| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 5, March 2019, pages 31-42
Vesicle properties and health benefits of milk phospholipids: a review
Zhiguang Huanga, b, c, Hui Zhaoa, *, Wenqiang Guana, Jianfu Liua, Charles Brennana, b, c, *, Don Kulasirib, Maneesha S. Mohanb
aTianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
bDepartment of Wine, Food and Molecular Biosciences, Faculty of Agriculture and Life Sciences, Lincoln University, Lincoln, Christchurch 7647, New Zealand
cRiddet Research Institute, Palmerston North, New Zealand
*Corresponding author: Hui Zhao and Charles Brennan, Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China. E-mail:;
DOI: 10.31665/JFB.2019.5176
Received: December 20, 2018
Revised received & accepted: January 20, 2019
| Abstract | ▴Top |
Phospholipids are important ingredients in milk. They serve as bioactive components with processing functionalities, despite representing only a small proportion of total milk lipids. There has been increasing interest in vesicle properties and health effects of milk phospholipids. However, there are limited reports on industry-scale manufacturing of related commercial products. This contribution aims to elucidate the industrial processes of manufacturing milk phospholipid products including phospholipid extraction and fraction as well as summarizing determination assays of milk phospholipids. In addition to industrial production, this review elaborates on application aspects, such as the biological properties of milk phospholipids and their technical importance as delivery vesicles of liposomes and phytosomes. In addition, new insights on large-scale production of milk phospholipids and new applications such as phytosomes and antioxidant properties are discussed.
Keywords: Milk phospholipids; Solvent extraction; Liposome; Phytosome; Health effects; Vesicle properties
| 1. Introduction | ▴Top |
Milk contributes about one third of human dietary lipid intake (USDA, 2017). Milk phospholipids have been used as materials for nutrient carriers since the early 2000s. Thompson (2005) first used milk phospholipids to fabricate three kinds of liposome to encapsulate bioactive compounds. Since then, milk phospholipid-based liposomes have been prepared to encapsulate ascorbic acid (Farhang et al., 2012) and lactoferrin (Liu et al., 2013). More recently, milk phospholipid liposomes were applied to improve the storage stability of encapsulates (Gulseren and Corredig, 2013), the encapsulate solubility and encapsulation efficiency (Jin et al., 2016; Liu et al., 2012) and the bioavailability of encapsulate (Maswadeh et al., 2015), showing better efficiency than soy lecithin (Liu et al., 2012). Furthermore, in terms of biological effects, several review papers have summarized various health benefits of milk phospholipids, with emphasis on therapeutic aspects (Castro-Gómez et al., 2015), infant’s gut development and cognitive functions (Ortega-Anaya and Jimenez-Flores, 2018), and physiological functionalities (Verardo et al., 2017).
This contribution aims to summarize the industrial processes of manufacturing milk phospholipids, to update last five-year results on using phytosomes or liposomes to enhance bioavailability of bioactive compounds. It also reports on the recent trends on biological activities of milk phospholipids including antioxidant potential.
| 2. Structure, composition and occurrence | ▴Top |
2.1. Molecular structure
Milk phospholipids include two subclasses, glycerophospholipids and sphingolipids. Glycerophospholipids consist of a glycerol moiety with two fatty acids (lipophilic) esterified at positions of sn-1 and sn-2 and a hydroxyl group at sn-3 position, linked to a phosphate group and a hydrophilic residue. The structural details of the latter determine the types of glycerophospholipids, namely phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidyl-glycerol (PG), and phosphatidic acid (PA) (Verardo et al., 2017). The amphiphilic structure (lipophilic tail and hydrophilic head) provides milk phospholipids with emulsification properties.
Sphingolipids consists of sphingosine backbone (2-amino-4-octadecene-1,3-diol), linked to a fatty acid through an amide bond and a polar head. Sphingomyelin (SM) is a prominent subclass of sphingolipids, having a phosphocholine head group. A minor constituent of sphingolipids in milk is glycosphingolipid, whose polar group comprises carbohydrate groups (glucose, galactcose, and lactose) (Ortega-Anaya and Jimenez-Flores, 2018).
