| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 1, March 2018, pages 41-55
Inositol phosphates: health implications, methods of analysis, and occurrence in plant foods
Quynh H. Duonga, Karen G. Lapsleyb, Ronald B. Pegga, *
aDepartment of Food Science & Technology, College of Agricultural and Environmental Sciences, The University of Georgia, 100 Cedar Street, Athens, GA, 30602, USA
b, Almond Board of California, 1150 Ninth Street, Suite, 1500, Modesto, CA, 95354, USA
*Corresponding author: Dr. Ronald B. Pegg, Department of Food Science & Technology, The University of Georgia, 100 Cedar Street, Athens, GA 30602-2610, USA
DOI: 10.31665/JFB.2018.1126
Received: January 5, 2018
Revised received & accepted: January 15, 2018
| Abstract | ▴Top |
Inositol phosphates (InsPs), especially myo-inositol hexakisphosphate (InsP6), are important binders of phosphorus and minerals in plant seeds. However, they have long been considered as anti-nutritional components of plant foods due to their possible negative effects on the absorption of minerals and proteins in mammals. On the other hand, recent findings have found InsPs to be ubiquitous in eukaryote cells and actively participating in multiple cell functions. In vivo and in vitro studies have also documented the preventive potential of these compounds against the development of a wide range of diseases. In light of these findings, interest in the relationship between these compounds and human health has been renewed. It is suggested that the interactions of InsPs with other nutrients in the gut are complex, that the absorption of dietary InsPs might be implied but is not certain, and that the disease fighting capabilities of InsPs hold both promises and limitations. At the same time, the analysis of these compounds in foods and biological samples still faces many challenges, calling for more advanced modification and developments in the future.
Keywords: Inositol phosphates (InsPs); Phytic acid; Phytate; Antioxidant; Chemoprevention
| 1. Introduction | ▴Top |
Phytic acid (myo-inositol hexakisphosphate, InsP6), also frequently referred to by its salt form phytate and phytin, is an important component of plant seeds and a controversial constituent in the human diet. The compound is commonly consumed in high amounts from staple cereals and legumes, in which it serves as the main phosphorus reservoir and one of the major binders of minerals for the seeds (Kornegay, 2000). While plant seeds possess endogenous phytases to hydrolyze InsP6 and its salts upon germination, humans produce very minimal amount of this enzyme group endogenously and must rely on microbial phytases, which are often insufficient for the complete digestion of the InsP6 content in foods (Lopez et al., 2002). At the pH levels of the human digestive tract, InsP6 can form insoluble salts and complexes with minerals and macronutrients, rendering these nutrients unavailable for absorption or other activities (Konietzny et al., 2006; Kumar et al., 2010). As a result, this compound has been implied to be an antinutrient even though its negative effects have not been well described in vivo. On the other hand, emerging research is showing that InsP6 as well as lower forms of inositol phosphates (InsPs), including myo-inositol mono-, bis-, tris-, tetrakis-, and pentakisphosphate (InsP1–5), are widely present in eukaryote cells and may have important physiological functions (Irvine and Schell 2001; Sauer and Cooke 2010; Shears 1998). InsP6 has also been found to have anti-inflammatory and anticancer effects in various types of cell lines (Vucenik et al., 2004). At the same time, it is still unclear whether dietary InsPs can be absorbed in the human digestive tract and mobilized for metabolic activities, as no carrier for these compounds has been detected, but some studies have reported intracellular InsPs to be dependent on extracellular InsPs (Grases et al., 2002). Overall, there remain many questions on the role of dietary InsPs in human health and disease prevention. This contribution aims to provide an overview on the development of the understanding pertaining to the relationship between dietary InsPs and human health, the efforts to quantify InsPs in major food products, as well as the on-going challenges that are important to the study of these compounds.
| 2. Chemical properties of inositol phosphates | ▴Top |
InsPs refer to the phosphorylated compounds of inositol (cyclohexane-1,2,3,4,5,6-hexol), in which one or more hydroxyl moieties on the six-carbon ring of inositol is replaced with a phosphate group. Inositol exists in nine possible stereoisomers, of which myo-inositol (Figure 1) is the most abundant species found in nature. As a result, “inositol phosphate” commonly refers to a phosphorylated myo-inositol. Depending on the number of phosphate groups on the inositol ring, a compound in this family can be referred to as myo-inositol mono-, bis-, tris-, tetrakis-, pentakis, or hexakisphosphate (InsP1–6). All lower inositol phosphates (InsP1–5) display isomerism.
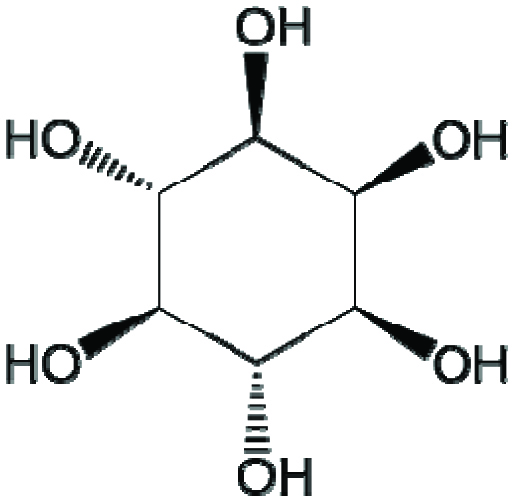 Click for large image | Figure 1. Chemical structure of myo-inositol. |
To date, myo-inositol hexakisphosphate (InsP6 –Figure 2), also commonly referred to as phytic acid, has garnered the most attention due to its capability to chelate mineral cations in the digestive system and its high apparent concentration in plant seeds. The compound is sterically stable when one phosphate is in the axial position and five phosphates are in the equatorial position (1ax/5eq) (Bohn et al., 2008). Depending on the pH of the medium and the presence of other cations, InsP6 can invert into the sterically hindered form, in which one phosphate is in the equatorial position and five are in the axial position (5ax/1eq). Under the effect of heat, acid treatment, or enzymatic activity by the enzyme group phytase, InsP6 can be dephosphorylated into InsP1–5 as well asmyo- inositol.
