| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 6, June 2019, pages 110-117
Potential cardioprotective peptides generated in Spanish dry-cured ham
Marta Gallego, Leticia Mora, Fidel Toldrá*
Instituto de Agroquímica y Tecnología de Alimentos (CSIC), Avenue Agustín Escardino 7, 46980, Paterna (Valencia), Spain
*Corresponding author: Fidel Toldrá, Instituto de Agroquímica y Tecnología de Alimentos (CSIC), Avenue Agustín Escardino 7, 46980, Paterna (Valencia), Spain. Tel: +34963900022 ext.2112; Fax: +34963636301; E-mail: ftoldra@iata.csic.es
DOI: 10.31665/JFB.2019.6188
Received: April 5, 2019
Revised received & accepted: June 29, 2019
| Abstract | ▴Top |
Food-derived bioactive peptides are promising compounds for the prevention and treatment of cardiovascular diseases, the main cause of mortality in developed countries. The aim of this work was to determine the in vitro anti-inflammatory, antioxidant, and angiotensin I-converting enzyme (ACE-I) inhibitory activities of twenty-four peptides that were identified in Spanish dry-cured hams. For the first time, some peptides such as PSNPP, HCNKKYRSEM and FNMPLTIRITPGSKA showed anti-inflammatory activity expressed as platelet-activating factor-acetylhydrolase, autotaxin, and lipoxygenase inhibition. Peptides MDPKYR and TKYRVP were the strongest antioxidants, whereas GGVPGG, TKYRVP, and HCNKKYRSEM showed the highest ACE-I inhibitory activity. Additionally, several peptides such as KPVAAP, MDPKYR, TKYRVP, HCNKKYRSEM exerted more than one of the assayed activities, increasing their health-enhancing potential. More studies are needed to evaluate the bioavailability of such peptides and their in vivo effect. This would contribute to consider dry-cured ham as a source of peptides beneficial for cardiovascular health.
Keywords: Bioactive peptides; Anti-inflammatory activity; Antioxidant activity; ACE-I inhibitory activity; Multifunctional peptides; Cardiovascular health
| 1. Introduction | ▴Top |
Cardiovascular diseases (CVD) are currently the main cause of mortality in developed countries, being hypertension, dyslipidaemia, and diabetes important risk factors associated with the development of heart diseases. Food-derived bioactive peptides constitute interesting compounds for the prevention and treatment of CVD as alternative to available pharmaceutical drugs that entail high costs and negative-side effects in the organism (Erdmann et al., 2008). Bioactive peptides are generally 2–20 amino acids in length and are capable to exert positive health effects in the human body such as antihypertensive, antioxidant, anti-inflammatory, and antidiabetic activities, among others. Bioactive peptides may be generated, or lose part of its bioactivity, by the action of proteolytic enzymes during food processing and gastrointestinal digestion (GI). Then, peptides must reach their target sites in an active form and significant quantity to exert their beneficial effects (Udenigwe and Aluko, 2012).
Many bioactive peptides have been reported to be naturally generated during the processing of Spanish dry-cured ham through the action of endogenous muscle enzymes along processing. Among them, angiotensin I-converting enzyme (ACE-I) inhibitory peptides are outstanding (Escudero et al., 2013a, 2014). Such ACE-I inhibitors prevent the formation of the vasoconstrictor angiotensin-II and the release of aldosterone in the Renin-Angiotensin-Aldosterone System (RAAS). Additionally, blood pressure regulation can be performed though the endothelin and nitric oxide (NO) systems, as well as other less studied mechanisms related to oxidative stress, vascular inflammation, and sympathetic nervous system (Majumder and Jianping, 2015).
Antioxidant peptides (Escudero et al., 2013b; Mora et al., 2014), and dipeptidyl peptidase-IV (DPP-IV) inhibitory peptides with potential antidiabetic activity (Gallego et al., 2014a) have been also identified in Spanish dry-cured hams. These bioactive peptides might participate in the regulation of vascular functions, blood pressure, and inflammatory responses due to the link between metabolic pathways. In fact, oxidative stress is often associated with inflammatory processes, but exist other complex mechanisms of action for anti-inflammatory peptides related to RAAS, pro-inflammatory cytokines, pro-inflammatory signalling kinases, and integrin-dependent signalling (Chakrabarti et al., 2014; Schulz et al., 2011).
