected via cardiac puncture. The blood sample was centrifuged at 4,000 rpm for 15 min at 4 °C, the serum was collected and stored at −80 °C until analyses.
2.4.3. Oral glucose tolerance test (OGTT)
OGTT was carried out on the 25th day of treatment according to the method described by Ablat et al. (2019). All the animals were fasted overnight (16 h) before starting the experiments. By using oral gavage, Group 1 (normal control) and Group 2 (diabetic control) were treated with vehicle, Group 3, 4, and 5 were treated with 50, 100, and 200 mg/kg of M. glabra leaves extract respectively, and Group 6 was given 10 mg/kg of sitagliptin. After 30 min, all groups of rats were administered orally with 2 g/kg of α-D-glucose (Sigma-Aldrich, St. Louis, USA). At 0 (right after glucose load), 30, 60, 90, and 120 min, blood samples were collected from the tail vein and the blood glucose levels were determined by using the glucometer. Glucose responses of the rats during the OGTT were analyzed by calculating the total area under the curve (AUC) using the trapezoidal rule formula:
2.4.4. Determination of serum DPP-4, GLP-1, and insulin
The quantification of DPP-4, GLP-1, and insulin in the serum was performed by enzyme-linked immunosorbent assay (ELISA) using commercial kits for rat (Elabscience, Wuhan, China), GLP-1 ELISA Kit (Cusabio Biotech, Wuhan, China), and rat insulin ELISA kit (Mercodia AB, Uppsala, Sweden) respectively, according to the manufacturer’s instructions.
2.4.5. Serum biochemical analyses
Serum biochemical included alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatinine, urea, and electrolytes (sodium, potassium, chloride) were determined by automatic chemistry analyzer (Biolis 241 Premi-um., Biorex diagnostics, UK). Lipid profile included total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were characterized by using chemistry analyzer (Siemens Dimension Xpand Plus, Siemens AG, Munich, German).
2.5. Assay-guided isolation of phytoconstituents from M. glabra leaves extract
The CHCl3 extract of M. glabra leaves (20.0 g) was subjected to fractionation due to higher DPP-4 and α-amylase inhibitory activities. The fractionation was performed using CC packed with silica gel 60 (70–230 mesh ASTM) and eluted with increasing polarity of solvent systems starting from hexane, hexane: acetone, acetone:MeOH to MeOH. A total of 109 fractions of 200 mL each were collected from the CC. All the fractions were subjected to the TLC technique and the fractions with similar TLC profiles were pooled to give five major fractions, F1 (fractions 1 to 23), F2 (fractions 24 to 39), F3 (fractions 40 to 56), F4 (fractions 57 to 73) and F5 (fractions 74 to 109). Further fractionation of the most active fraction, F4 (5.3 g) on silica gel 60 (230–400 mesh ASTM) with eluting solvent systems of hexane, hexane:CHCl3, CHCl3:MeOH, and MeOH to give 20 fractions (F4.1-F4.20) of 50 mL each. F4.3 (522 mg) was purified by CC on Sephadex LH-20 eluted with CHCl3:MeOH (1:1) to yield 10 sub-fractions, F4.3.1-F4.3.10 of 20 mL each where F4.3.4 was identified as p-geranyl coumaric acid (1) (6.8 mg). F4.7 (745 mg) was subjected to CC on silica gel 60 (230–400 mesh) and eluted with hexane:CHCl3 (1.5:3.5) to yield stigmasterol (2) (9.2 mg). F4.10 (1.1 g) was re-chromatographed by using CC over silica gel 60 (230–400 mesh) with eluting solvent systems of increasing polarity starting from hexane:CHCl3 (4:1) to CHCl3:MeOH (1.5:3.5) and gave seventeen sub-fractions, F4.10.1-F4.10.17 of 20 mL each. Isocratic purification of F4.10.3 on silica gel with eluting solvent hexane:CHCl3 (1:1) yielded scopoletin (3) (4.0 mg). Purification of F4.10.11 over silica gel 60 (230–400 mesh) with eluting solvent hexane:CHCl3 (1:4) yielded evolitrine (4) (6.7 mg). F4.17 (341 mg) was subjected to CC over silica gel 60 (230–400 mesh) with eluting solvent hexane:EtOAc (1:4) to obtain pachypodol (5) (5.6 mg).
2.6. Structural elucidation of isolated phytoconstituents
A combination of Nuclear magnetic resonance (NMR) spectroscopy (JEOL 500 MHz NMR), mass spectrometry, and FT-IR were used to identify the isolated compounds. Tetramethyl silane (TMS) was used as an internal standard for NMR analysis. Mass spectral data of the pure compounds were obtained using a Shimadzu QP 5050A Spectrophotometer (Shimadzu Corporation, Kyoto, Japan) with a direct injection probe. One-dimensional (1D) (1H, 13C) and two-dimensional (2D) (COSY, HMQC, HMBC, DEPT) NMR spectra of pure compounds were processed using MestReNova software ver. 14.1.2 (Mestrelab Research S.L., Santiago de Compostela, Spain). The structures of the isolated compounds were confirmed by comparing their spectroscopic data with literature data.