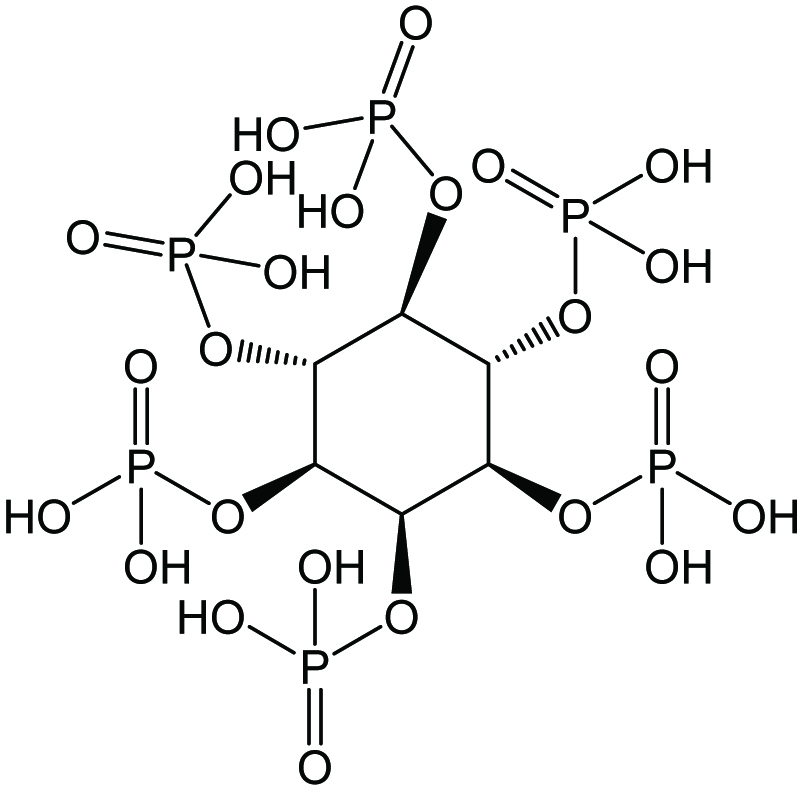 Click for large image | Figure 2. Chemical structures of myo-inositol hexakisphosphate (InsP6) or phytic acid. |
As indicated by their prefixes, the phosphate groups of InsP2–6 are not internally connected and these compounds are polydentate ligands (Bohn et al., 2008). InsPs are capable of binding mineral cations such as calcium, zinc, copper, cobalt, manganese, iron, and magnesium, forming the salt phytate (phytin, if it is a mixed salt). The binding capability varies among InsPs and is dependent on the type of mineral. For example, in vitro studies at pH 3 to 8 have found that InsPs typically have a greater affinity with copper, zinc, and nickel than with other mineral cations (Persson et al., 1998; Tsao et al.,1997; Vasca et al., 2002). InsP6 and InsP5have higher binding strength than InsP3 and InsP4, and the distribution of the phosphate groups on the inositol ring also differentiates binding affinity among stereoisomers. InsPs and their salts with monovalent cations, such as sodium and potassium, are typically water soluble, while salts with multivalent cations have varying degrees of solubility depending on the pH of the environment and the presence of competing cations.
At low pH levels, InsP6 can bind to the cationic groups of proteins and forms insoluble complexes (Ellis and Morris, 1983). This binding might include interactions with the α-NH2 terminal group, the ε-NH2 group of lysine, the imidazole group of histidine, and the guanidyl group of arginine. At pH levels higher than these proteins’ isoelectric points, soluble protein–mineral–phytate complexes may form (Gemede, 2014).
| 3. The physiological roles of extraneous inositol phosphates | ▴Top |
InsPs are ubiquitous in seeds, which include cereals, legumes, and nuts that are frequent components in human and animal diets. InsP6 is said to typically comprise >85% of InsP composition and serves as a mineral binder, phosphorus source, and energy source for the seed (Kornegay, 2000). During germination, phosphorus and minerals will be released from phytate by phytases. Many animals, especially ruminant types, have been found to depend on microbial phytases in the gut for InsP catabolism. In humans, phytase secretion appears to be limited, and microbial phytases in the large intestine are likely to be important to the digestion of InsPs (Iqbal et al.,1994; Lopez et al., 2002; Schlemmer et al.,2009).
3.1. Inositol phosphates as an antinutrient
Dietary InsPs are often viewed as undesirable due to their capability to chelate mineral cations and to form complexes with protein, carbohydrate, and lipid, which reduce the availability of these nutrients in the body (Kumar et al., 2010). InsP6 and InsP5 are the most abundant InsPs in foods and are also the InsPs with the strongest binding capabilities. In many countries where whole grain cereals and legumes are staples, InsPs, along with other components, such as inorganic phosphates, polyphenols, and nondigestible fiber, may exacerbate on-going nutrient deficiency problems (Schlemmer et al., 2009). Of most concern are the potential adverse effects on the absorption of zinc and iron–two minerals with prevalent deficiencies around the world–as well as calcium and magnesium. Furthermore, the formation of insoluble phytates/phytins and complexes also render phosphorus unavailable for the body (Konietzny et al., 2006).
Zinc is strongly sequestered by InsP6, and zinc phytate forms readily within the pH range of 3 to 7, which includes the pH range of the duodenum, where the absorption of this mineral occurs (Persson et al., 1998). Among InsP3–6, only InsP6 and InsP5 have been found to exert a significant inhibitory effect on zinc absorption (Lönnerdal et al.,1989). The World Health Organization (WHO) considers zinc absorption to be low when it is <15% (compared to ∼30% in most diets), which can be caused by a phytate:zinc mole ratio of more than 15 (World Health Organization, 2014). On the other hand, the International Zinc Nutrition Consultative Group (IZiNCG) set the low level of zinc absorption to be 18% in adult men and 25% in adult women, which may occur when the phytate:zinc mole ratio is more than 18 (IZiNCG et al., 2004). The difference between these prediction models highlights the need for more human studies on this topic. It is notable that other than the phytate:zinc mole ratio, the level of inhibition can also be affected by the presence of secondary cations, such as calcium and magnesium ions, as secondary cations can either displace Zn2+ from InsPs’ binding sites or form insoluble phytins in conjunction with Zn2+(Forbes et al., 1984; Gemede, 2014; Walter et al., 2000).The impact of these interactions in humans is not yet fully understood, and while IZiNCG currently considers calcium and protein to be insignificant factors in predicting zinc absorption, the organization also emphasizes that more studies on the interactions of these nutrients with zinc and phytate during the digestion process are necessary. For example, in a study with forty human subjects, Fredlund et al. (2006) experimented by adding various levels of InsP6 ranging from 0 to 1344 µmol to a low-phytate bread roll containing 47µmol zinc and 66.6 mmol calcium. The author found that zinc absorption decreased from 21.5% to 14.45% when the InsP6 content in the roll reached a phytate:zinc mole ratio of 5.7:1 (260 µmol InsP6/roll). By the time the phytate:zinc mole ratio surpassed 15:1, the overall zinc absorption was only 7.4%, which was much lower than what was predicted by WHO and IZiNCG.
Of the two types of dietary iron, heme and non-heme iron, dietary InsP6 and InsP5 have been implicated with decreased non-heme iron absorption (Reddy et al., 1996; Sandberg et al., 1999). As heme iron only comes from animal foods, there is concern for populations whose diet contains little to no meat, as a high proportion of phytate-containing grains may experience exacerbated iron deficiency. With regard to non-heme iron, InsP4 and InsP3 did not exhibit an inhibitory effect on absorption when they were the only InsPs in foods, but when present in combination with InsP5 and InsP6, the inhibitory efficacy was stronger than when only InsP5 and InsP6 were in the samples (Sandberg et al., 1999). This finding suggested that there could be a synergistic effect between InsP5–6 and lower InsPs, where an iron ion at a high degree of covalency could bind with two or three different InsP molecules simultaneously, some of which could be InsP3–4. The authors also remarked that different isomeric forms of InsP3–4 could have exerted varying levels of effect in this case, as myo-1,2,6-inositol trisphosphate and InsPs with the 1,2,3-trisphophate grouping can form more stable complexes with Fe3+. On the other hand, the negative effect of InsPs on iron absorption was not found in all studies, showing that this effect can be influenced by factors other than the levels of InsPs and iron that are yet to be identified (House and Welch, 1987; Simpson et al., 1981). Hallberg et al. (1987) have observed that iron phytate in foods can be present in notable quantities as monoferric phytate, which is well absorbed. Yet, when sodium phytate or a mixture of magnesium phytate and potassium phytate were added to the samples, iron absorption decreased, likely due to the formation of insoluble phytins.