Although there is an absence of reports on the anti-inflammatory activity of peptides isolated from dry-cured ham, two recent studies in humans have suggested that the regular intake of dry-cured hams could contribute to cardiovascular health related to glucose and lipid metabolism, hypertension, and inflammatory processes (Martínez-Sánchez et al., 2017; Montoro-García et al,. 2017). Thus, the aim of this work was to determine the in vitro anti-inflammatory, antioxidant, and ACE-I inhibitory activities of twenty-four peptides isolated from dry-cured hams and identified in a previous study.
| 2. Materials and methods | ▴Top |
2.1. Chemicals and reagents
Angiotensin I-converting enzyme from rabbit lung, captopril, fluorescein, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich, Co. (St. Louis, MO, USA). O-aminobenzoylglycyl-p-nitro-L-phenylalanyl-L-proline (Abz-Gly-p-nitro-Phe-Pro-OH) trifluoroacetate salt was from Bachem AG. (Bubendorf, Switzerland) while potassium persulfate was from Panreac Química, SAU (Barcelona, Spain). PAF acetylhydrolase inhibitor screening assay kit (Item No. 10004380), autotaxin inhibitor screening assay kit (Item No. 700580), and lipoxygenase inhibitor screening assay kit (Item No. 760700) were supplied by Cayman Chemical Company (Ann Arbor, MI, USA). All chemicals and reagents were of analytical grade.
2.2. Peptide synthesis
Twenty-four peptides were selected, from a range of previously identified peptides in Spanish dry-cured hams, according to potential bioactivity based on the length, molecular weight, sequence, and composition in amino acids (Table 1). The selected peptides were synthesised (GenScript Corporation, Piscataway, NJ, USA) and the purity checked through liquid chromatography-mass spectrometry (LC-MS) analysis. Peptide solutions (1 mM) were prepared for subsequent bioactivity assays.
 Click to view | Table 1. Peptides previously identified in Spanish dry-cured ham and synthetised for testing their bioactivity |
2.3. Anti-inflammatory activity
2.3.1. Platelet-activating factor-acetylhydrolase inhibition assay
The platelet-activating factor-acetylhydrolase (PAF-AH) inhibitor kit was assayed in accordance with the manufacturers’ instructions. The substrate 2-thio PAF is hydrolysed by the enzyme PAF-AH, generating free thiols that are colorimetrically detected using 5,5′-dithio-bis-(2-nitrobenzoic acid) (DNTB). Samples were assayed in triplicate and results expressed as percentage of PAF-AH inhibition.
2.3.2. Autotaxin inhibition assay
The autotaxin (ATX) inhibitor kit was assayed as described by the manufacturer. ATX cleaves bis-(p-nitrophenyl) phosphate, releasing the yellow product p-nitrophenol that is spectrophotometrically measured. Samples were assayed in triplicate and results expressed as percentage of ATX inhibition.
2.3.3. Lipoxygenase inhibition assay
Lipoxygenase (LOX) inhibitor kit was assyed as described by the manufacturer. In this assay, the hydroperoxides produced in the lipoxygenation reaction using 15-lipoxygenase are detected and measured. Samples were assayed in triplicate and results expressed as percentage of LOX inhibition.
2.4. Antioxidant activity
2.4.1. Oxygen radical absorbance capacity (ORAC)
Different concentrations of the peptides (5–500 μM) were assayed in triplicate. For that, 140 μL of sample in 75 mM phosphate buffer (pH 7.4) was added 70 μL of 200 nM fluorescein and kept 15 min at 37 °C. After adding 70 μL of 80 mM AAPH, fluorescence was read every minute for 100 min. The excitation and emission wavelengths were 485 and 538 nm, respectively. Trolox (2–16 μM) was used as standard and tryptophan as positive control. The ORAC values were determined by integrating the relative fluorescence curve and plotted against trolox concentration in order to obtain a standard curve. Results were expressed as nanomoles of trolox equivalents (TE) per mg of sample.
2.4.2. ABTS radical scavenging capacity
In order to perform the ABTS assay, 7 mM of ABTS was dissolved in 2.45 mM potassium persulfate, keeping it in the dark for 12–16 h at room temperature to produce the radical cation ABTS•+. The ABTS•+ solution was diluted with 50 mM phosphate buffer saline (PBS; pH 7.4) to obtain an absorbance of 0.70 ± 0.02 at 734 nm. Different concentrations of the peptides (0.1–2 mM) were assayed in triplicate. For that, 10 μL of sample and 990 μL of ABTS•+ solution was incubated 6 min and the absorbance measured at 734 nm. The standard was trolox (0.05–2 mM), ascorbic acid the positive control and PBS the negative control. The ABTS radical scavenging activity was determined and plotted against the trolox concentration. Results were expressed as nanomoles of TEAC (trolox equivalent antioxidant capacity) per mg of sample.