2.7. Dipeptidyl peptidase-4 (DPP-4) inhibitory assay
DPP-4 inhibitor screening kit (Cayman Chemical, Ann Arbor, MI, USA) was used to analyse the DPP-4 inhibitory activity the of plant extracts, fractions, and compounds based on a fluorescence assay method. The fluorogenic substrate in this assay was Gly-Pro-aminomethylcoumarin (AMC) in which its peptide bond can be cleaved by DPP-4 to release the free AMC group. Initially, the test samples were dissolved and diluted to required concentrations using dimethyl sulfoxide (DMSO). 10 μL of samples with different concentrations (0.078–10.0 mg/mL) were then each pipetted into a 96-well plate followed by the addition of diluted assay buffer (30 μL), a diluted human-recombinant DPP-4 enzyme solution (10 μL) and diluted fluorogenic substrate (50 μL). For the negative control well, the sample was replaced by solvent. Sitagliptin standard was used as the positive control. The mixture in the 96-well plate was shaken and subjected to incubation for 30 minutes at 37 °C for the reaction to occur. After incubation, the fluorescence of the free AMC group generated from the reaction was measured on an excitation wavelength of 350–360 nm and emission wavelength of 450–465 nm using a microplate reader. Percentage of inhibition was calculated using the formula:
2.8. α-Amylase inhibitory assay
The α-amylase inhibition assay was conducted according to the method described by Abdullah and Kassim (2017) with slight modifications. Initially, 40 μL of test samples with varying concentrations (0.078–10.0 mg/mL) was mixed with 30 μL of 0.1 M sodium phosphate buffer (pH 6.9) in a 96-well microplate prior to the addition of 10 μL of α-amylase (1 U/mL) into the wells. The plate was subjected to incubation at 37 °C for 15 minutes, followed by the addition of 30 μL of soluble starch (1.0%), and re-incubated at 37 °C for 30 minutes. The reaction in the mixture was halted by adding 30 μL of hydrochloric acid (1.0 M) and 30 μL of iodine reagent. The absorbance was measured at a wavelength of 620 nm. Acarbose and phosphate buffer was used as the positive and negative controls, respectively. The α-amylase inhibition activity was calculated using the equation as follows:
2.9. Statistical analysis
The data were expressed as mean ± standard deviation. Statistical analysis was performed using One-Way Analysis of Variance (ANOVA) followed by Tukey post-hoc test for multiple comparisons. P values < 0.05 were considered significant.
| 3. Results | ▴Top |
3.1. Acute toxicity study of M. glabra leaves extract
During the main and sighting study, the rats that were treated respectively with 300 and 2,000 mg/kg (2000MG) of M. glabra leaves extract did not show mortalities nor any visible signs of behavioral changes and abnormalities in the skin, fur, eyes, and mucous membrane. Conditions like tremors, salivation, convulsions, and coma were also absent. Furthermore, none of the experimental animals suffered a loss in body weight. From the onset up to the end of the study period, a gradual increase in mean body weight was observed in both control and treated groups. Both groups showed a consistent gain in weekly body weight with the percentage ranging from 11.20 to 11.62% for the control group and 11.60 to 11.95% for the 2000MG group (Table 1). Additionally, no statistically significant difference (p > 0.05) was observed for the serum ALT, AST, ALP, urea, creatinine, sodium, potassium, and chloride levels between control group and treated group (Table 1).
 Click to view | Table 1. Effects of M. glabra leaves extract on body weight gain and selected liver and kidney function indices in acute toxicity study |
3.2. Antidiabetic study of M. glabra leaves extract
3.2.1. Effects of M. glabra leaves extract on body weight
The effects of M. glabra on the body weight of experimental animals on the 0, 14th, and 28th days of the treatment period were summarized in Table 2. Diabetic treated groups which received 50, 100, and 200 mg/kg of M. glabra extracts were denoted as 50MG, 100MG, and 200MG groups, respectively. Meanwhile, diabetic treated group which received 10 mg/kg of sitagliptin was presented as SITA group. At 0 day of treatment, the initial body weight of the diabetic control and the diabetic treated groups (50MG, 100MG, 200MG, and SITA) were significantly (p < 0.05) higher than the normal control group due to consumption of HFD. Throughout the treatment period, the diabetic control and 50MG groups showed a continuous decreased in body weight. The diabetic control group showed a drastic decreased in body weight by 10.99%. Contrarily, the normal control group showed an increase in body weight by 9.26%. Despite the continuous weight loss, the diabetic rats in the 50MG group experienced a significantly (p < 0.05) less reduction in body weight (2.45%) as compared to the diabetic control group. The diabetic rats from 100MG, 200MG, and SITA groups showed significantly (p < 0.05) higher body weight than those of diabetic control. 100MG, 200MG, and SITA treated groups demonstrated an in-crease in body weight by 1.66, 4.40, and 6.86% respectively.
