| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 20, December 2022, pages 72-79
Volatile sulfur compound in Pinot noir wines affected by vineyard irrigation, tillage, and nitrogen supplementation
Yu Fang, Barney Watson, Danye Zhu, I-Min Tsai, Michael C Qian*
Department of Food Science and Technology, Oregon State University, Corvallis, OR 97330, USA
*Corresponding author: Department of Food Science and Technology, Oregon State University, Corvallis, OR 97330, USA. Michael C. Qian, 100 Wiegand Hall, Corvallis, OR 97331, USA. Tel: (541) 737-9114; Fax: (541) 737-1877; E-mail: Michael.qian@oregonstate.edu
DOI: 10.31665/JFB.2022.18330
Received: November 30, 2022
Revised received & accepted: December 26, 2022
| Abstract | ▴Top |
The effects of vineyard nitrogen fertilization, tilling, and irrigation on the contents of volatile sulfur compounds in Pinot noir wines were investigated in this study. Wines were made from two field blocks of twelve combinations of irrigation (dry or irrigated), tillage (tilled or not tilled), and fertilization (none, foliar nitrogen supplementation or soil applied nitrogen) from three vintages of Vitis vinifera cv. Pinot noir. The concentrations of volatile sulfur compounds were quantified using solid-phase micro-extraction and gas chromatography/ pulse flame photometric detection (HS-SPME-GC/PFPD). Multivariate analysis of variance (MANOVA) showed that vintage year, irrigation, and nitrogen can affect volatile sulfur compounds (p < 0.01). Foliar nitrogen supplementation or soil nitrogen application significantly increased the contents of H2S (p < 0.01) and methanethiol (MeSH) (p < 0.01) in Pinot noir wines. Irrigation treatment yielded higher H2S and MeSH than non-irrigation treatment, and with tillage treatments also yielded higher H2S and MeSH. ANOVA demonstrated the interaction factor (irrigation × nitrogen) had significant impact on concentration of H2S and MeSH in wines. The combination of irrigation and soil nitrogen supplement had the highest amount of both H2S and MeSH. Dimethyl sulfide (DMS), methionol, methyl thioacetate (MeSOAc), and ethyl thioacetate (EtSOAc) were mainly affected by vintage.
Keywords: Volatile sulfur compounds; Pinot noir wines; nitrogen fertilization; Vineyard nutrient management; SPME-GC/PFPD
| 1. Introduction | ▴Top |
Volatile sulfur compounds are known to have potent and characteristic odors to many vegetables (Friedrich et al., 2022; Marcinkowska and Jeleń, 2022) and fruits (Cannon and Ho, 2018; Wanikawa and Sugimoto, 2022). They are also important to the appealing or off-flavor of foods and beverages (McGorrin, 2011; Jo et al., 2019), including alcoholic beverages (Yan et al., 2020; Parr et al., 2021; Wanikawa and Sugimoto, 2022). When presented at concentrations above their sensory detection thresholds, many volatile sulfur compounds can cause off-flavors such as reduced, sulfury, and rotten egg in the wine (Bekker et al., 2021). Since many factors, such as deficiencies of nutrients (amino acids and vitamins) and sulfite residues, are associated with the formation of volatile sulfur compounds (Bekker et al., 2021; Jimenez-Lorenzo et al., 2021), optimizations of vine health and fruit quality are among the top topics of grape growing and winemaking.
The mechanisms of forming these compounds in wine are still not fully understood. Most studies indicate that the sulfur amino acids of grape juice, especially methionine, seem to act as precursors of some sulfur compounds (Moreira et al., 2002; Pripis-Nicolau et al., 2004). The evidence showed that yeast breaks down the extra-cellular proteins and leaves sulfide residues of the sulfur-containing amino acids behind when a deficiency of nitrogenous components occurs in the must (Spiropoulos et al., 2000). Although some studies also showed that the presence of elemental sulfur from vineyard sprays could also cause hydrogen sulfide (H2S) formation during fermentation (Rauhut and Kuerbel 1994; Jastrzembski et al., 2017), the result was not always consistent (Thomas et al., 1993). H2S can also act as a precursor for other volatile sulfur compounds (i.e., mercaptans) that also impart off-odor to wine (Lambrechts and Pretorius, 2000).