2.5. ACE-I inhibitory activity
The methodology developed by Sentandreu and Toldrá (2006) was followed. 50 µL of sample was mixed with 50 µL of ACE-I (3 mU/mL in 150 mM Tris buffer, pH 8.3). Then, 200 µL of substrate Abz-Gly-p-nitro-Phe-Pro-OH (0.45 mM in 150 mM Tris buffer with 1.125 mM NaCl, pH 8.3) was added to start the reaction and the fluorescence measured after incubating 45 min at 37 °C. The excitation and emission wavelengths were 355 and 405 nm, respectively. The assay was done in triplicate and captopril used as positive control. Results were expressed as percentage inhibition of ACE-I.
2.6. Statistical analysis
One-way analysis of variance (ANOVA) and Fisher’s multiple range tests were performed using the software XLSTAT 2011 v5.01 (Addinsoft, Barcelona, Spain). Results were expressed as the mean of 3 replicates ± standard deviations, and differences were considered significantly at P < 0.05.
| 3. Results and discussion | ▴Top |
3.1. Anti-inflammatory activity of dry-cured ham peptides
The anti-inflammatory activity of the peptides was evaluated by measuring inhibition percentages of PAF-AH, ATX, and LOX. Recently, a clinical study evaluated the cause-effect relationship between dry-cured ham consumption and cardiovascular effects, analysing the changes in monocytes and platelets as involved agents in inflammatory and thrombotic responses, which resulted affected together with the levels of plasmatic P-selectin, monocyte chemoattractant protein-1 (MCP-1) and interleukin 6 in healthy subjects (Martínez-Sánchez et al., 2017).
Nineteen of the assayed peptides showed PAF-AH inhibitory activity, with values ranged from 1.28% to 26.06% (Figure 1a), and FNMPLTIRITPGSKA was the peptide showing the highest PAF-AH inhibition. Recently, PAF-AH inhibitory peptides were found after the digestion of GI endogenous proteins such as lysozyme and trypsin (Dave et al., 2016), and after simulated GI digestion of dry-cured ham by-products (Gallego et al., 2019). Also a papain protein hydrolysate and seven identified peptides from the macroalga Palmaria palmata showed PAF-AH inhibitory activity, being NIGK the peptide with the highest inhibition, 