 Click to view | Table 2. Body weight of experimental rats on 0, 14th, and 28th day of treatment period |
3.2.2. Effects of M. glabra leaves extract on fasting blood glucose (FBG)
The FBG levels of experimental rats in the antidiabetic study were summarized in Table 3. As shown, the initial FBG level of the diabetic control and all the diabetic treated groups were significantly (p < 0.05) higher than the normal control, indicating hyperglycemic condition resulted from diabetic induction procedures. After 28 days of the treatment period, the reduction of FBG levels in 50MG, 100MG, 200MG, and SITA groups can be observed. For 200MG and SITA groups, a significant (p < 0.05) decrease in FBG levels as compared to the initial day was shown after two weeks of oral administration. In comparison, the onset of FBG reduction in the 100MG group was slower than 200MG and SITA groups in which significant (p < 0.05) FBG level reduction can only be observed at the end of the treatment period. The percentage reduction of FBG levels after the treatment period for 100MG, 200MG, and SITA groups were 14.02, 25.63, and 37.87% respectively. Although the FBG level of the 50MG group that received the lowest dose of 50 mg/kg of the extract was gradually reduced during the treatment period, the reduction was not significant (p > 0.05) when compared to the FBG level at initial day. Without the interference of the treatment, no significant variation was noted in the FBG levels within the diabetic control and normal control groups throughout the treatment period of the antidiabetic study.
 Click to view | Table 3. Fasting blood glucose (FBG) levels of experimental rats on 0, 14th, and 28th day of treatment period |
3.2.3. Effects of M. glabra leaves extract in oral glucose tolerance test (OGTT)
The effects of M. glabra leaves extract in OGTT of experimental animals and the AUC values after 120 min of glucose load were presented in Figures 1 and 2, respectively. During the OGTT, the FBG of the experimental animals were recorded at 0 min and then every 30 min interval for 2 h. From the results, blood glucose levels in all groups were increased to maximum values at 30 min time point after glucose load (2 g/kg) which then gradually decreased in the following minutes. Compared with those of normal control, the rats in the diabetic control and all the diabetic treated groups showed significantly (p < 0.05) higher blood glucose levels at every time point. Nevertheless, all the treated groups exhibited significantly (p < 0.05) lower blood glucose levels when compared to the diabetic control. In relative to the FBG readings at 30 