Similarly, the inhibitory effect of phytate on calcium and magnesium ion absorption may be suggested, but experimental values have been inconclusive (Crea et al.,2006). Miyazawa et al. (1996) reported a negative effect on the apparent absorption of magnesium but not that of calcium in rats. Lönnerdal et al. (1989) observed inhibition of calcium ion uptake by InsP6 and InsP5, but not by InsP4 and InsP3. It is also unclear whether lowered absorption of these minerals actually translates into health concerns. A survey study by López-González et al. (2008) assessed that dietary phytate is not a risk factor of osteoporosis, and increasing phytate consumption can even be linked with higher bone mineral density.
In pig and poultry nutrition, phytase supplementation to the fodder has been demonstrated to improve amino acid digestibility, which implied that InsPs impedes amino acid absorption in the gut (Selle et al., 2000). The form of InsP can influence this effect, as the magnesium–potassium salt of InsP6 showed a higher inhibitory strength than free InsP6 (Onyango et al., 2008). InsP6 has also been shown to inhibit several digestive enzymes such as α-amylase, lipase, and various proteinases (e.g., pepsin, trypsin, chymotrypsin) in fish and poultry (Denstadli et al., 2006; Khan and Ghosh, 2013; Liu et al., 2010). These effects may be explained by the non-specific bindings of InsPs with cationic moieties of amino acids and proteins, followed by the precipitation of these complexes at low pH levels. In additional, ternary protein–mineral–phytate complexes may form, with a divalent mineral cation serving as the cationic bridge between phytate and an anionic moiety of a protein. Examples of these anionic moieties include the unprotonated imidazole group of histidine and the ionized carboxyl groups of other proteins (Selle et al., 2000). The solubility of these complexes is also dependent on pH. Among soluble mineral–phytate complexes and ternary protein–mineral–phytate complexes, an equilibrium may exist. Preference for either type of complex is influenced by ion concentrations as well as the degree of strain placed on the inositol ring by each type of complex (Champagne et al., 1990). For instance, the equilibrium involving calcium ion can be illustrated as follows:
From these perspectives, InsPs appear to be generally antinutritional. Breeding, genetic modification, germination, soaking, heat treatment, and fermentation are methods that have been investigated and recommended to reduce InsP levels in plant foods. On the other hand, it must be noted that InsPs are only some among many dynamic factors that can interact with each other in the digestive tract to affect mineral absorption. For instance, the inhibitory effect of InsPs on zinc ion absorption can be reduced with dietary protein, while administration of ascorbic acid with a phytate-containing meal has been demonstrated to improve iron absorption (Davidsson et al., 1994; Sandström et al., 1989; Siegenberg et al., 1991). Regarding magnesium, many unrefined plant foods rich in phytate are also rich in this mineral, and it has been argued that the eventual amount of magnesium absorbed from these foods may be equal to or higher than from low-phytate containing foods (Levrat-Verny et al., 1999). In rats, resistant starch has been shown to be able to negate the effects of phytic acid on the absorption of iron, zinc, and copper (Lopez et al., 1998). The authors suggested that a potential mechanism behind this effect is the production of short-chain fatty acids (SCFA) during the fermentation process, which adjusts the pH of the environment. Additionally, these SCFA can compete with InsPs to form complexes with mineral cations, which may be low in charge and can enter the enterocytes.
The dynamic of InsP6 protonation and complex formation is difficult to study and summarize because it is strongly affected by several factors at the same time. The molecule can donate up to twelve protons and exhibits multiple acid dissociation constants across a wide range of pH values, depending on the ionic strength of the medium and the presence of other ions (Crea et al., 2008; Heighton et al., 2008). The stability and solubility of the complexes formed between InsP6 and any type of ion are also affected by these aspects. Meanwhile, the condition of the digestive system can vary strongly. The average pH level in the human gastrointestinal tract varies between 1 and 7.5, with possible modifications caused by foods consumed in the meal (Evans et al., 1988; Kong and Singh, 2008).Taking these effects into account, improving mineral absorption by maintaining a diverse diet might be a more positive approach to avoid mineral deficiency than reducing InsP contents in foods. Some amount of dietary InsPs may be important, because InsPs’ general capability to chelate mineral ions may help reduce the absorption of toxic trace elements such as cadmium and lead (Schlemmer et al., 2009). Moreover, new findings on the roles of InsPs in normal body functions and disease states propose that InsPs may be essential and beneficial to human health.
3.2. Inositol phosphates as participants in cell functions and the role of dietary inositol phosphates
In recent decades, insights on the importance of InsPs to eukaryote cells have emerged.Ins(1,4,5)P3, Ins(1,3,4)P3, Ins(1,3,4,5)P4, and Ins(1,3,4,5,6)P5 have been found to work dynamically in different Ca2+ signaling pathways (Irvine and Schell, 2001; Lückhoff and Clapham, 1992; Streb et al.,1983; Tsubokawa et al., 1996; Vajanaphanich et al., 1994). Both myo-inositol-1,3,4,5,6-pentakisphosphate and InsP6have important roles in mRNA export, genomic stability, and apoptosis (Loss et al.,2013; Majerus et al., 2008; Monserrate and York, 2010). More than half of the 63 possible InsP isomers have been identified in various types of cell (Irvine and Schell, 2001; Shears 1998). Different reactions by InsP pools to specific stimulations imply that these compounds may actually have many more functions to be investigated (Irvine and Schell, 2001; Otto et al., 2007).
Many different pathways for the synthesis of InsPs by cells have been discovered in plants, molds, and animals. Phosphatidylinositol 4,5-bisphosphate can be hydrolyzed into Ins(1,4,5)P3, which, together with inositol, can be rapidly converted by plant and slime mold cells into other InsPs through a series of positionally selective and sequential kinase reactions. In mammalian cells, glucose-6-phosphate is believed to serve as the precursor for Ins (1,4,5) P3, providing a second pathway that is independent of inositol lipid metabolism (Irvine and Schell, 2001; Sasakawa et al., 1995). Yet, both pathways have been found to proceed at extremely slow rates, despite some evidences indicating that the conversion of Ins (1,4,5) P3 into InsP6 in cells can be very fast. The synthesis of InsPs in animals remains an ongoing topic for examination.