50.74% at 1 mg/mL (Fitzgerald et al., 2013). The PAF-AH enzyme plays a relevant role in vascular inflammation because it degrades oxidised phospholipids and generates the pro-inflammatory lysophosphatidylcholine and oxidised non-esterified fatty acids. So, this enzyme has been described to be a promising therapeutic target for the prevention of inflammation and atherosclerotic lesions (Wilensky et al., 2008).
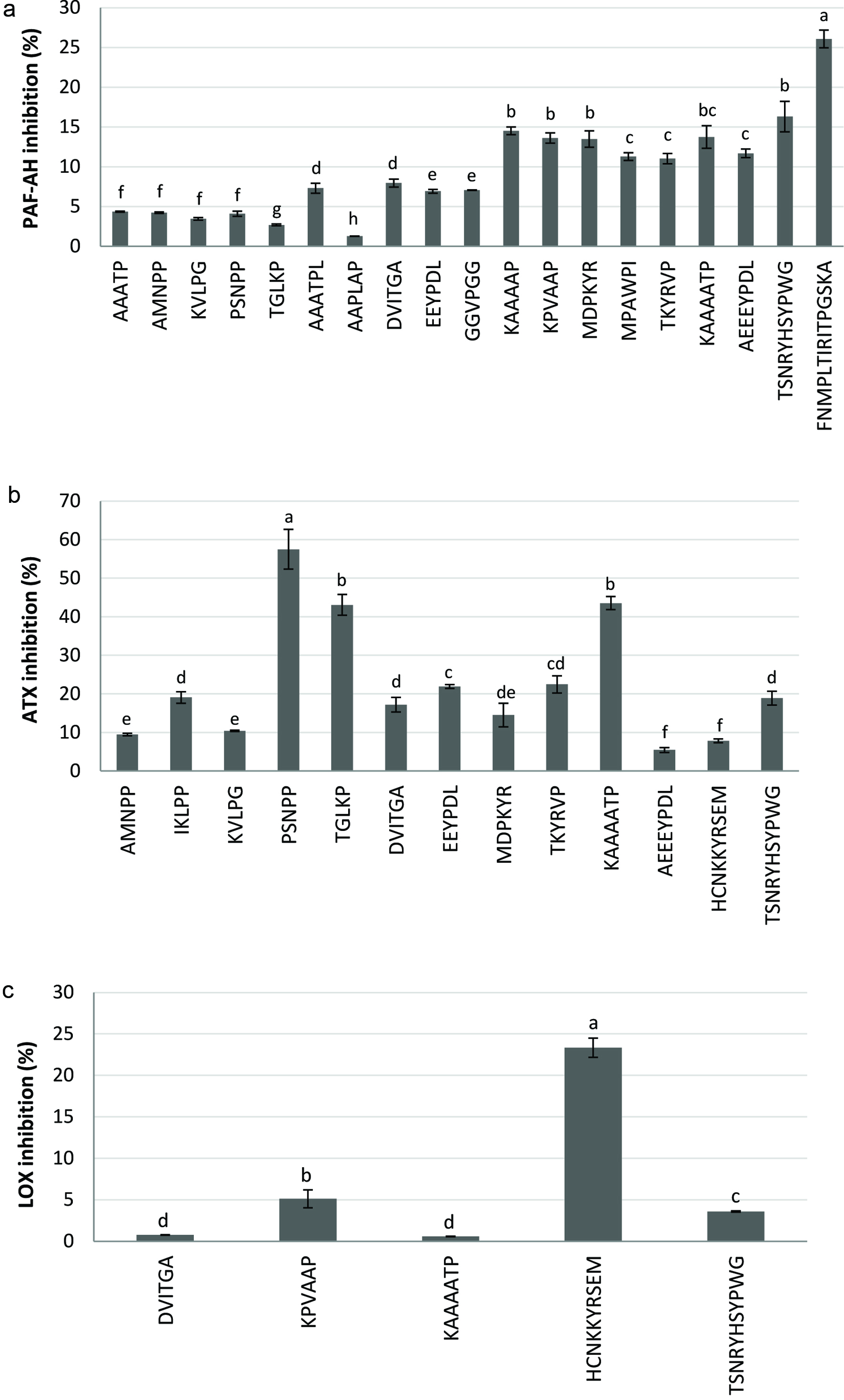 Click for large image | Figure 1. Anti-inflammatory activity of the dry-cured ham peptides expressed as a) platelet-activating factor-acetylhydrolase (PAF-AH) inhibitory activity, b) autotaxin (ATX) inhibitory activity, and c) lipoxygenase (LOX) inhibitory activity. Bar letters indicate significant differences among the values at P < 0.05. |
The ATX enzyme is a lysophospholipase D that hydrolyses lysophosphatidylcholine to generate lysophosphatidic acid (LPA), which stimulates multiple cell signaling mechanisms. The ATX-LPA pathway is implicated in several pathologies including inflammation, tumor progression and nervous system diseases (Gierse et al., 2010). As can be seen in Figure 1b, thirteen peptides presented ATX inhibition, with values between 5.44% and 57.49%. The peptide PSNPP was found as the strongest inhibitor, followed by TGLKP and KAAAATP.
When assaying LOX inhibition, only five of the assayed peptides showed inhibitory activity, with a maximum value of 23.33% for the peptide HCNKKYRSEM (see Figure 1c). The LOX enzyme plays a key role in unsaturated fatty acid metabolism, generating reactive hydroperoxides and lipid oxidative products. Besides, the LOX catalyses the formation of signal molecules such as leukotrienes and lipoxins, that when increased or after inappropriate production can promote the development of inflammation, atherosclerosis, and cancer (Schurink et al., 2006). Some peptides derived from β-casein (Schurink et al., 2006) and collagen proteins (Chen et al., 2018) as well as hydrolysates and peptide fractions obtained from edible insects (Zielińska et al., 2017) have been reported to exert in vitro anti-inflammatory potential expressed as LOX inhibition.