min time point, the diabetic control, 50MG, and 100MG groups showed significantly (p < 0.05) lowered blood glucose at 90 min time point with a percentage reduction of 13.88, 17.85, and 20.81% respectively. When com-pared to 100MG, the 200MG and SITA groups showed better improvement in glycemic response where significant (p < 0.05) reduction in blood glucose can be observed at 60 min time point after glucose load by 21.83 and 22.48% respectively. Based on the AUC readings, the AUC of the diabetic control drastically spikes up to 5,780.25 mmol/L·min while the AUC of normal control was only 991 mmol/L·min. The 50MG, 100MG, 200MG, and SITA groups demonstrated significantly lowered AUC of OGTT as compared to diabetic control with AUC values of 4,566.75, 4,127.63, 3,598.65, and 3,255.45 mmol/L·min respectively.
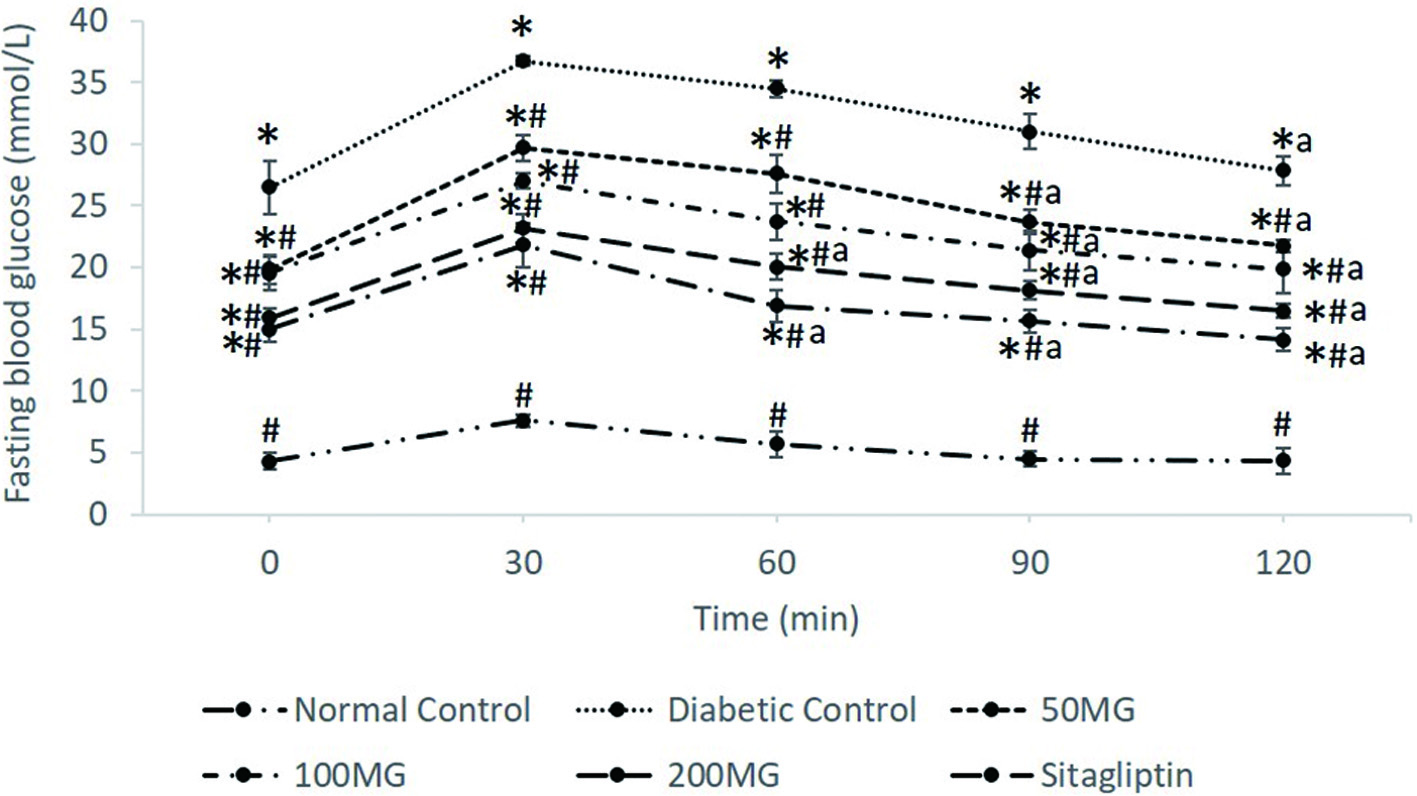 Click for large image | Figure 1. The effects of M. glabra extract on blood glucose levels in OGTT. Data are shown as mean ± standard deviation. *(p < 0.05) versus normal control; #(p < 0.05) versus diabetic control. a (p < 0.05) versus FBG at 30 min. 50MG: Group received 50 mg/kg of M. glabra extract; 100MG: Group received 100 mg/kg of M. glabra extract; 200MG: Group received 200 mg/kg of M. glabra extract; SITA: Group received 10 mg/kg of sitagliptin. |
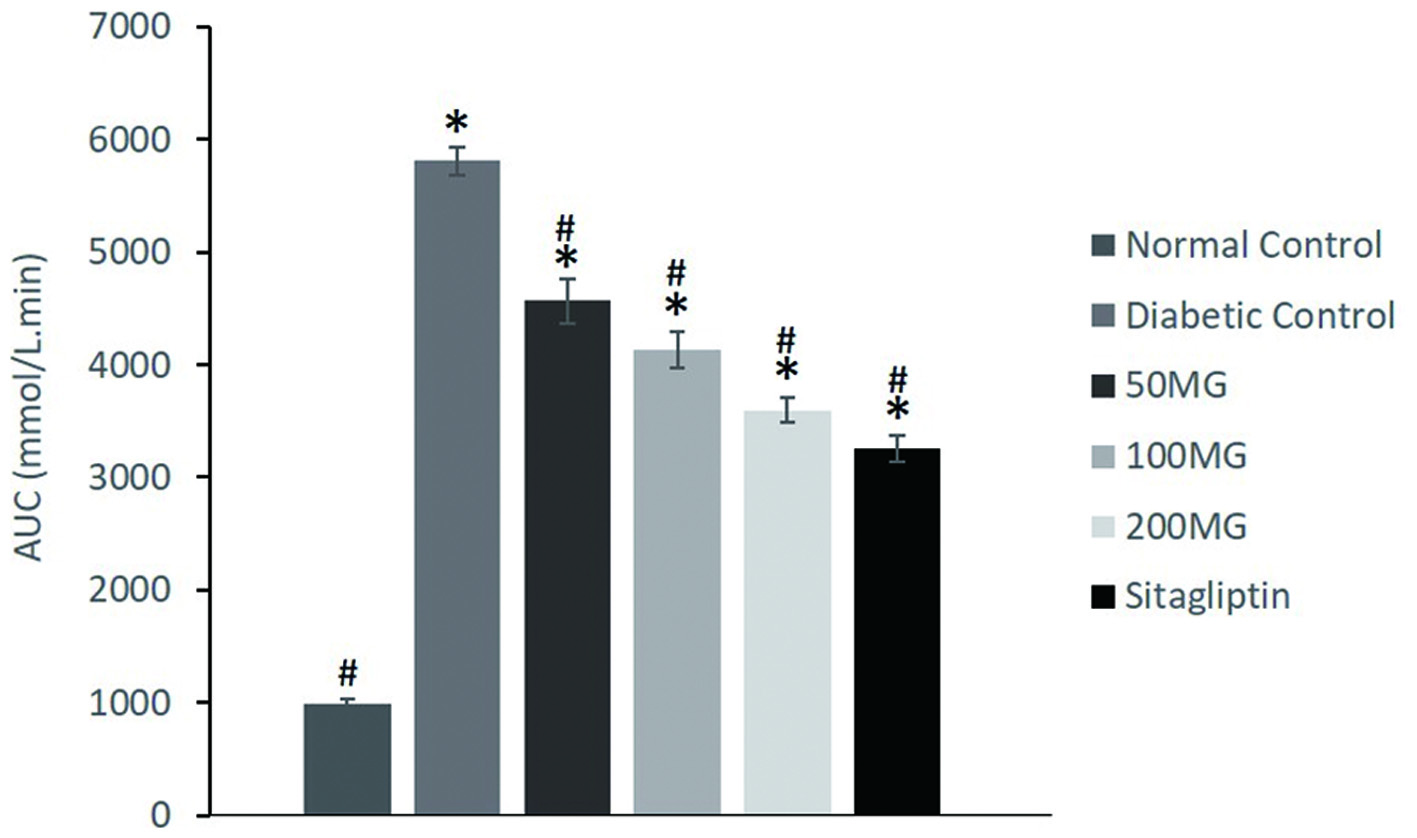 Click for large image | Figure 2. The effects of M. glabra extract on AUC of glucose values at 0–120 min after glucose load of experimental rats. Data are shown as mean ± standard deviation. *(p < 0.05) versus normal control; #(p < 0.05) versus diabetic control. 50MG: Group received 50 mg/kg of M. glabra extract; 100MG: Group received 100 mg/kg of M. glabra extract; 200MG: Group received 200 mg/kg of M. glabra extract; SITA: Group received 10 mg/kg of sitagliptin. |
3.2.4. Effects of M. glabra leaves extract on serum DPP-4, GLP-1, and insulin levels
The effects of M. glabra leaves extract on serum DPP-4, GLP-1, and insulin levels were summarized in Table 4. The normal control recorded the lowest DPP-4 level of 3.76 ng/mL while the serum DPP-4 level of diabetic control was significantly (p < 0.05) elevated to 4.96 ng/mL when compared to any other groups. In contrast to serum DPP-4 level, normal control showed the highest GLP-1 concentration at 24.01 pmol/L while the diabetic control showed the lowest amount at 7.50 pmol/L. Corresponded to the serum GLP-1 level, normal control subsequently showed the highest serum insulin concentration of 4.36 ng/mL. A significantly (p < 0.05) lower serum insulin was observed in diabetic control with a concentration of 1.29 ng/mL.