It is also worthwhile to mention a class of molecules closely related to InsPs, namely inositol pyrophosphates (PPx-InsPs). Using InsP6 as precursor, these compounds are synthesized by linking additional phosphate(s) to an existing phosphate group on the inositol ring via pyrophosphate bond(s) (Williams et al., 2015). So far, a few members of this family have been described, including several stereoisomers of triphosphoinositol pentakisphosphate (PPP-InsP5), diphosphoinositol pentakisphosphate (PP-InsP5), bis-disphosphoinositol trisphosphate ((PP)2-InsP3), and bisdiphosphoinositol tetrakisphosphate ((PP)2-InsP4). These compounds are sometimes abbreviated as InsP7 and InsP8, although these names fail to signify the important presence of the high-energy diphosphate or triphosphate chain(s). Similar to InsPs, PPx-InsPs are ubiquitous in eukaryote cells and have been inferred to participate in many cell functions, including energy sensing, inorganic phosphate sensing, and immune response (Williams et al., 2015; Wilson et al.,2013). Because PPx-InsPs are present in very small amounts, dietary PPx-InsPs are unlikely to be absorbed at significant levels by the human body. Consequently, the body may have to rely on the synthesis of these compounds from InsP6.
At the moment, the contribution of exogenous sources to the maintenance of InsP pools in the human body is also not fully understood. Depending on the pH, InsPs can be negatively charged, which hinder their absorption in the gut. The small size of the inositol ring also leads to a high-charge density. So far, no carrier has been detected for InsPs in the human digestive track. Nevertheless, existing studies suggest that cellular intake of these compounds is possible and that InsP levels in the body are influenced by dietary intakes.
Grases et al. (2002) have demonstrated that in rats fed with different levels of InsP6, the concentration of extracellular InsP6 (in plasma and interstitial fluid) increases with dietary intake, while the concentration of extracellular InsP3 is not significantly affected. In the same study, human epithelial and mesenchymal cells grown in culture media with inositol reached an InsP6 concentration of ∼16 μmol/kg, whether or not InsP6 was supplied in the media, indicating the cells’ capability to synthesize InsP6 from inositol. When these cells were treated with 1.8 mM InsP6 for 1 hour, the intracellular InsP6 content did not change, whereas the intracellular InsP3 content doubled. This effect was attributed to dephosphorylation activity by the cell, which varies in rate depending on cell type (Vucenik and Shamsuddin, 1994). The concentrations of InsP3 and InsP6 in plasma fluid and in cells were thus concluded to have partial links to exogenous InsP6.
By monitoring InsP6 intake through diet and administration of oral doses, Grases et al.(2001) showed that the plasma level and urinary excretion of InsP6 in humans are affected by oral ingestion in both the short and long term. A positive correlation was detected between these two measurements. Under the test conditions, the urinary excretion levels were always much lower than the intake level and would plateau at a certain InsP6 dose, indicating that humans have a limited ability to absorb and/or excrete InsP6. Another study also found that InsP6 absorption is independent of the condition of the stomach (whether empty, empty with an alkalinizer, or full), suggesting that absorption may occur during intestinal transit (Grases et al., 2006).
Using the ileostomy model, Agte et al. (2005) were able to describe the apparent absorption of all six InsPs from traditional Indian vegetarian meals. The level of apparent phosphorus absorption was found to increase from InsP6 to InsP1, although it must be noted that higher InsPs contributed more phosphorus than lower forms due to their higher relative abundance. InsP1, which was consumed in minimal amount, was completely degraded, while absorption for phosphorus from InsP2 only reached 64%. For InsP3–6, which were responsible for ∼96% of the phosphorus amount bound by InsPs in these meals, the % phosphorus absorption varied between 29 and 39. The net absorption of InsP phosphorus from these meals was 35%. As only three subjects were able to complete the trial (two men and one woman), this study was severely limited by sample size. For InsP1–5 output results, it was not possible to distinguish between the amount of InsPs retained from the original meals and the quantity of InsPs generated by the degradation of higher forms. Nevertheless, these results still suggest that InsPs might be degraded and absorbed at a significant degree in the human digestive system.
In a controlled trial with Korean women, Joung et al.(2007) found that the degradation of dietary InsP5 and InsP6 is greater in elderly women compared to that in young women. A rise in dietary phytate levels also induced a significant rise in phytate excretion in young women, but not in elderly women. In both subject groups, phosphorus absorption was not affected when phytate became the main dietary phosphorus source. These results imply that the human body may have a mechanism to alter phytate degradation activity in the digestive tract according to its needs.
It is important to remember that biological samples are particularly complex to analyze, and some of the studies mentioned above used indirect assays for the measurement of InsPs, which might result in inaccurate detection. By using a specific assay, Letcher et al. (2008) have found InsP6 to be undetected in the serum and platelet-free plasma (<1 nM InsP6) as well as in the urine (<5 nM InsP6), suggesting that InsP6 was unlikely to be absorbed in the gut. Wilson et al. (2015) also reached this conclusion when they applied a different specific assay on the same types of samples.As a result, the absorption of dietary InsPs still requires further investigation, especially with direct analysis methods.
3.3. Inositol phosphates’ disease fighting abilities
Recently, many findings have highlighted InsPs’ disease fighting capabilities. These include the prevention of pathological calcification, antioxidative activities, and anticancer effects. InsP6 was first considered as an agent against pathological calcification in hypercalcuria patients due to its possible interference with calcium absorption (Nassim and Higgins, 1965). Later, InsP6 was found to have strong crystallization inhibiting property against these unwanted calcium salts, which allows it to be effective even at a relatively low concentration. Experimentation with synthetic urine has demonstrated that phytic acid can completely stop the growth of calcium oxalate stones, which is the predominant form of kidney stones (Costa-Bauzá et al., 2005). In animal models, InsP6 has been shown to reduce calcium deposition in kidneys and related organs, as well as slow down the growth of dystrophic calcification plaques in the cardiovascular system (Grases et al., 1996; Grases et al., 2004b). In humans, lower urinary concentration of InsP6 has been associated with active calcium oxalate stone-formers, and high intake of dietary InsP6 has been suggested to decrease the risk of kidney stones in these subjects (Conte et al., 1999; Curhan et al.,2004; Grases et al., 2000). As a crystallization inhibitor, InsP6 may contribute to the prevention of sialolithiasis and dental calculus formation (Grases et al., 2009; Grases et al.,2003).
InsPs may exert their antioxidative property via generic as well as specific chelating activities. Other than binding iron and copper ions in general, some InsPs has the 1,2,3-trisphosphate arrangement which, upon interacting with Fe3+, would occupy all six co-ordination sites of this ion with hydroxyl groups (Phillippy and Graf, 1997). As a result, these compounds inhibit iron’s catalytic action on lipid peroxidation and hydroxyl radical formation in the Fenton reaction. A similar effect has been observed with ferrous ion. Phytate can be used to prevent myoglobin oxidation in meat processing, which changes the color of homogenized meat from red to brown (Park et al., 2004; Stodolak et al.,2007). From in vitro studies on rodent liver, Rimbach and Pallauf (1998) did not find phytate to have the capability to reduce oxidation markers in rats, while Bhowmik et al. (2017) found that InsP6 significantly reduced the oxidative stress caused by iron overload in mice. Intravenously-injected phytate appears to provide some degree of myocardial protection to rats subjected to cardiac excision, but not to rat hearts that were removed from hypothermic storage (modeling a heart transplant) (Kazimoglu et al., 2004; Rao et al.,1991). These results suggest that InsPs’ performance as antioxidants in vivo may be limited to specific working conditions and routes of administration (Iemma et al., 2007).