The effects of different yeast strains on H2S production have already been widely studied (Ugliano et al., 2009; Jimenez-Lorenzo et al., 2021). The effect of vinification parameters,such as addition of sulfite or sulfur-containing amino acid to the must, and fermentation temperature, on the development of volatile sulfur compounds in wines and grape musts (Karagiannis and Lanaridis, 1999; Moreira et al., 2002; Kinzurik et al., 2020).
However, little information is available on the formation of highly volatile sulfur compounds other than H2S in wine. One of the major reasons is that it is challenging to measure these highly volatile sulfur compounds due to their extremely low concentration in wine and high reactivity (Mestres et al., 2000). With the advancement of analytical instrumentation, more quick and sensitive analytical methods have ben developed for the quantification of trace amounts of volatile sulfur compounds in wines and various alcoholic beverages (Fang and Qian, 2005; Davis and Qian, 2019a, b; Ontañón et al., 2019; Dziekońska-Kubczak et al., 2020; Yu et al., 2022). Among various analytical methods, solid-phase microextraction (SPME) coupled with GC-pulsed flame photometric detection (PFPD) is a simple and sensitive technique (Fang and Qian, 2005). With this method, the quantified volatile sulfur compounds could go as low as 0.5 µg/L, which is lower than their sensory detection thresholds in Pinot noir wines (Tsai and McDaniel, 2011).
In some parts of Oregon, grapevines are subject to low soil water availability, accompanied by high solar irradiance levels during the sumer. Under these conditions, photosynthesis is significantly reduced, particularly toward the end of the growing season. The inability of vines to photosynthesize prior to harvest results in a shortage of carbohydrates and a reduction of nitrogenous compounds in grapes at harvest (Lohnertz et al., 2000). Low assimilable nitrogen levels at harvest may cause slow and sluggish fermentation, producing wines with high residual sugar or off-flavors at undesirable level. To improve fruit quality, various vineyard practices such as additional nitrogen supplementation, irrigation, and tillage have been studied on vine health and fermentation behavior. This study reported the effect of vineyard practices used for nitrogen management on volatile sulfur compounds in Pinot noir wines.
| 2. Materials and methods | ▴Top |
2.1. Vine treatments and wine preparation
Three consecutive vintage Pinot noir wines were produced from Oregon State University viticulture trials with grapes grown at Benton-Lane vineyard in the Oregon Southern Willamette Valley appellation. Pinot noir clone FPMS 2A vines were grafted onto 7-year-old Teleki 5C rootstocks. There were 24 wine samples: 12 treatment combinations and two field replications (Table 1). The treatments included nitrogen supplement (three levels: none, foliar applied, and soil applied), irrigation (two levels: dry and irrigated), and tillage (two levels: alternate in-row tilling and not tilled). The irrigation treatment involved water applied at the rate of 0.5gal/hour for four hours daily for a total of 200 hours during ripening. Tilling was done in early spring to encourage nitrogen utilization and reduce nutrient and water competition. Fertilizer was applied to either soil or foliar: soil nitrogen was applied manually one time in May at the rate of 39 Kg urea/ha. Foliar N was split into two applications of 1.5 kg/ha applied by spraying on the leaves.
 Click to view | Table 1. The experimental design for the Vineyard treatments |
After harvest, grapes from each treatment were collected, crushed, stemmed, treated with 50 mg/L sulfur dioxide, and fermented separately (inoculated 1 g/L Lavin RC 212 Bourgorouge yeast). The musts were punched down twice daily during fermentation and pressed after seven days of fermentation. After wines were settled and racked off the primary yeast lees, 0.025g/gallon OSU 1-step (Lalvin) malolactic bacteria was used to induce secondary malolactic fermentation. The new wines were cold stabilized, racked, bottled with the addition of 25 mg/L of sulfur dioxide, aged for nine months, and stored in the experimental winery at 18 °C. All the wine samples were analyzed at the same time.