 Click to view | Table 4. Serum DPP-4, GLP-1, and insulin levels of experimental rats |
Among the extract-treated groups, the 100MG and 200MG groups showed significant improvement in serum DPP-4 levels. In relative to the diabetic control, the 100MG and 200MG groups showed a significant reduction (p < 0.05) in serum DPP4 by 12.5 and 16.73% respectively. The 100MG group showed increments in GLP-1 and insulin secretion of 33.87 and 82.17% but the increments were not significant when compared to the diabetic control. The 200MG group, however, showed significant (p < 0.05) increments in serum GLP-1 and insulin levels of about 74.00% and more than 2-fold, respectively. The treatment group of 50MG decreased the serum DPP-4 by 9.68% and increased the serum GLP-1 and insulin by 14.27 and 41.09% respectively, however, no significant (p > 0.05) variations were observed when compared to those of diabetic control group. The SITA group showed a remarkable decrease in serum DPP-4 level of diabetic rats to around 4.06 ng/mL with no significant difference (p < 0.05) than those of normal control. The greatest increment of serum GLP-1 and insulin levels were also observed in the SITA group with an increment of more than 2-fold for both indices.
3.2.5. Effects of M. glabra leaves extract on serum lipid, liver, and kidney profile
The effects of M. glabra leaves extract on serum lipid profile were assessed based on TC, TG, LDL, and HDL levels in the serum as showed in Table 5. In this study, the rats from the diabetic control group presented uncommon lipid profiles which involved an obvious increment of TC, TG, and LDL followed by a reduction in serum HDL when compared to the normal control group. Compared to the diabetic control, the 50MG group significantly (p < 0.05) decreased the TC, TG, and LDL levels by 25.26, 37.83, and 24.14% respectively while the 100MG group significantly (p < 0.05) decreased the TC, TG, and LDL levels by 28.42, 49.73, and 26.72% respectively. The 50 and 100MG groups respectively increased the serum HDL by 89.58% and more than 1-fold compared to that of diabetic control. Among the extract treated groups, the 200MG group showed the highest re-duction in the TC, TG, and LDL levels by 34.74, 64.32, and 56.90% respectively. SITA group significantly (p < 0.05) reduced the TC, TG, and LDL levels by 33.33, 57.84, and 53.04% respectively. The HDL levels of both the 200MG and SITA groups were increased by approximately more than 2.5-fold relative to that of diabetic control.
 Click to view | Table 5. Serum lipid, liver, and kidney profile indices of experimental rats. |
The liver function of experimental rats was assessed based on the levels of ALT, AST, and ALP in the serum (Table 5). Compared to those of normal control, rats from diabetic control showed notable elevations of ALT, AST, and ALP. Diabetic rats from 50MG group significantly (p < 0.05) lowered the ALT, AST, and ALP levels by 42.52, 48.33, and 65.87% respectively. Meanwhile, diabetic rats in the 100MG group showed a significant (p < 0.05) reduction of ALT, AST, and ALP levels by 58.41, 63.66, and 62.87%, respectively. Greater reduction of ALT (70.03%), AST (66.95%), and ALP (71.73%) can be observed in the rats from the 200MG group. Similar efficacy in the reduction of serum ALT, AST, and ALP levels in the diabetic rats was demonstrated by the SITA group that received sitagliptin treatment.
The effects of M. glabra leaves extract on the serum kidney function indices included urea, creatinine, and electrolytes (sodium, potassium, and chloride) of diabetic rats were presented in Table 5. Diabetic control rats showed significant (p < 0.05) elevated levels of urea and creatinine together with significant (p < 0.05) reduction of serum sodium when compared to the normal control rats. Treatment with 50 mg/kg of M. glabra extract lowered the serum urea and creatinine levels while increased the sodium level when compared to the diabetic control but the changes were not statistically different (p > 0.05). Oral administration of 100 mg/kg of M. glabra extract in diabetic rats resulted in a significant (p < 0.05) reduction in serum urea and creatinine levels by 37.76 and 17.37% respectively. However, no significant increment of sodium can be observed in the 100MG group. The 200MG and SITA groups caused significant (p < 0.05) changes in the levels of serum urea, creatinine, and sodium of diabetic rats. The 200MG group showed a respective decrement in serum urea and creatinine by 48.74 and 15.80% together with an increment of serum sodium by 4.78%. The sitagliptin treatment received by the SITA group led to the lowering of serum urea and creatinine levels by 55.44 and 19.30% respectively while the serum sodium level was increased by 9.56%.