2.2. Composition
Milk lipids represent approximately 4% of bovine milk (Bylund, 2015). Among total milk fat, only 0.32–1% represents phospholipid compounds (Le et al., 2015). Thus, it takes 2.5–8 liters of raw milk to produce one gram of phospholipids. Phospholipids are structured, functional lipids (Jala and Kumar, 2018). In all the three phospholipid sources, PC and PE contributes to the major proportion (52, 55 and 90% for milk, soy and egg yolk, respectively) of polar lipids. Compared to soy lecithin and egg yolk lecithin, milk phospholipids have a more balanced distribution in each subclass. SM and PS (24 and 12% in milk phospholipid profile, respectively), being regarded as functional ingredients for brain development (Castro-Gómez et al., 2015; Higurashi et al., 2015), are virtually absent in other sources, such as soy (0 and 0.5%, respectively) and egg yolk lecithin (1.5 and 0%, respectively) (Li, 2014).
Apart from SM and PS profile, milk phospholipids have advantages over the other two sources due to their natural origin, oxidative stability and color compatibility. Milk phospholipids have lower content of polyunsaturated fatty acids (PUFA 7.2–7.9% (Lopez et al., 2008)) than soy lecithin (60.37% (Imran et al., 2015)) and egg lecithin (23.2% (Asomaning and Curtis, 2017)). Unsaturated fatty acids had a proportion of approximately 46.14% for mature bovine milk phospholipids (Zou et al., 2015), and 33–44.8% for two kinds of bovine milk polar lipids fed on maize silage and linseed (Lopez et al., 2008), while for the lecithin of soy and egg yolk, this percentage was 79.58 (Imran et al., 2015) and 54.6 (Asomaning and Curtis, 2017), respectively. Thus, milk phospholipids are more resistant to oxidation than other phospholipids and they also have less color intensity for this kind of fatty acid profile (Ireland, 2014).
In terms of fatty acid profile of phospholipids, bovine milk, soy and egg yolk all have predominant distribution of long chain fatty acids (LCFA 13–21), and the abundance of their LCFA is above 90% (Asomaning and Curtis, 2017; Butina et al., 2017). The top two prominent fatty acids of phospholipids for milk and egg yolk are oleic and palmitic acids, which together account for more than 60%. The principal fatty acids of soy lecithin are linoleic and palmitic acids, contributing to 63.4 and 16.4%, respectively (Lopez et al., 2014).
2.3. Occurrence
In intact raw bovine milk, phospholipids take the form of milk fat globule membrane (MFGM: 0.1–20 μm in diameter, 10–50 nm in thickness (Arranz and Corredig, 2017)). The triple-layer membrane consists of a surface-active inner monolayer enveloping triacylglycerols (TAG) in the center and an outer bilayer in contact with the aqueous phase of milk (Castro-Gómez et al., 2015). The milk fat globule membrane is composed of polar lipids, proteins, glycoproteins, enzymes and minor neutral lipids (Zhao et al., 2019).
In dairy products, the triple-layer membrane structure becomes disrupted during processing and milk phospholipids redistribute into such products as buttermilk (BM) and beta serum powder (BSP, >60% lipid), which is an aqueous dairy stream through phase inversion from an oil-in-water to a water-in-oil emulsion (Fletcher et al., 2006).
| 3. Industrial manufacturing | ▴Top |
3.1. Phospholipid extraction from dairy products
Milk phospholipid concentrated streams are related to butter processing, anhydrous milk fat (AMF) or whey fraction. Commercial milk phospholipid products are usually derived from dairy products, such as butter serum AMF, buttermilk, or acid butter whey. The level of phospholipids in these streams can be as high as 11.54, 2.03 and 1.84%, respectively (Le et al., 2015). Butter serum powder represents the highest level of phospholipid concentrate among those dairy streams. Therefore, it is a preferred feed for making milk phospholipid.