2.2. Quantification of volatile sulfur compounds in wines
The quantification of volatile sulfur compounds in wines was performed by a previously published method (Fang and Qian, 2005). In general, five milliliters of wine samples and 100 μl of internal standard solution, which included 500 µg/L (w/w) of EMS, 2 µg/L (w/w) of IsoProDS, and 100 mg/L (w/w) of 4-methylthiobutanol, were placed in 20 ml pre-flushed autosampler vials. The sulfur volatiles were equilibrated for 15min at 30 °C, and extracted at the same temperature for 30min with agitation by an 85µm CarboxenTM-PDMS StableFlexTM SPME fiber (SUPELCO, Bellefonte, PA, USA). After extraction, the SPME fiber was injected directly into GC injection port with the splitless mode at 300 °C. The GC/PFPD analyses were made on a Varian CP-3800 gas chromatography equipped with a pulsed flame photometric detector (PFPD) (Varian, Walnut Creek, CA, USA) operating in sulfur mode. The separations were performed using a DB-FFAP capillary column (30m × 0.32 mm I.D., 1 µm film thickness, Agilent, Palo Alto, CA, USA).
The purified chemicals, H2S, methanethiol (MeSH), ethanethiol (EtSH), dimethyl sulfide (DMS), diethyl sulfide (DES), dimethyl disulfide (DMDS), diethyl disulfide (DEDS), dimethyl trisulfide (DMTS), methyl thioacetate (MeSOAc), ethyl thioacetate (EtSOAc), and methionol were used to build up calibration curves as presented in the previous publication (Fang and Qian, 2005). The sulfur responses of target compounds were calculated by the square root of peak area. Triplicate analysis was performed on all samples, and amounts of sulfur compounds in wines were determined by comparing their own standard curves.
2.3. Statistical analysis
A complete randomized block design was used in the present study. The four treatments, vintage (year), irrigation (irrigate), tillage (till), and nitrogen were fixed effects, and replication (rep) was considered as a block effect. The data were first analyzed by multivariate analysis of variance (MANOVA) to examine whether significant differences were found in concentrations of eight volatile sulfur compounds (H2S, MeSH, DMS, DMDS, DMTS, MeSOAc, EtSOAc, and methionol) in wine samples with different treatments. Year, irrigate, till, nitrogen, and rep were considered as the main effects. All 2-way, one 3-way (irrigate × till × nitrogen) and one 4-way (year × irrigate × till × nitrogen) interactions were included in the MANOVA model. The level of significance (α) was 0.05. The MAVONA results (Wilk’s λ) showed that rep and the interactions containing rep were not statistically different from various levels of treatments (p > 0.05). Therefore, year, irrigation, till, nitrogen and all their interactions were included in the four-way ANOVA model on the eight volatile sulfur compounds individually. To understand the paired mean differences, mean concentrations of volatile sulfur compounds in different treatments were compared by multiple comparisons adjusted by Tukey-HSD method. Principle components analysis (PCA) was also performed on the mean data with a varimax rotation. The minimum of 0.7 for the correlation of original sulfur compounds with the new factor generated was used as a selection criterion. All statistical analyses were performed using SPSS 13.0 for windows (SPSS Inc., Chicago, IL). PCA was performed by MetaboAnalyst 5.0 (https://www.metaboanalyst.ca)
| 3. Results and discussion | ▴Top |
In all wine samples, ethanethiol and diethyl disulfide were not detected. Diethyl sulfide was detected in only a few wine samples, and its concentrations were very low (not shown). The results from this present study confirmed the previous results in commercial wines (Fang and Qian, 2005) that highly volatile sulfur compounds containing an ethyl group were not the major products during normal winemaking. A previous sulfur survey in Oregon wines conducted in this laboratory also showed that trace amounts of ethanethiol could generate off-flavor problems (Fang et al., 2005).