3.3. Isolation of phytoconstituents of M. glabra leaves
Bioassay-guided isolation work on M. glabra leaves extract afforded five known compounds namely p-geranyl coumaric acid (1) (Guzman et al., 2014), stigmasterol (2) (Muhit et al., 2010), scopoletin (3) (Quynh et al., 2018), evolitrine (4) (Saputri et al., 2019), and pachypodol (5). Compounds 1, 2, and 4 were isolated for the first time from M. glabra. 1D and 2D NMR along with ESI-MS of the isolated compounds agreed with the literature which effectively confirmed their identification. The EI-MS and NMR spectra of compounds 1–5 can be found in Figures S1–S5. Figure 3 shows the structures of compounds 1 to 5.
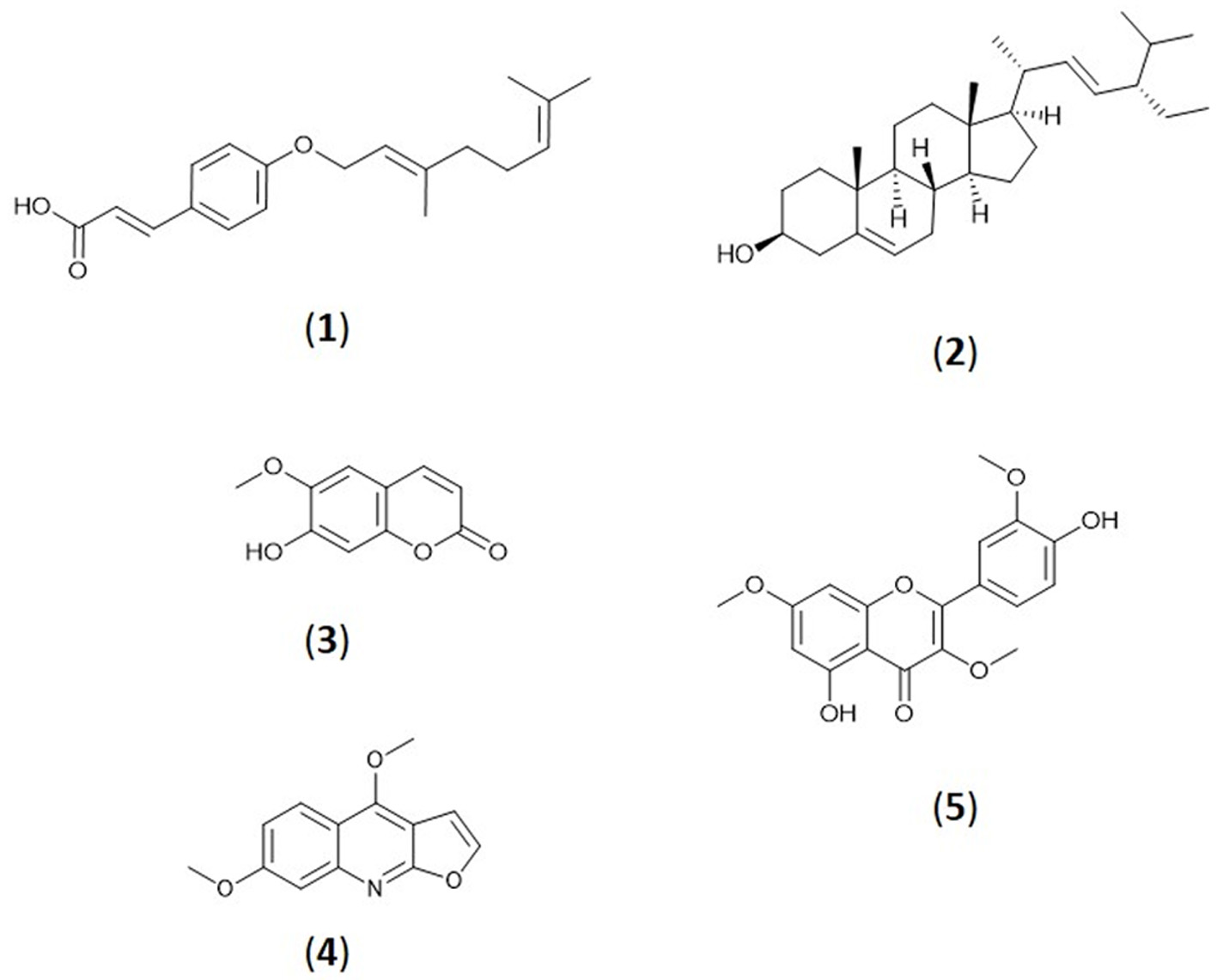 Click for large image | Figure 3. Structures of compounds 1 to 5. |
3.4. DPP-4 and α-amylase inhibitory activities of isolated phytoconstituents
The DPP-4 and α-amylase inhibitory activities of compounds 1 to 5 were summarized in Table 6. With regard to inhibition against DPP-4, scopoletin (3) showed the high-est DPP-4 inhibition activity with IC50 value of 36.34 ± 2.80 μM followed by pachypodol (5) which possessed DPP-4 inhibitory activity with IC50 value of 66.34 ± 2.30 μM. Compounds 1, 2 and 4, however, were found to be inactive in inhibition against DPP-4 where their IC50 couldn’t be determined even when tested at the highest concentration of 1 mg/mL. Meanwhile, Stigmasterol (2) was the most active in inhibiting α-amylase with an IC50 value of 304.02 ± 16.20 μM which was then followed by scopoletin (3), and pachypodol (5) which possessed an IC50 value of 412.36 ± 18.31 and 608.20 ± 49.95 μM against α-amylase, respectively.