Despite the assayed dry-cured ham-derived peptides showed, in general, low in vitro anti-inflammatory activity, further studies could elucidate the sequences of other peptides contributing to the inhibitory activities as well as evaluate possible synergistic effects among peptides. Current challenges in the study of anti-inflammatory peptides are mainly due to the diversity and complexity of the inflammatory responses. In fact, each peptide could have a specific structure for their activity according to the followed molecular mechanism (Guha and Majumder, 2018). Up to date, several natural anti-inflammatory peptides derived from animal, plant, bacteria and marine organisms as well as synthetic peptides have been reported as potential candidates for reducing inflammation (Dadar et al., 2019). For example, the ACE-inhibitory tripeptide LSW from a soy protein hydrolysate has shown anti-inflammatory activity on vascular smooth muscle cells through its participation in several signaling pathways (Lin et al., 2017), whereas the casein-derived VPP modulated monocyte adhesion to vascular endothelium through its effect on a MAP kinase pathway (Aihara et al., 2009). The tripeptides IRW and IQW from egg ovotransferrin protein were able to limit the expression of pro-inflammatory cytokines and oxidative stress in endothelial cells (Liao et al., 2016; Majumder et al., 2013), and recently, longer egg-derived peptides such as DEDTQAMPFR and MLGATSL also showed anti-inflammatory effects on Caco-2 cells (Zhang et al., 2019). These studies suggest the importance of peptide structure in the regulatory pathway in which they participate to exert anti-inflammatory activity.
3.2. Antioxidant activity of dry-cured ham peptides
The antioxidant activity was measured by using ORAC and ABTS radical scavenging capacity assays because the lack of a single standard methodology for characterising the complete antioxidant activity of a sample (Huang et al., 2005)
The antioxidant results obtained using the ORAC assay are shown in Figure 2a, excluding peptides with values lower than 100 nmol TE/mg. Six peptides presented a significant antioxidant activity according to the ABTS assay (Figure 2b). Peptides MDPKYR and TKYRVP were the strongest antioxidants, showing ORAC values of 3,087.5 and 2,886.8 nmol TE/mg, respectively, and ABTS values of 5,444.30 nmol TEAC/mg and 6,987.86 nmol TEAC/mg, respectively. The presence of tyrosine (Y) and the positive charged amino acids arginine (R) and lysine (K) in these peptide sequences could play an important role in this observed antioxidant activity, as well as could contribute to the antimicrobial activity of these peptides as previously reported by Castellano et al. (2016). Also other residues such as tryptophan (W), methionine (M), cysteine (C), histidine (H) and phenylalanine (F) contained in the sequence, as well as the structure and hydrophobicity of the peptides would determine their bioactivity and bioavailability (Liu et al., 2016).