AMF, derived either from fresh cream or butter, contains purified milk fat (>99.8%) with removal of water and non-fat solid (Bylund, 2015). Butter serum AMF consists of highest proportion of phospholipids, with 11.54, 1.25 and 48.4% in terms of dry matter (DM), wet base and lipid base, respectively (Pimentel et al., 2016; Smithers and Augustin, 2013). Buttermilk, a product of churn process, is the serum of butter, containing the most of original milk whey proteins and less fat than butter (Chandan and Kilara, 2010). Buttermilk phospholipids are less abundant than those of butter serum, with 2.03% of dry matter (DM) content. Acid buttermilk whey has a DM-based protein percentage of approximately 84.7%, containing 1.84 and 0.1% phospholipids for dry and wet products, respectively (Smithers and Augustin, 2013). Intact milk fat globule membrane contains 30–70% polar lipids. However, it is generally only regarded as a laboratory source of phospholipids (Holzmüller and Kulozik, 2016; Lu et al., 2016).
Solvent extraction is one of the common methods to isolate milk phospholipids from dairy lipid concentrates. Ethanol is the most used solvent to extract milk lipids, for instance, hot alcohol (90%) extraction at 70 °C rendered around 90% recovery rate (Price et al., 2018). Ethanolic extraction of lipids from proteins resulted in high purity (75%) phospholipids (Burling and Graverholt, 2008). In a laboratory up-scaling test, supercritical carbon dioxide and 20% ethanol was utilized to extract lipids and final product had a purity of 56.24 ± 0.07% (Barry et al., 2017). Supercritical carbon dioxide can only dissolve triacylglycerols without phospholipids, but together with near-critical dimethyl ether, it extracts both neutral and polar lipids (Fletcher et al., 2006). Hexane is also a solvent that is occasionally used for lipid extraction (Shulman et al., 2011). Phospholipids are acetone-insoluble, but triacylglycerols dissolves in acetone. This selectivity in solubility also provides an approach to purify milk phospholipids (Le et al., 2015; Zou et al., 2015).
To obtain a high purity of phospholipids, lactose and protein (casein and whey protein) need be isolated from lipids. Proteins can be denatured thermally or in acid solution (pH 4.6) (Ferreiro et al., 2016; Price et al., 2018), the aggregated particles are then sieved by subsequent filtration. Starting with whey protein phospholipid concentrate, ethanol at 60–80 °C denatures proteins, resulting in phospholipid concentration of ca. 45.8% in the filtrates (Price et al., 2018). Proteolysis is also a viable way to remove proteins, in which whey and casein break into peptides and amino acids. Then the small molecules enter permeate after ultrafiltration (UF) or microfiltration (MF) operation (Barry et al., 2017; Konrad et al., 2013). Alcalase (E.E. 3.4.21.62), a serine type endoprotease with esterase activity, catalysed amino esters at pH 7.5 and 35–60 °C (Barry et al., 2017), while tryptic and peptic hydrolysis may be carried out at 42 °C for 2–16 h, with pH at 7.7 and 2.0, respectively (Konrad et al., 2013). Lactose is a smaller molecule than lipid ant it also goes into permeate (Levin et al., 2016).
The process flow diagrams of industrial milk phospholipid manufacturing are not available due to commercial confidence. However, according to previous research reports, a block diagram was proposed to illustrate the principle of typical industrial production processes of milk phospholipids (Figure 1). Starting from butter serum or buttermilk, milk phospholipid concentrate can be refined by sequential unit operations of delactosing, deproteinising, and defatting (Fletcher et al., 2006; Ireland, 2014; Zou et al., 2015).