The concentrations of other target sulfur compounds in wine samples were presented in Tables 2. H2S levels in wines were from 0.08 to 7.3 µg/L, methanethiol concentrations were from 0.79 to 4.9 µg/L, and dimethyl sulfide concentrations were from 7.2 to 23.4 µg/L. The most abundant sulfide compound in wine samples was methionol, which ranged from 1.07 to 3.35 mg/L. Less than 1µg/L of DMDS and DMTS were found in all these wines. Concentrations of these sulfur compounds in experimental Pinot noir wines were below their detection thresholds in wine (Mestres et al., 2000). Neither off-flavor nor sulfur-related aroma difference was found in any of these wines, which was consistent with the instrumental analysis performed in the present study.
 Click to view | Table 2. The concentration of volatile sulfur compounds in three vintage wines (µg/L). |
MANOVA results showed that vintage year, irrigation and nitrogen were significantly different for the various levels of treatments (p < 0.05) (Table 3). Significant two-way interactions were year × nitrogen and irrigation × nitrogen (p < 0.05). The results indicated that the concentrations of the eight volatile sulfur compounds in wine samples were dependent on vintage year, irrigation and nitrogen , but also on a combination of vintage year and nitrogen, and a combination of irrigation and nitrogen.
 Click to view | Table 3. The MANOVA results using SPSS 13.0 (α = 0.05) |
The four-way ANOVA results on each target compound showed that vintage year greatly influenced concentrations of the volatile sulfur compounds in wine samples (p < 0.05) except for MeSH. Table 4 reported the concentration means of volatile sulfur compounds in three years. Vintages bearing different superscripts were significantly different at p < 0.05 by ANOVA and Tukey’s HSD. Multiple comparison results showed that the wine samples from vintage year 1 contained higher H2S, DMS, DMDS, DMTS and methionol, but lower MeSOAc and EtSOAc. However, the reason was not clear yet.
 Click to view | Table 4. The means of sulfur volatile compound concentrations (µg/L) in Pinot noir wine from three vintage years (n = 24) and from different vineyard treatments (n = 36, except for nitrogen supplements n = 24) |
Table 4 also showed the concentration means of target compounds in wine samples by different viticulture treatment. Treatments bearing different superscripts were significant different at p < 0.05 by ANOVA and Tukey’s HSD. There was no significant difference between viticulture treatments on DMMS, EtSOAC, DMTS, and methionol. Multiple comparison results showed that the wine samples without nitrogen supplementation had significant lower concentrations of H2S and MeSH. Irrigation treatment yielded higher H2S and MeSH than non-irrigation treatment, and with tillage treatments yielded higher H2S and MeSH. Moreover, irrigation significantly increased the amount of MeSOAc, and tillage significantly increased the amount of DMS.
ANOVA demonstrated interaction factor (irrigate × nitrogen) had significant impact on concentration of H2S and MeSH in wines. In Figure 1, the combination of irrigation and soil nitrogen supplement had the highest amount of both H2S and MeSH, and followed by the combination of irrigation and foil nitrogen supplement. The results indicated that the effects of nitrogen supplement on these two compounds were further amplified by irrigation treatment.