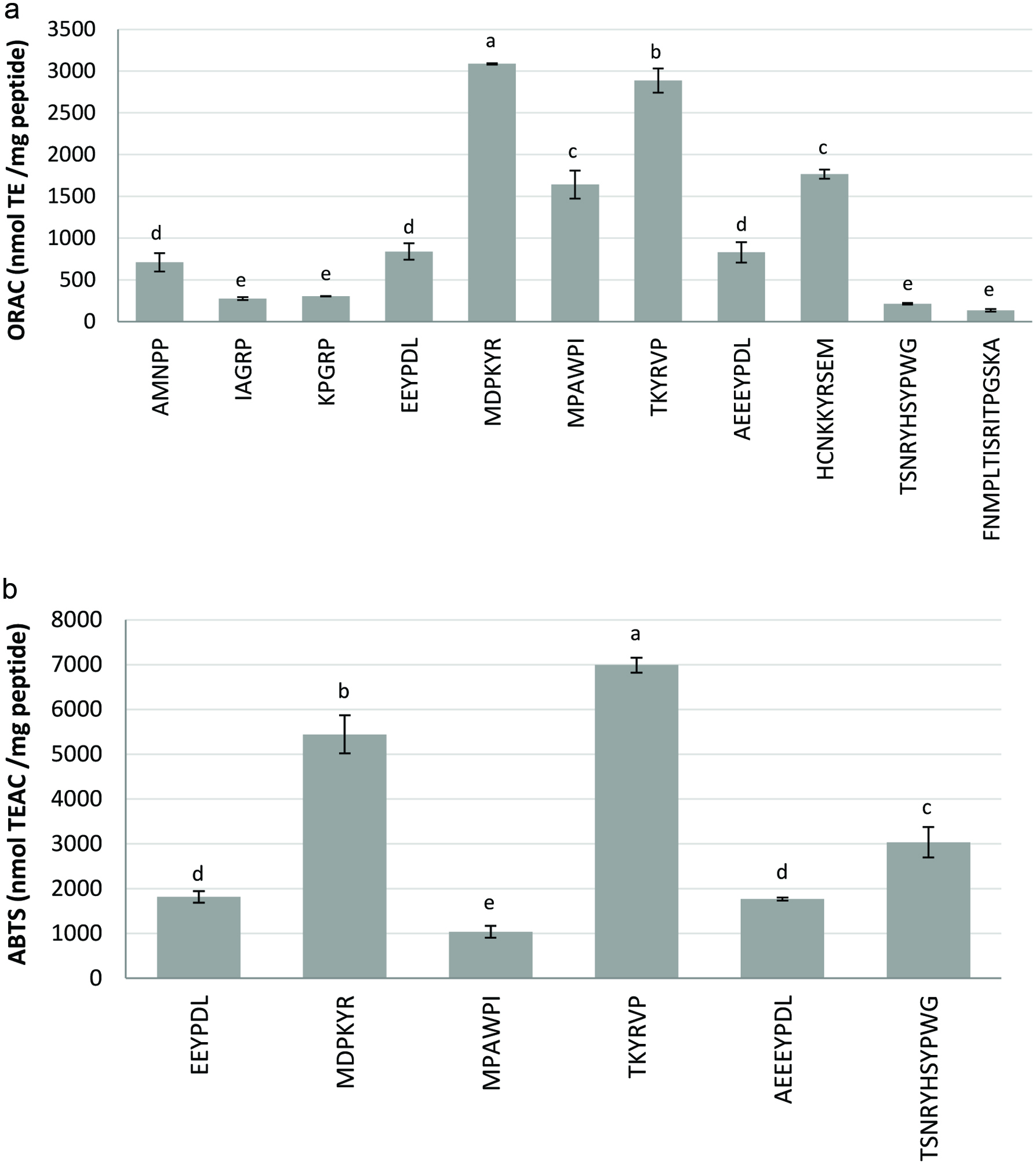 Click for large image | Figure 2. Antioxidant activity of the dry-cured ham peptides determined by a) oxygen radical absorbance capacity (ORAC) assay, and b) ABTS radical scavenging capacity. Bar letters indicate significant differences among the values at P < 0.05. |
The scavenging of reactive oxygen species (ROS) by antioxidant peptides prevents or retards protein and lipid oxidative processes in food, and can protect the body against oxidative stress and CVD. ROS may alter vascular function by oxidative modifications of nucleic acids and proteins, decrease NO bioavailability, and promote inflammation, apoptosis and other alterations. These processes can lead to functional and structural changes on the vascular system associated with hypertension and other CVD (Chakrabarti et al., 2014; Schulz et al., 2011).
3.3. ACE-I inhibitory activity of dry-cured ham peptides
The ACE-I inhibitory activity of the assayed peptides is shown in Figure 3. The highest activity (99.34% inhibition) was observed for the peptide HCNKKYRSEM, whereas GGVPGG and TKYRVP presented inhibitory percentages over 80%. Peptides TSNRYHSYPWG and FNMPLTIRITPGSKA also showed good ACE-I inhibition, with values around 70%. Peptides HCNKKYRSEM and TKYRVP were previously assayed for antilisterial activity (Castellano et al., 2016) and GGVPGG for antioxidant activity measured by DPPH radical-scavenging and ferric-reducing power assays (Mora et al., 2014). Escudero et al. (2014) reported the ACE inhibitory activity of several peptides generated in Spanish dry-cured ham. Peptides IAGRP, AAPLAP, KAAAAP, and KPVAAP showed over 80% inhibition at a concentration of 1 mM, whereas peptides AAATP, IKLPP, KPGRP, TGLKP, and KAAAATP presented values ranged from 51% to 73% (data not shown in Figure 3). The stability of the peptide IAGRP against in vitro digestion and heat treatments was also described (Escudero et al., 2014). Additionally, a previous study using a Caco-2 cell monolayer evidenced the transepithelial transport of the intact peptide KPVAAP as well as the generation and absorption of fragments derived from the peptides AAPLAP and KPVAAP that maintained their ACE-I inhibitory activity (Gallego et al., 2016). The size of peptides as well as the composition and location of the amino acids contained in their sequences determine to a large extend their resistance to enzymatic hydrolysis and thus their bioactivity (Segura-Campos et al., 2011). The presence of amino acids such as proline (P), alanine (A), and aromatic or aliphatic amino acids close to the C-terminus as well as hydrophobic residues at the N-terminus has been reported to promote its binding to ACE. In general, hydrophobic peptides display high affinity to the sub-sites located on the active site of ACE and inhibit the enzyme. Moreover, peptides containing proline and hydroxyproline have been reported to have high resistance to digestive enzymatic hydrolysis, improving their bioavailability (Segura-Campos et al., 2011).