InsP6 has demonstrated a remarkable preventive effect on tumor development in different cancer cell lines, namely blood, colon, liver, lung, mammary, melanoma, pancreas, prostate, skin, soft tissue, and uterine cervix (Vucenik and Shamsuddin, 2006). Multiple effects beyond antioxidant activity have been recognized, such as the inhibition of cell proliferation, metastasis, angiogenesis, and inflammation (Gu et al., 2010;Kumar et al., 2004;Norazalina et al., 2010; Raina et al., 2013). InsP6 has been shown to support apoptosis as well as the differentiation and maturation of malignant cells, which can recover the normal phenotype (Deliliers et al., 2002;Shamsuddin and Yang, 1995; Singh et al., 2003; Vucenik et al.,2004). Some studies observed that InsP6 enhances the activity of natural killer cells and reduces the depression of these cells by carcinogens (Baten et al., 1989; Zhang et al., 2005). These activities vary depending on dose, time, and the origin of the cells. A synergistic effect upon combination with inositol has also been suggested in a number of animal studies on different cancer types (Shamsuddin et al., 1989; Vucenik et al., 1995).
Dietary phytate exhibits an antineoplastic effect in non-ruminant animal models equally well whether consumed in a fluid or non-fluid system, highlighting the possibility of effective absorption mechanisms for extraneous InsPs that do not depend on microbial phytases. However, a human’s ability to absorb InsPs cannot be directly extrapolated from rodent models because phytase activity in our tissue is ∼30 times lower than that of rat tissues (Iqbal et al., 1994). On the other hand, it has been hypothesized that the anticancer activities of InsP6depend on the compound’s dephosphorylation to lower InsPs, even though the exact metabolism pathways and mechanisms of action still need to be elucidated (Vucenik and Shamsuddin, 2006). From this point of view, extraneous InsP1–5 may also play important roles against cancer.
In mouse, InsP6 has demonstrated a protective effect against Alzheimer’s disease by decreasing lipid peroxidation and increasing cytochrome oxidase levels in the brain (Anekonda et al., 2011). In the same study, the compound was found to prevent the apoptosis of neurons by protecting MC65 cells against cytotoxicity by amyloid-β peptide. This action is opposite to the effect InsP6 exerts on malignant cells, which has been described above, implying that InsP6 is likely to serve as an apoptosis modulator. Similar protective activities have also been observed in other cell conditions. In studying the rat mesencephalic dopaminergic cell line (N27), Xu et al. (2008) reported that InsP6 decreased pathological apoptosis induced by 1-methyl-4-phenylpyridinium and increase cell viability, suggesting that InsP6 may offer protective therapy for Parkinson’s disease. In pig small intestines, InsP6 was found to reduced cell proliferation, apoptosis, and cyclooxygenase-2 expression, which are associated with inflammation (Silva et al., 2014a). The compound also protected cells against hypoxia-induced morphological changes. These effects were attributed to InsP6’s antioxidant property, which may have inhibited the production of cytosolic reactive oxygen species. Notably, InsP6 from rice was found to be more efficient than InsP6 from corn, which was hypothesized by the authors to be due to the different numbers of sodium and water moieties attached to each InsP6 molecule between the two sources (i.e., InsP6·12Na·8H2O from rice versus InsP6·11Na·7H2O from corn).
Some positive effects of phytate on type-2 diabetes mellitus have been suggested, as phytate intake has been found to negatively correlate with blood glucose response. In mice fed a high-fat diet, InsP6 exerted an antihyperglycemic effect by regulating the activities of glucose-6-phosphatase, glucokinase, and phosphoenolpyruvate carboxykinase (Kim et al., 2010). Larsson et al. (1997) also demonstrated that InsP6 may play a role in regulating insulin secretion, possibly by inhibiting the activity of serine–threonine protein phosphatases, thus opening intracellular calcium channels and leading to insulin release. InsP5 could exert similar effects on calcium ion channels, but required a higher amount than InsP6, while InsP4 was not able to provide any action of the type.
Jariwalla et al. (1990) reported phytate to significantly lower serum cholesterol and triacylglycerol levels. A lower serum zinc level and zinc–copper ratio were observed in conjunction with these effects. Zinc and copper ions share the same mucosal carrier systems, and excess zinc in the diet can lead to reduced copper absorption, which has been hypothesized to cause coronary heart disease (Klevay, 1975). Because InsP6 has a greater binding affinity for zinc than copper ions, its presence may have helped in lowering the zinc–copper ratio (Persson et al., 1998). However, in experimenting with rats fed a high-sucrose diet, Onomi et al. (2004) noted that the hepatic concentrations of total lipids and triacylglycerols, as well as the hepatic activity of glucose-6-phosphate dehydrogenase, were reduced when the sodium phytate level in the feed was as low as 0.02%. Meanwhile, the serum zinc level was not affected until the sodium phytate content reached 2.5%. These authors also detected reductions in cholesterol level and fatty acid synthetase activity, but the effects did not show a clear dose-dependent relationship. As a result, the mechanisms behind the reductive effects of dietary phytate on serum cholesterol still need to be investigated.
Several in vitro studies have been conducted on InsP6’s antiviral potential on the human immunodeficiency virus (HIV). The compound was found to inhibit the cytopathic effect of this virus and the expression of the HIV-specific antigen (Kumar et al.,2010). In a T-cell line, InsP6 was also shown to be able to suppress the replication of HIV-1 (Otake et al., 1999). Recently, Tateishi et al. (2014) found InsP6 to be able to tightly bind the matrix domain of the Pr55Gag protein, which is a critical component for the assembly of the HIV-1 virus. Subsequently, these authors coupled InsP6 with diacylglycerol moieties to synthesize myo-phosphatidylinositol 2,3,4,5,6-pentakisphosphate derivatives. These derivatives were found to have an even stronger binding capacity with the Pr55 Gag protein’s matrix domain, suggesting a potential molecular design for anti-HIV agents.
The effects of InsP6 against mycotoxins have been investigated, with some results. InsP6 has been shown to impede the histopathological alterations caused by aflatoxin B1 in albino rats, which include heightened oxidative stress and decreased reproductive function (El-Saad and Mahmoud, 2009). In swine, InsP6 exhibited protective effects on the cytoplasmic membrane of intestinal cells when these cells were exposed to deoxynivalenol and fumonisin B1 (Silva et al.,2014b).