 Click to view | Table 6. DPP-4 and α-amylase inhibitory activities of isolated phytoconstituents of M. glabra leaves extract |
| 4. Discussion | ▴Top |
Medicinal plants are the quarry of therapeutic agents which can be utilized for the management and/or treatment of various human maladies and diseases. Toxicity study is suggested before in vivo efficacy studies and human consumptions of medicinal plants. Specifically, the extracts, vehicles, and dosages chosen in the experimental design for in vivo efficacy studies should not impose any toxicity or interfering effects on the living cells or animal models. Thus, the acute oral toxicity study of M. glabra leaves chloroform extract was performed before the commencement of in vivo antidiabetic study of the extract. In this study, M. glabra leaves chloroform extract at a dose of 2,000 mg/kg did not produce any mortalities or abnormal clinical signs or behaviors in the rats. Insignificant alterations in body weight gain as well as liver and kidney function indices were observed between the normal control and the group that was administered with the extract. This indicated that the M. glabra leaves extract did not caused any toxicity to the rats at a dose of 2,000 mg/kg.
In the antidiabetic study, the induction of diabetes in experimental animals using a combination of HFD and low dose of STZ injection has been considered as a suitable model for the evaluation of hypoglycemic agents’ efficacy against type 2 diabetes. The present study showed that the untreated diabetic control rats experienced drastic weight loss accompanied by high FBG and poor glucose tolerance performance in OGTT when compared to the normal control rats. The body weight loss was due to the high catabolism of proteins in the tissue and fats because of insulin insufficiency (Miaffo et al., 2020). Administration of HFD was shown to increase adipose tissue or fat mass as well as enlarging fat cells which eventually leads to insulin resistance (Panigrahi et al., 2016). Meanwhile, injection of low-dose of STZ to the rats caused partial damage of pancreatic β-cells resulting in inadequate insulin production (Magalhães et al., 2019).
The untreated diabetic rats also showed significantly lowered serum DPP-4, GLP-1, and insulin levels. The hyperglycemic condition was seemingly associated with the serum DPP-4 level. Our observations were in line with the previous study which reported that the hyperglycemia condition increased the biosynthesis and secretion of DPP-4 in the endothelial cells (Kirino et al., 2009). Moreover, kidney damage in diabetic rats may cause the leakage of the DPP-4 enzyme from the kidney into the circulation. The higher levels of DPP-4 resulted in the reduction of circulating GLP-1 due to the extensive hydrolysis by DPP-4. Subsequently, this contributed to the significantly lower serum insulin level in the diabetic control rats. The same findings were reported in the previous studies (Lalitha et al., 2020; Purnomo et al., 2015)
Hyperlipidemia has been associated with type 2 diabetes due to abnormal lipid metabolism. It is one of the major complications that could lead to morbidity and fatality in diabetics. The diabetic control rats showed the occurrence of hyperlipidemia with an obvious increment of TC, TG, and LDL followed by a reduction in serum HDL when compared to the normal control group. Previous study had likewise revealed the occurrence of hyperlipidemia in the diabetic rats that were induced by HFD in a combination of a low dose of STZ. Compared to those of normal control, rats from diabetic control also showed notable elevations of ALT, AST, and ALP. This agrees with Pottathil et al. (2020) who reported the increments of ALT, AST, and ALP in the serum of diabetic rats. High levels of these hepatic function enzymes in the serum indicated liver damage and impaired membrane permeability. Many reports had denoted that high blood glucose and hyper-lipidemia contributed to the damage of liver membrane structure and caused the leakage of liver enzymes from the liver into the circulation, resulting in the increased levels of the liver enzymes in the serum (Dubey et al., 2020; Kurup and Mini, 2017; Tang et al., 2017).
Oral administration of M. glabra leaves extract was found to improve the hyperglycemia disorders in the diabetic rats. Less reduction of weight loss, lower FBG, and better glucose tolerance were observed in the treated diabetic rats especially those that were treated with higher dose (200 mg/kg) of the extract. Thus, the hypoglycemia effect of M. glabra leaves extract was proposed to work in a dose-dependent manner. The significant increment of serum GLP-1 and insulin levels observed in the extract treated diabetic group suggested that the M. glabra leaves extract could exert an antidiabetic effect in diabetic rats partly by obstructing the DPP-4 enzymes to increase the half-life of GLP-1 or by stimulating the secretion of GLP-1 and insulin. These results were in accordance with previous findings from the in vitro assay where the leaves extract of M. glabra were found to be effective on the inhibition of DPP-4. Besides that, the in vitro inhibition of the extract on α-amylase enzyme suggested that the plant extract could additionally suppress α-amylase enzyme in diabetic rats and reduced the digestion of carbohydrates which subsequently lower the absorption of glucose into the blood. Therefore, the plasma glucose lowering effect by the M. glabra leaves extract could be depicted in different mechanisms that were targeting different key enzymes involved in the pathogenesis of type 2 diabetes.