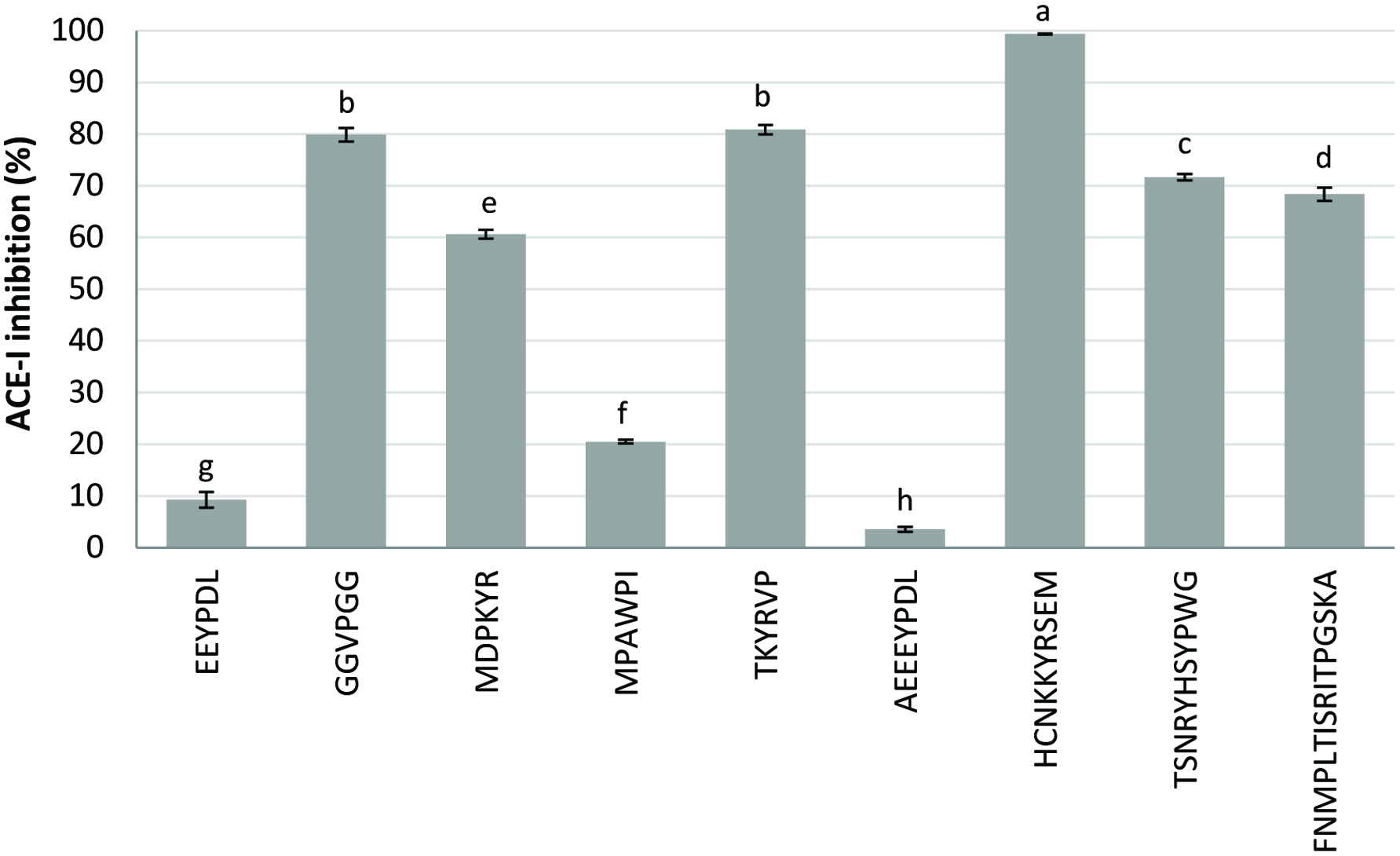 Click for large image | Figure 3. Angiotensin I-converting enzyme (ACE-I) inhibitory activity of the dry-cured ham peptides. Bar letters indicate significant differences among the values at P < 0.05. |
The action of ACE-I inhibitory peptides in the RAAS by lowering blood pressure has been widely reported, although the activity observed in vitro does not always correlate with in vivo effects. The digestion by GI enzymes would determine the availability of peptides, since the effectiveness to inhibit ACE depends on the peptide structure and the absorption is greatly influenced by the peptide size. Furthermore, there are several pathways involved in regulating blood pressure, so peptides may also interact with enzymes other than ACE-I (Wu et al., 2017). Several studies have evidenced the antihypertensive action in spontaneously hypertensive rats (SHR) of dry-cured ham peptides (Escudero et al., 2013a; Mora et al., 2015) as well as of pork meat peptides generated by the action of digestive enzymes (Escudero et al., 2012). Moreover, a recent study with human volunteers suggested that the regular consumption of dry-cured ham would not modify arterial blood pressure and urine sodium excretion despite increasing daily salt intake (Montoro-García et al., 2017), probably due to the participation of different antihypertensive mechanisms (Wu et al., 2017). ACE-I inhibitory peptides can prevent both the accumulation of the pro-inflammatory angiotensin II and the hydrolysis of bradykinin, leading to a reduction of free radical levels and an increase of NO release in the vascular system. Thus, these peptides might be used not only for the treatment of hypertension, but also to prevent oxidative stress and inflammation-associated CVD (Ferrario and Strawn, 2006).
3.4. Multifunctional peptides
Some food-derived bioactive peptides have been described to be multifunctional as they can exert two or more health-promoting activities that may or may not be related (Li and Aluko, 2010; Udenigwe and Aluko, 2012). So, proteins with high proportion of positively charged and hydrophobic residues would be a good source of multifunctional peptides (Rao et al., 2012). In our study, several peptides have displayed more than one bioactivity. The peptide KPVAAP exhibited high ACE-I inhibition, and certain anti-inflammatory activity expressed as PAF-AH and LOX inhibition. As mentioned previously, this peptide could be transported intact through the intestinal epithelium (Gallego et al. 