Because there is no unambiguous evidence that dietary InsPs can be absorbed, it is still unclear as to whether InsPs in foods can provide significant disease prevention. Yet, the growing number of findings pertaining to the physiological functions and potential benefits of InsPs promise that much remains to be learned about these compounds, and there is a need to separate and quantify InsPs in foods. While accurate and sensitive methods are becoming more available, the diversity of food and biomedical systems also require that modifications be investigated for each sample type.
| 4. Methods of separation and quantification for InsPs | ▴Top |
Although InsP6 was identified as far back as 1903, the detection and quantification of InsPs have been of continuous interest for researchers to this day. These compounds do not possess a characteristic spectrophotometric absorbance maximum and are available only at relatively low concentrations. Currently, an optimal method for the complete separation and measurement of all InsPs with their isomers has yet to be developed.
4.1. Non-specific methods
Heubner and Stadler (1914) introduced a non-specific method for the determination of phytic acid in ground cereal powder (Rather, 1917). In this method, phytic acid is extracted with hydrochloric acid (HCl) and then titrated with a ferric chloride solution in the presence of ammonium thiocyanate to form a pink-colored chromogen. The quantity of Fe3+ that reacts is used to calculate the phytic acid content. Because ferric phytate forms as a white precipitate, the exact end point of this titration is difficult to detect. Furthermore, the mole ratio between Fe3+ and InsP6 in the complex is inconsistent. Using the assumption that the total phosphorous content of the sample would originate from phytate, McCance and Widdowson (1935) later modified this method by quantifying the phosphorus content of the ferric phytate precipitate instead of Fe3+. A major disadvantage of assays that depend on ferric phytate is the overestimation of phytic acid in samples with high contents of inorganic phosphorus and/or non-InsPs organic phosphates, as these compounds can also precipitate with the ferric ion. Even today similar research continues: Burgos-Luján and Tong (2015) recently described a method based on the back-titration of ferric phytate with EDTA for the measurement of phytic acid in fluid food samples. McKie and McCleary (2016) also reported a modified colorimetric molybdenumblue assay to measure the amount of inorganic phosphate released from extracted phytic acid of foods and animal feeds as a result of dephosphorylation with phytase and alkaline phosphatasetreatment.
To eliminate inorganic phosphorus from samples, researchers have attempted to develop quantitative methods based on ion-exchange interactions. Most notable is a method by Harland and Oberleas (1977), in which the supernatant from an HCl extraction of the sample is placed on an AG 1-X8 chloride form anion-exchange column. The column is rinsed with water and 0.05 M NaCl to elute inorganic phosphates. Organic phosphates trapped on the resin are then eluted with 0.7 M NaCl. Assuming that phytates accounts for all forms of organic phosphates in the sample, an acid digestion of the final fraction followed by the measurement of inorganic phosphates via the Fiske-Subbarow colorimetric method can be used to infer the phytate content. In the Fiske-Subbarow assay, the sample is treated with ammonium molybdate to form phosphomolybdate, which can be reduced to molybdenum blue in the presence of a reductant, such as aminonaphthosulfonic acid (Goldenberg and Fernandez, 1966). The intensity of the blue-colored chromogen, with maximum absorbance at λ = 640 nm, is directly correlated to the concentration of phosphate in the sample. In an improvement of this method, Ellis and Morris (1983) added EDTA and NaOH to prevent the binding of InsP6 to proteins and metal ions in the extraction. This version was later adopted as the official AOAC method (986.11) for determining phytate in foods (AOAC International,2005).
Due to the non-specificity of this chromatographic method, the InsP6 concentration can still be heavily overestimated in samples where InsP1–5 make up more than 20% of the InsP content (Lehrfeld and Morris 1992). Additionally, sugar phosphates, phospholipids, nucleotides, and phosphate derivatives of thiamine also contribute to the organic phosphate levels in foods. Overestimation problems are of particular importance, because foods and diet patterns reported to have high phytic acid contents would often be associated with mineral deficiency concerns.
4.2. Specific methods
While working with soil, Smith and Clark (1952) were able to extract InsP3–6 from hydrolyzed sodium phytate via anion-exchange chromatography, utilizing a weak-base exchange resin and stepwise elution with increasing HCl concentration (Cosgrove, 1963a). The content of each InsP was deduced via the phosphorus–inositol ratio in each collected fraction. Later, Cosgrove (1963b) demonstrated that utilizing the strong anion exchange resin AG 1 for this method allowed for separation of different InsP5 isomers. The author, however, did not perform a quantification of InsPs in food samples.
To achieve a shorter elution time, Tangendjaja et al. (1980) then applied high-performance liquid chromatography (HPLC), particularly reversed-phase chromatography, using a μBondapak C18 column and sodium acetate as mobile phase. Graf and Dintzis (1982) later improved this method by adding a purification step with the AG 1 resin after extraction. With both methods, however, the retention time of InsP6 was less than 2 min and other InsPs could not be identified. In a modification, Sandberg and Ahderinne (1986) used formic acid/methanol as the mobile phase and added tetrabutylammonium hydroxide as the ion-pair reagent, thus affording an increase in the retention time and differentiation between InsP3–6, although their stereoisomers were still indiscriminable.
Around this time, the application of gas chromatography (GC) in InsP analysis was also explored. Heathers et al. (1989) derivatized InsP fractions collected from HPLC separation into hexatrimethylsilyl derivatives for quantification by GC, and successfully applied this method to isomers of InsP1–3. The need for a derivatization process and the inability to measure InsP4–6 are considered drawbacks for this method.
Overall, HPLC is still the most common separation technique for InsPs due to its versatility. While ion-pair reversed-phase HPLC has been succesfully used to separate InsPs, anion-exchange HPLC has become more popular due to its ease of use. In recent decades, many different mobile phase gradients and anion-exchangers for HPLC have been applied to separate InsPs down to their isomers. Nevertheless, it remains that InsP3–6 are better eluted with an acidic mobile phase while InsP1–2 prefer an alkaline mobile phase (Schlemmer et al., 2009). Good separation of all InsPs and their isomers is unlikely to be achieved in the same run, while time-effective separation of InsP1-6 in one run will sacrifice information on isomers. It should be noted that a high pH level may reduce the life time of a silica-based stationary phase; when an alkaline mobile phase is utilized, a polymer-based column is preferable. Therefore, there might be some limitations to the choice of column for the sensitive detection of InsP1–2 by HPLC (Sjöberg et al., 2016).
Taking advantage of the exchange of Fe3+ from the ferric sulfosalicylic acid complex to phytate, Latta and Eskin (1980) developed a colorimetric detection method using a modified Wade reagent. Reaction with phytate in a sample extract would result in a loss in the absorbance at λ = 500 nm for this purple reagent. Rounds and Nielsen (1993) then modified this assay by replacing the AG 1 resin in the preceding method with another polystyrene-based strong anion-exchanger equipped on HPLC. Effluent from the analytical column was combined with a post-columnreagent of 0.015% FeCl3•6H2O (w/v) plus 0.15% (w/v) sulfosalicylic acid; this allowed for decrease absorbance readings at λ = 500 nm to be monitored as the eluting phosphates complexed with iron in the reagent. Using this approach, InsP2–6 could be separated within 30min. Recently, Agostinho et al. (2016) introduceda similar post-column detection method based on the inhibitory effect by InsP6 on the formation of the red complex between glyoxal bis(2-hydroxyaniline) and calcium ions in alkaline.