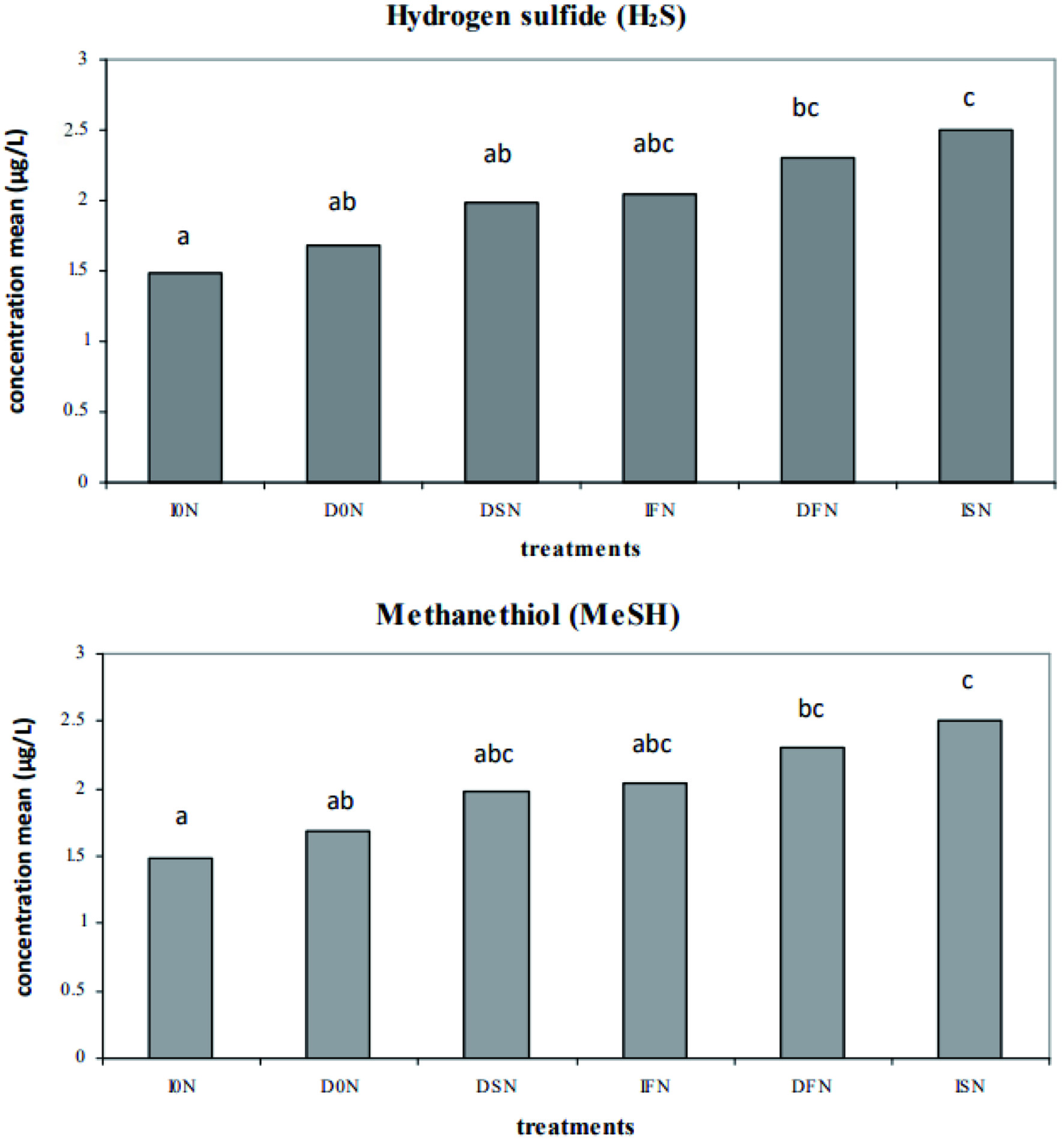 Click for large image | Figure 1. The concentration means of hydrogen sulfide (H2S) and methanethiol (MeSH) by different irrigation and nitrogen treatment combination. Treatments bearing different superscripts are significant different at p < 0.05 by ANOVA and Tukey’s HSD. |
The principal component analysis (2D scores plot) with 95% confidence intervals of sulfur volatiles in differently treated of Pinot noir wines were showed in Figure 2. PC1 and PC2 accounted for 45.6% and 26.8% of the variation of the sulfur volatiles on Year 1 samples (Figure 2a), 45.8% and 27.2% Year 2 samples (Figure 2b), 48.3% and 25.1% on Year 3 samples (Figure 2c), 49.6% and 22% on all samples (Figure 2d), respectively. More than 70% of the score plots from PC1 and PC2 indicated that the PCA model was an effective technique for exploring the different treatments effect on sulfur volatiles in wines. Dry-No till-No nitrogen (D NT 0N) had separate clusters in both Year 1 and Year 3 vintages compared to Dry-No till-Foliar nitrogen (NT FN) and Dry-No Till-Soil nitrogen (D NT SN), respectively. “Dry” treatment reduced nitrogen absorption by the vine, and “No till” treatment further decreased vine nutrient uptake due to soil nutrient and water competition from cover crops. Nitrogen supplementation either by soil application or foliar application was effective for the separation. Similarly, Irrigation-No till-no nitrogen (I NT 0N) showed own clusters in both Year 1 and Year 3 vintages compared to Irrigation-No till-Foliar nitrogen ( I NT FN) and Irrigation-No till-Soil nitrogen (I NT SN). In addition, Irrigation-Till-No nitrogen (I T 0N) also had own clusters in Year 2 compared to Irrigation-Till-Foliar nitrogen (I T FN) and Irrigation-Till-Soil nitrogen (I T SN). Figure S1 showed that most of the Year 3 wines were positively correlated with sulfite esters (MeSOAc and EtSOAc) and negatively correlated with dimethyl sulfide compared to Year 1 and Year 2 vintages. Furthermore, the Year 2 wines contributed more methionol than the Year 1 and Year 3 vintages. Besides, the results indicated that the contribution to H2S and MeSH was significantly increased when nitrogen supplementation was applied in the vineyard (Figure S1). H2S and its off-flavor have been studied the most in wine. Except its low detection threshold, it can further react with wine components and generate mercaptans, which are more difficult to eliminate in winemaking (Lambrechts and Pretorius, 2000). Therefore, the concentrations of H2S and MeSH in wines are generally correlated to each other.