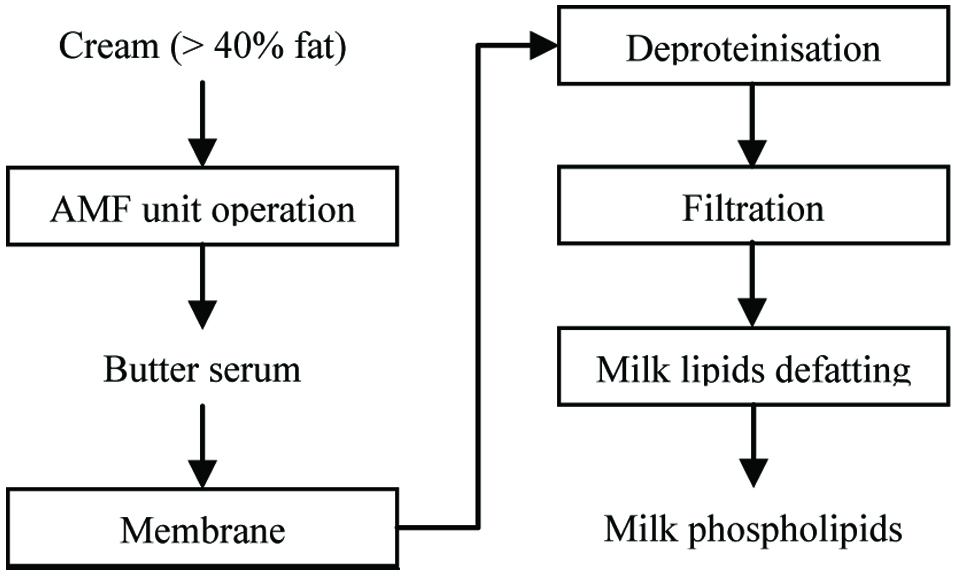 Click for large image | Figure 1. Block process flow diagram to illustrate a typical routine of milk phospholipid (PL) isolation and purification. |
Apart from the combined methods of solvents, filtration and centrifugation, milk phospholipids can be synthesized by using lecithin phosphatidylcholine and milk L-serine (WO2005027822). First, the choline group of soy PC is cleaved with Phospholipase D, and replaced with an L-serine group in the presence of calcium salt. The synthesized PS 20/60 (21 and 62% PS, respectively) can acquire an unpleasant taste and may become undrinkable. Thus, oil capsule was formulated to alter the flavor of PS. PS20/60 are physically unstable, and as they come from impure origin, these PS products (PS 20/60) were restricted by the public health authorities as described in WO2006-128465A1 (Table 1).
 Click to view | Table 1. Phospholipid composition of three typical dairy products |
3.2. Available commercial products and related patents
Among the milk phospholipid portfolio of Fonterra Co-operative Group Ltd, Phospholac 600 consists of approximately 75% phospholipid, representing one of the most concentrated milk phospholipids in large-scale commercial products (Li, 2014). Its Phospholac 500/600/700 and Gangolac 600 have a phospholipid content of 34, 70, 62, and 15%, respectively (Li, 2014; Thompson, 2005). Additionally, Arla Foods amba have commercialized a series of phospholipid rich dairy milk concentrated (PRDMC) products, including Lacprodan® MFGM 10 and Lacprodan® PL 20/75. Lacprodan® MFGM 10 has been claimed to support physiological development of infant gut and provide infants with similar phospholipid benefits as breastfed infants because the fatty acid profile of Lacprodan® is similar to that of human milk (Sokol et al., 2015). In addition, PL 20 is made out of serum phase of butter oil product (AMF) with membrane filtration, yielding over 20% phospholipids in total solids, which is a pure-natural nutraceutical with properties that is not discovered in conventional phospholipid sources including soy. PL 75 is a further ethanolic extract from PL 20, with 75% of phospholipids and protein-free. PL 20 and 75 targets infant milk formula and skin care, respectively (Arla, 2018).
As illustrated in Table 2, ethanol has frequently been used to extract milk lipids during industrial processes of manufacturing milk phospholipids. To further purify phospholipids, acetone (or dimethyl ether) is a common solvent to dissolve triacylglycerols. Most industrial milk products are generated from buttermilk (BM) and butter serum powder (BSP), except for some other origins such as whey protein concentrate (WPC) by Morinaga Milk Industrial Co Ltd. Tatua Co-operative Dairy Company produces a milk phospholipid concentrate from beta serum powder, an aqueous dairy ingredient separated from dairy streams comprising more than 60% lipids which has been made by phase inversion from an oil-in-water to a water-in-oil dispersion (Tatua, 2018). For instance, Lipidex, a derivative from beta serum powder by Synlait Milk Limited, contains 5–7% phospholipids and 26.6% fat in total (Moukarzel, 2016). Bovine milk SM (#860063, 25–200 mg) by Avanti has a purity of 99% (Lopez et al., 2014). Lecico Lipamine M20 comprises 20% of phospholipids including sphingomyelin, ceramides and ganglioside. This product has been produced with a special membrane technology, which used only water without using other solvents (Lecico, 2018).