In 1988, another post-column dye-detection method was developed based on the exchange of yttrium ionfrom the Y3+-4-(2-pyridylazo)resorcinol complex to InsPs, which reduces the absorbance at λ = 546 nm of this colored complex (Mayr, 1988). In a modified method utilizing the same approach, Guse et al. (1993) were able to quantify InsP3–6 in cell extracts with high sensitivity: the detection limits were approximately 15 pmol for InsP3, 10 pmol for InsP4, 5 pmol for InsP5, and 1 to 3 pmol for InsP6. Yet, this method requires great care to avoid contamination of the eluent with ions such as Fe3+ and Zn2+, as these ions can complex with both InsPs and 4-(2-pyridylazo)resorcinol.
Ligand exchange reactions between InsPs and fluorescent complexes have also been introduced, including the use of a ferric methylcalcein blue complex, copper(II) gelatin complex, and copper(II) 2,2′-bipyridine complex (Cao et al., 2011; Chen et al., 2007; Irth et al., 1990). Interestingly, InsP6 has been found to activate the oxidation of 1,1′-dipyridyl ketone hydrazone (DPKH) in the presence of Cu2+, resulting in an intense fluorescent product (March et al., 1999). The authors suggest that the copper(II)–phytate complex may have heightened catalytic activity compared with Cu2+ alone on the reaction between DPKH and oxygen. This method is useful for the detection of phytic acid in foods and urine.
A method for on-line detection of InsPs has always been in demand. In qualitative studies on cells and tissue, a number of authors have chosen to radiolabel their samples with myo-[3H]inositol or [31P]PO43−. In working on several isomers of InsP1–4, Taylor et al.(1990) coupled this technique with separation via HPLC for the quantitation of InsPs. They successfully recorded changes in the distribution of the analytes overtime, proposing a potential approach for the study of InsPs in biological processes, such as cellular signaling. Due to the lack of absolute concentration data, this method is considered unsuitable for many research purposes. Furthermore, it is laborious and less sensitive than some off-line analyses. Instead, several studies have investigated the application of chemically–suppressed conductivity and the researchers were able to separate different phosphorylated organic compounds, including InsPs, in both physiological and food samples (Smith and MacQuarrie, 1988; Talamondet al.,2000). Notable is the coupling of isotachophoresis and zone electrophoresis with contact conductivity, as demonstrated by Kvasnička et al. (2011).
When multiple InsPs are being identified or when matrix effects can cause the elution times of InsPs to deviate from those of standard compounds, reliance on retention time for detection in HPLC methods can also risk inaccurate interpretation. Nuclear magnetic resonance (NMR) spectroscopy, atomic emission spectroscopy (AES), and mass spectrometry (MS) are among detection techniques that can reduce this risk in susceptible samples. 31P-NMR spectroscopy is prized as a noninvasive and highly specific method for InsP determination. Because it can be applied on intact tissues and cell suspensions, it is especially suitable for the study of InsP metabolism, binding activities, and degradation (Heighton et al., 2008; Zhuang et al., 2010). Alternatively, some authors have used 1H-NMR to study the interactions between InsP6 and paramagnetic metal ions such as ferric and ferrous ions (Heighton et al., 2008). NMR can be used to identify and confirm stereoisomers that often coelute during chromatographic separation (Phillippy, 1989). The disadvantages of NMR, however, are that the technique is relatively insensitive, cost-prohibitive, and spectra can be very complex (Kemme et al., 1999; Schlemmer et al., 2009).
In 1996, a method using inductively coupled plasma (ICP) combined with AES for InsP6 determination in urine was reported, but the sample treatment process is long, complicated, and requires a large amount of sample (Grases and Llobera, 1996). Later, a more efficient and sensitive method was developed, attaining a limit of detection of 64 μg/L and limit of quantification of 213 μg/L (Grases et al.,2004a). Although still less sensitive than ICP coupled with MS, this method is considered suitable for routine determination. A preliminary study by Amaro et al. (2004) suggested that coupling HPLC with ICP-AES can improve sensitivity.
Mass spectrometry (MS) and tandem mass spectrometry (MS/MS) are powerful approaches to attain high sensitivity and can be coupled with HPLC, GC, and ICP for different purposes. ICP-MS is suitable for biomedical samples such as urine (Muñoz and Valiente, 2003). For food samples, the use of sector field ICP-MS has been investigated with initial success (Helfrich and Bettmer, 2004). GC-MS has also been applied for food and biological samples, such as rat organs, human plasma, urine and kidney stones (de Koning, 1994; March et al., 2001), but this method still requires a derivatization step of InsPs to trimethysilyl derivatives. HPLC-MS can achieve comparable results without calling for derivatization of the sample (Perelló et al.,2004). Further modification may even eliminate all together the need for a separate chromatographic extraction step preceding detection. Using negative mode electrospray ionization (ESI) with anion-exchange HPLC–MS/MS, Liu et al. (2009) were able to simultaneously separate and quantify InsP1–6 from various foods and cell samples, with sensitivity reaching as low as the pmol range and elution time of less than 30 min. This can be considered one of the most efficient methods for measuring InsPs in samples without regard to stereoisomers. A similar method was later successfully applied for the qualitative detection of InsPs from environmental matrices, including soil, manure, and aquatic sediments (Sjöberg et al., 2016). Recently, Zhang et al. (2017) introduced a method coupling ion-pair chromatography with ESI/MS/MS that could detect InsP1–6 within 15 min of sample injection and achieved very low limits of detection for InsP1–5 (less than 1 pmol). Another notable method which utilized MS/MS was described by Shelor et al. (2015), in which hydroxide eluent ion chromatography was coupled with MS/MS. In addition to simultaneously detecting InsP1–6, this method was able to fully separate several stereoisomers of InsP2–6 in a 50min run. Unfortunately, the authors did not attempt to identify the structure of these isomers. An advantage of using MS/MS compared to ICP-MS is the potential of discriminating structural isomers based solely on mass spectrometry information. Utilizing positive-ion mode ESI with low-energy collisional-activated dissociation MS/MS, Hsu et al. (2003) were able to differentiate the stereoisomers of InsP1, InsP2, and InsP3 in bovine brain extract. This method was unable to identify the stereoisomers of InsP4 and InsP5, however, and how it can be applied for the quantification of InsP1–3 isomers still needs to be examined.
| 5. Inositol phosphates in plant foods | ▴Top |
The distribution of InsPs in plant seeds varies greatly, with the major concentration sites being the bran, germ, and the cotyledon. For cereals, InsP6 has been found to occur mainly in the germ of corn, the aleurone of wheat, the bran of rice, and the germ and bran of pearl millets (O’Dell et al., 1972; Reddy et al., 1989). On the other hand, legumes deposit InsP6 throughout the cotyledon. Further structural characteristic of InsP depositions can be attained via toluidine blue staining, where these compounds would appear red-violet under an optical microscope. In almond meal and hazelnuts, for example, these depositions have been found to be small pockets densely surrounded by proteins, suggesting the storage location of InsPs in these foods (Andriotis et al., 2005; Dourado et al., 2004).