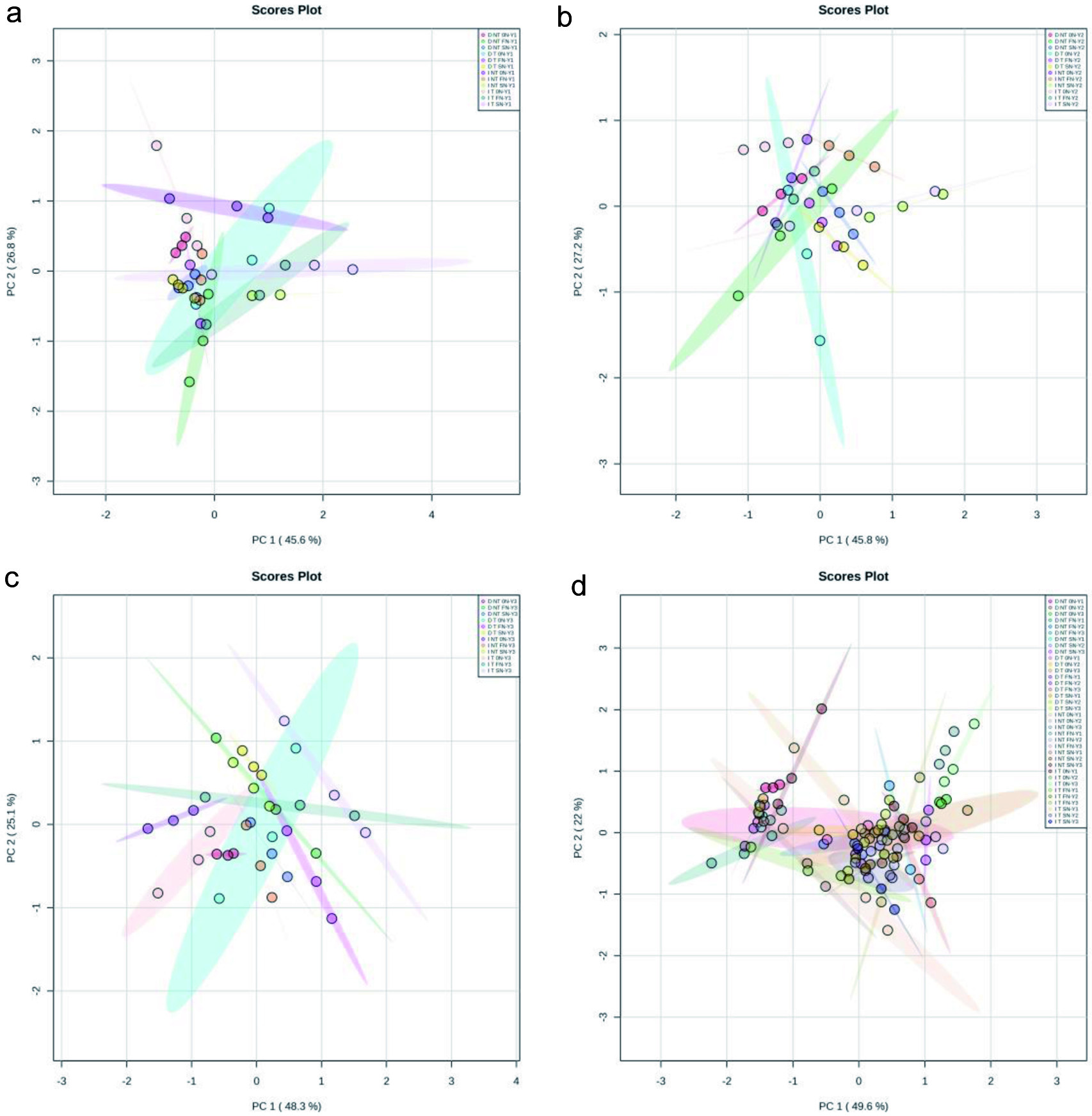 Click for large image | Figure 2. Principal component analysis (2D scores plot) of sulfur volatiles in differently treated of Pinot noir wines. (a) Year 1 samples; (b) Year 2 samples; (c) Year 3 samples; (d) All samples. |
Nitrogen compounds are required by yeast for the production of cell biomass, the synthesis of DNA, RNA, and the proteins and enzymes necessary for the biochemical processes of fermentation. The readily fermentable nitrogen content in juice and musts is composed primarily of ammonia (NH3) and the alpha-amino acids (particularly arginine, serine, glutamate, threonine, aspartate, and lysine). An approximation of the total yeast fermentable nitrogen may be taken as the sum of the nitrogen available from ammonia and the alpha-amino acids present in the juice or must (Bisson, 1991; Dukes and Butzke, 1998; Jiranek et al., 1995). If the levels of fermentable nitrogen are too low, the total cell biomass produced will be low, the yeast fermentation will be slow, and the fermentation may stop or ‘stick’ before all the fermentable sugar is utilized.
It is well known that nitrogen deficiency in grape must is one of major reasons to elevated formation of H2S off-flavor, but formation of H2S is much more complex (Spiropoulos et al., 2000). Sea et al. measured the production of H2S during wine fermentation for two seasons, and reported poor correlation between H2S and nitrogen concentrations in must during wine fermentation (Sea et al., 1998). In addition, some researches reported that H2S production was even significantly higher when the concentration of yeast assimilable nitrogen content (YANC) was increased