 Click to view | Table 2. Industrial manufacturing of milk phospholipids |
3.3. Analysis: sample preparation, fractionation and chromatography
For analysis proposes, milk lipid samples are usually prepared with solvent extraction. The Folch and Bligh extraction method using both chloroform and methanol, a common formula to dissolve milk lipids. Though dichloromethane (DCM, less toxic than chloroform) has recently been introduced to replace chloroform (Claumarchirant et al., 2016), the principal methods of lipid extraction remain to be the Folch extraction (Bourlieua et al., 2018), the Bligh method (chloroform:methanol:water is 1:2:0.8, v/v/v) (Cheema et al., 2017) or the Röse-Gottlieb extraction of ammoniacal ethanolic solution of milk samples with diethyl ether and light petroleum (Barry et al., 2016; Ferreiro et al., 2016). Total lipids of samples may be measured using gravimetric determination, Gerber-van Gulik butyrometer, infrared spectral method specified in International Dairy Federation (IDF) (Ferreiro et al., 2016), or gas chromatograph equipped with a flame ionization detector (FID) (Rodríguez-Alcal et al., 2015).
Milk phospholipid fractions are usually further purified by a solid-phase extraction (SPE) before a determination assay of phospholipids and their subclasses, as illustrated in Table 3. Silica gel bonded cartridge is the most used SPE column to fractionate phospholipids from neutral lipids. First, the column is conditioned with hexane, then it is eluted by hexane (C6) and diethyl-ether (DEE) mixture to separate triacylglycerols. After that, another elution with chloroform, methanol and water will bring phospholipids out of the SPE column, which will be collected for solvent evaporation by using rotary evaporation. The final product (phospholipids) after solvent drying is stored at -20 °C before using (Haddadian et al., 2018). In addition, chloroform and methanol have been used as SPE conditioning and elution solvents (Walczak et al., 2016). Some SPE was performed with silica gel plate instead of silica gel bonded cartridges (Zou et al., 2015). Total phospholipids in milk samples can be determined by IDF molybdate assay (Vilamarim et al., 2018), Fourier transform infrared (FTIR) spectroscopy (Kala et al., 2018) or enzymatic method measuring the choline content (Shrestha et al., 2017).
 Click to view | Table 3. HPLC assays to determine milk phospholipids |
Nuclear magnetic resonance (NMR) using 31P is a standard assay to quantify milk phospholipids and their subclasses (Hickey et al., 2017; Xu et al., 2015). However, chromatography is the more common assay to determine milk phospholipids. Thin layer chromatography (TLC) is a convenient assay without sophisticated instruments. A formula of TLC elution solvent mixture containing hexane, diethyl ether and acetic acid (80:20:1, v/v/v) has often been applied on a silica gel plate. The fractionated subclasses are then visualized on the plate with iodine vapor (Fuller et al., 2012; Zou et al., 2015).
High-performance liquid chromatography (HPLC) remains the most commonly used method, because it can accurately quantify the total phospholipids and each of their subclasses than TLC. For each HPLC assay, 5–10 µL sample (approximately 5–100 μg/mL) is the necessary amount to perform chromatographic analysis (Cheema et al., 2017). As shown in Table 3, HPLC was usually coupled with such detectors as ultraviolet (UV) absorbance, evaporative light-scattering detector (ELSD) and mass spectroscopy (MS). Due to the polarity of milk phospholipid, silica column has often been used to separate the subclasses of milk phospholipids. To further fractionate the species of specified milk phospholipid subclasses, reverse phase (RP) HPLC with C18 column can be employed (Dugo et al., 2013). The binary solvents of chloroform and methanol or acetonitrile and ammonium acetate are frequently used as an elution medium. The change of formula of elution solvents leads to the different detection order of phospholipid subclasses in the chromatogram, as illustrate in Table 3. In some cases, pH of mobile phase was modulated by trimethylamine or ammonia hydroxide (pH 3 and 6, respectively) and formic acid has shown benefits in providing a flat baseline (Ferreiro et al., 2014).
| 4. Vesicle properties | ▴Top |
4.1. Liposomes
Milk phospholipid concentrate has good emulsification properties due to its amphiphilic molecular structure. Milk phospholipids can also be used to deliver nutraceuticals and bioactive compounds in food and bio-pharmaceutical industries, achieving better stability, solubility and bioavailability of the encapsulate (Livney et al., 2016). In a recent report, the vesicle properties of milk phospholipids was thoroughly reviewed (Arranz and Corredig, 2017).