2016), suggesting its potential to exert several bioactive actions in the organism. Also peptides MDPKYR, TKYRVP, HCNKKYRSEM, and TSNRYHSYPWG, which were previously described as antibacterial peptides by Castellano et al. (2016), have displayed several in vitro bioactivities. So, MDPKYR and TKYRVP showed ACE-I inhibitory, antioxidant capacity in ORAC and ABTS assays, PAF-AH inhibitory and ATX inhibitory activities. The peptide HCNKKYRSEM presented ORAC antioxidant activity and ACE-I, ATX and LOX inhibition, whereas TSNRYHSYPWG inhibited ACE-I, PAF-AH, ATX, and LOX and displayed certain antioxidant activity in both ORAC and ABTS assays. Additionally, peptides TGLKP and KAAAATP have shown to be good ACE-I inhibitors and presented PAF-AH and ATX inhibitory activities. These results suggests the potential of some peptides generated in dry-cured ham as suitable candidates for further research on natural and multifunctional food ingredients to control and prevent CVD. Multifunctional peptides can act on two or more different receptors with complementary mechanism of action to gain additive or synergistic effect compared to single-activity peptides. Statistical modelling is a useful tool to study structure-activity relationships since the size and structural characteristics of the peptides are the key factors determining their molecular mechanisms and functions (Agyei et al., 2015). Bioavailability studies are also required to confirm potential health benefits of such peptides using animal models or clinical trials.
| 4. Conclusions | ▴Top |
Different peptides from Spanish dry-cured hams have shown for the first time in vitro anti-inflammatory activity determined as PAF-AH, ATX, and LOX inhibition as well as confirmed ACE-I inhibition, and antioxidant activity. These results would support the beneficial effects on cardiovascular health previously observed in clinical trials in relation to hypertension and inflammatory processes. Additionally, this study has revealed that some of the assayed peptides such as KPVAAP, MDPKYR, TKYRVP, and HCNKKYRSEM can exert more than one bioactivity, increasing their health-enhancing potential. Additional studies are needed to evaluate the bioavailability and in vivo efficiency of these peptides.
Acknowledgments
Grants AGL2014-57367-R and AGL2017-89831-R and FEDER funds from the Spanish Ministry of Economy, Industry and Competitiveness and Intramural 201870E006 grant from CSIC are acknowledged. Ramón y Cajal postdoctoral contract to LM is also acknowledged.
| References | ▴Top |