Existing data on the InsP6 content of plant seeds have mainly reported only InsP6 and the values have shown strong variations, especially with tree nuts, where the highest InsP6 value is can be 6- to 10-fold higher than the lowest value in the range (see Table 1 and 2). Significant variations in cereal InsP6 content due to cultivar, location, crop year, and fertilization have been observed in some but not all studies (Barrier-Guillot et al., 1996;Hídvégi and Lásztity, 2002;McCall et al., 1953; Nahapetian and Bassiri, 1976; Singh and Sedeh, 1979; Steiner et al., 2007).The contribution of InsPs phosphorus to total phosphorus content in plant seeds hasbeen found to vary widely, ranging from 30 to 95% in cereals and legumes, and 25 to 75% in tree nuts (Deshpande, 2002; Lolas et al., 1976; O’Dell et al., 1972; Reddy et al., 1989; Rodehutscord et al., 2016). Steiner et al. (2007) have identified a positive correlation between InsP6 phosphorus and total phosphorus content in some cereals and legumes, with the relationship stronger in legumes than in cereals.
 Click to view | Table 1. Content of myo-inositol hexakisphosphate in the grain/seed of major cereals and legumes |
 Click to view | Table 2. Content of myo-inositol hexakisphosphate in major tree nuts |
It is important to note that drawing a general consensus on the occurrence of InsP6 in food products is difficult because not all studies utilized specific methods and some studies have estimated the InsP6 content by assuming that a certain percentage of the total phosphorus content comprises phytic acid, which disregards possible deviations due to cultivation and processing factors. Furthermore, it is unclear whether indirect assays measure InsP6 as phytic acid or its salt forms (phytate/phytin), which can vary in number and the type of mineral cation bound to the molecule. In light of the recent findings on the potential roles of different InsPs in human health, it has become important to accurately assess the InsPs levels in foods with specific methods.
Although information on the abundance of InsP1–5 in plant seeds is still limited, some differences among different plants have been identified. Stepwise dephosphorylation of InsP6 by phytases extracted from the bran of spelt, rye, barley, and oat have identified the products to be Ins(1,2,3,5,6)P5, Ins(1,2,5,6)P4, Ins(1,2,6)P3, Ins(1,2)P2, and Ins(2)P (Greiner and Alminger, 2001).With phytase from rice bran, dephosphorylation can result in the same products, as well as Ins(1,2,3,4,5)P5, Ins(1,2,3,4)P4, and Ins(1,2,3)P3 (Hayakawa et al., 1990). Similarly, by using phytase extracted from wheat, the major hydrolysis pathway of InsP6would result the same products listed by Greiner and Alminger (2001), while a minor pathway may instead produce Ins(1,2,3,6)P4 and Ins(1,2,3)P3 as InsP4 and InsP3 products (Nakano et al., 2000). Lim and Tate (1973) have shown that Ins(1,3,4,5,6)P5, Ins(1,2,3,4,5)P5, Ins(1,2,3,4,6)P5, Ins(1,2,3,4)P4, and Ins(1)P may also be produced in wheat, suggesting that multiple types of phytase may be present. This effect has been observed with phytases from lupine seeds, in which the phytase LP2 produces Ins(1,2,3,4,5)P5 and Ins(1,2,3,4)P4 while the two phytases LP11 and LP12 produces Ins(1,2,4,5,6)P5 and Ins(1,2,5,6)P4. After generating these products, all three phytases would produce Ins(1,2,6)P3, Ins(1,2)P2, and Ins(2)P (Greiner et al., 2002).
Interestingly, in surveying multiple genotypes of barley, oats, rye, maize, triticale, and wheat, Rodehutscord et al. (2016) found Ins(1,2,4,5,6)P5 to be present in all samples, whereas Ins(1,2,3,4,5)P5 was only in barley, rye, triticale and wheat samples, and Ins(1,2,3,4,6)P5 was only in rye, triticale, and wheat samples. InsP4 was not identified in any sample. The only InsP3 isomer detected was Ins(1,5,6)P3, which was determined in selected genotypes of barley, maize, and rye. These findings are in agreement with previous results by Kasim et al. (1998), where InsP4 was not detected in the grains of barley, corn, oats, sorghum, and wheat. The only published value of InsP4 in cereal we have found was reported by Helfrich and Bettmer (2004) for corn (maize). We are also unaware of values pertaining to the levels of InsP1 and InsP2 in grains. Over all, cereal grains appear to be quite different from legumes and tree nuts, in which lower InsPs, including InsP1–2, were usually detected (Table 3).
 Click to view | Table 3. Lower inositol phosphate contents in selected plant seeds* |
Soaking and/or boiling have been found to significantly decreased InsP3–6 in both legumes and grains (El Tinay et al., 1989; Karle and Beleia, 2001; Lestienne et al., 2005a; Morris and Hill, 1996). In these processes, InsPs are hypothesized to be hydrolyzed into inorganic phosphorus inside the grain. Phytases are expected to be quickly degraded upon heat application. Lestienne et al. (2005b) found the inorganic phosphorus level to increase in soaked soybeans, whereas the soaking fluid showed no similar change or any significant phytase activity. Karle and Beleia (2001) also demonstrated that heat application after soaking did not further decrease the InsP6 content of soybeans compared to when only soaking was employed. Marfo et al. (1990) observed a significant decrease in the InsP6 level in several cereals and legumes after fermentation. This effect declined after 48 hours, which might be due to the inhibition of phytase activity by low the pH or increased abundance of inorganic phosphate. In processed foods containing cereals and legumes, the InsP levels can be affected by the processes mentioned above as well as the loss/removal of parts of the grain/seed and the inclusion of other ingredients, leading to great variation even within the same product type. For example, InsP6 has been found to range from <0.008 to 50 µmol/g in ready-to-eat wheat cereals and <0.008 to 16.1 µmol/g in wheat breads (Harland et al., 2004; Reddy et al., 1989).
| 6. Conclusions | ▴Top |
InsPs and their abundances in plant foods have long been a concern for nutrient absorption, especially for populations that may be more susceptible for nutrient deficiencies, including children, vegetarians, and residents in developing countries. This review has summarized some of the nutrients most affected by InsPs, as well as several factors that may influence InsPs capabilities to inhibit the absorption of this nutrition in the gut. Additionally, InsP roles in normal mammal biological functions, the potential for disease prevention, and the possibility for absorption from food sources were discussed. In each of these areas of interest, it is evident that many questions are still unanswered and further research is needed to improve the state of knowledge. Thus, more research on rapid and sensitive analytical methods to determine InsPs in food and biological systems is warranted.
| References | ▴Top |