if pantothenic acid was deficient (Wang et al., 2003). Results in the present study also showed that the concentrations of H2S and MeSH were significantly higher when nitrogen supplementation was applied in vineyard. Previous analysis showed that YANC of these vineyard treatments was not significantly different within the three vintage years (data not shown), which indicated that there was no directly correlation between concentrations of these sulfur volatiles and YANC. It has to be pointed out that the fermentation was considered as “normal”, and the concentration of the volatile sulfur compounds in this study was low and sulfur off-flavor was not detected in any of the wines.
Overall, in this study, the effects of nitrogen managements in vineyard practices on contents of volatile sulfur compounds in Pinot noir wines were investigated. The data showed that volatile sulfur compounds could be affected by vintage year, nitrogen supplement, and irrigation. Nitrogen supplementation could increase the H2S and MeSH levels in wines, but the concentrations were below the level trigging sulfur off-flavor in the wine.
| Supplementary material | ▴Top |
Figure S1. Principal component analysis (biplot) of sulfur volatiles in differently treated of Pinot noir wines. (a) Year 1 samples, shown as Y1; (b) Year 2 samples, shown as Y2; (c) Year 3 samples, shown as Y3; (d) All samples. Different number _1-_11 means I NT 0N, I T 0N, D NT 0N, D T 0N, I NT FN, I T FN, D NT FN, D T FN, I NT SN, I T SN, D NT SN, D T SN.
Acknowledgments
The authors thank the Oregon Wine Board and the USDA CSREE-NW Center for Small Fruit Research for funding this project.
| References | ▴Top |