Besides, the improvement in lipid profile found in M. glabra extract treated diabetic rats could be attributed to higher GLP-1 and insulin level of the rats. GLP-1 was proven to improve the insulin sensitivity of insulin responsive tissues particularly adipose tissue in diabetic and obese animals (Jiang et al., 2018). Insulin is important in stimulating lipoprotein lipase activity and achieving hydrolysis of triglycerides. Under the condition of inadequate insulin, hypertriglyceridemia could accelerate the exchange of triglyceride from very-low-density lipoproteins for cholesteryl ester found in HDL. The triglycerides-rich HDL that were more susceptible to lipolysis by hepatic lipase were quickly cleared from the plasma, thus causing lower serum HDL in diabetic rats (Galicia-Garcia et al., 2020). The amelioration of hyperglycemia and hyperlipidemia by M. glabra leaves extract then decreased the levels of hepatic enzymes (ALT, AST, and ALP) and kidney function indices (urea, creatinine) in the serum of treated diabetic rats. This finding was in line with a previous study that reported the blood glucose and hyperlipidemia lowering effect of M. lunu ankenda extract reduced the liver and kidney function indices in the serum of diabetic rats (Al-Zuaidy et al., 2017).
Previous literature has shown that many medicinal plants with active metabolites such as flavonoids, coumarins, and phenolics exhibit antidiabetic properties (Zhang et al., 2016). Among the compounds isolated from M. glabra leaves extract, both scopoletin (3) and pachypodol (5) exhibited inhibition activity against DPP-4. Our results were in line with the former works of literature where coumarins and flavonoids isolated from various plants have been reported as effective inhibitors of DPP-4 (Gao et al., 2020; Singh et al., 2020; Soni et al., 2019). In addition, scopoletin (3) was previously suggested as a potent DPP-4 inhibitor based on the in silico molecular docking analysis between the compound and DPP-4 receptor (Geng et al., 2013). Notably, this is the first time that scopoletin (3) was revealed to inhibit DPP-4 based on in vitro evaluation. Meanwhile, Stigmasterol (2) isolated in this study displayed considerable inhibition against α-amylase. Our results paralleled the previous studies which reported stigmasterol (2) as an inhibitor of α-amylase and exerted a hypoglycemic effect in diabetic rats (Kumar et al., 2013; Mitra et al., 2020). In addition, previous literature had also reported on the inhibition of scopoletin (3) against α-amylase and its blood glucose-lowering effect in diabetic rats and mice which supported the finding of our study (Choi et al., 2017; Jang et al., 2018; Thengyai et al., 2020) Thus, the presence of these bioactive compounds could have contributed to the antidiabetic effect of M. glabra leaves extract in the diabetic rats. However, further phytochemical screening is required to determine other potent antidiabetic agents from the M. glabra leaves.
| 5. Conclusions | ▴Top |
This study indicated that the M. glabra leaves extract could be effective as antidiabetic agent by lowering the blood glucose level and increasing the levels of GLP-1 and insulin in diabetic models. The plant could be natural source of antidiabetic metabolites and de-tailed phytochemical screening of other active metabolites in the plant is suggested for further investigation on its potential as alternatives for treatment of type 2 diabetes.
| Supplementary material | ▴Top |
Figure S1. (a) EI-MS (b) 1H NMR Spectrum (c) 13C NMR Spectrum of p-geranyl coumaric acid, 1.
Figure S2. (a) EI-MS (b) 1H NMR Spectrum (c) 13C NMR Spectrum of stigmasterol, 2.
Figure S3. (a) EI-MS (b) 1H NMR Spectrum (c) 13C NMR Spectrum of scopoletin, 3.
Figure S4. (a) EI-MS (b) 1H NMR Spectrum (c) 13C NMR Spectrum of evolitrine, 4.
Figure S5. (a) EI-MS (b, c) 1H NMR Spectrum (d) 13C NMR Spectrum of pachypodol, 5.
Acknowledgments
We would like to thank Universiti Putra Malaysia for the facilities that were made available for us to conduct this research.
This research was funded by the Malaysia Ministry of Higher Education (MOHE) under Fundamental Research Grant Scheme (FRGS/1/2019/WAB11/UPM/02/1).
Conflict of interest
The authors declare no conflict of interest.
Conceptualization, Alexandra Quek and Nur Kartinee Kassim; Data curation, Alexandra Quek; Formal analysis, Alexandra Quek; Investigation, Alexandra Quek, Dai Chuan Tan, Pei Cee Lim and Khozirah Shaari; Methodology, Alexandra Quek, Dai Chuan Tan, Pei Cee Lim and Nur Kartinee Kassim; Supervision, Nur Kartinee Kassim, Amin Ismail and Khozirah Shaari; Writing – original draft, Alexandra Quek; Writing – review & editing, Alexandra Quek, Dai Chuan Tan, Pei Cee Lim, Nur Kartinee Kassim, Amin Ismail and Khozirah Shaari.
| References | ▴Top |