Milk phospholipids-based liposomes have been proven to deliver lipophilic or hydrophilic components to improve the bioavailability of encapsulates, in either pharmaceutical or food industries. In the cosmetic area, liposomes have been used to facilitate dermal absorption of active compounds. Milk phospholipids-based liposomes have been applied to co-deliver beta-carotene within the membrane and ascorbic acid in the inner phase (Farhang, 2013). The complexing index increased when milk phospholipid concentration was improved from 5 to 10%, then plateaued at 26 ± 0.5% when milk phospholipid concentration was 10–15%. The size of carriers was 120 ± 2 nm using micro-fluidization unit. Due to the limited physical stability, the produced liposomes aggregated and stratified in one day (Farhang et al., 2012). The liposome carriers based on milk phospholipids were shown in Table 4.
 Click to view | Table 4. Milk phytosomes and liposomes as bioactive compound carriers |
4.2. Phytosomes
As illustrated in Table 4, phytosome carrier can also deliver bioactive compounds, both lipophilic and hydrophilic, in order to enhance oral bioavailability (Lu et al., 2018). Phytosomes are a durable complexes, with a simple manufacturing process (Gnananath et al., 2017). Complexing reaction of milk phospholipids and encapsulate (molecular ratio 1–5) was realized in either ethanolic or methanolic solution of 55°C. As a result, the bioavailability of encapsulate was enhanced by 3–5-fold (Freaga et al., 2018; Yu et al., 2016), while the solubility of 36-fold increase was evidenced (Telange et al., 2017).
Both milk phytosomes and liposomes are derived from milk phospholipids. Liposomes encapsulate bioactive compounds in either the core of phospholipid globule or in the phospholipid bilayer, whereas phytosomes are different from liposomes because phospholipids conjugate with encapsulates, hence they are more durable and efficient than liposomes (Karimi et al., 2015). Currently, the milk phospholipid-based phytosomes are not yet explored, and it should provide a prospective area to study.
4.3. Gastrointestinal digestion and absorption
Milk phospholipids do not hydrolysis in lingual and gastric tract, thereby they can be carriers of bioactive compounds (Castro-Gómez et al., 2015). Their digestion occurs in lumen, the upper part of intestinal gut. Phospholipase A/B/C/D acts on either sn-1 or 2 acyl (A), both sn-1 and 2 acryl (B), sn-3 phosphoric base (C) and sn-3 polar head (D), respectively (Gurr et al., 2002)). In human being, pancreatic phospholipase A2 (EC 3.1.1.4 (Venuti et al., 2017)) can act upon sn-2 position of phospholipids, resulting in lysophospholipids and fatty acids. The fatty acid group of lysphopholipids can be further cleaved by lysophospholiase (EC 3.1.1.5) (Winrow et al., 2003). Moreover, the pancreatic lysophospholipase of human being is most likely non-specific phospholipase, but carboxyl ester hydrolase (EC 3.1.1.1) (Duan and Borgström, 1993). In addition, sphingomyelinase (alk-SMase, EC 3.1.4.12) acts on phosphoric di-ester bond of sphingomyelin, generating ceramide and phosphocholine (Nilsson and Duan, 2019). Ceramide will be further split by mucosal ceramidase (N-CDase EC 3.5.1.23) (Mao and Obeid, 2008). The lipolysis products then cross the border of epithelial cells (mucosa) and enter the enterocyte to synthesize new phospholipids, which are then incorporated into chylomicrons (CM). After that, in approximately five hours postprandial, CM will enter into the lymph and blood circulation. Apart from absorption of hydrolysate of phospholipids (lyso-PLs and fatty acids), approximately 20% of phospholipids are passively absorbed in the intestinal lumen (Castro-Gómez et al., 2015). In addition, indigenous phospholipid excretion into bile is 10–20 g per day (Cohn et al., 2010), which was much higher than endogenous phospholipids (2–8 g phospholipid ingestion per day) (Lecomte et al., 2015). Therefore, phospholipids are not essential lipids though they are critical.
| 5. Health impacts | ▴Top |
The nutraceutical value of milk polar lipids has previously been reviewed, including the efficacy for modification of the trajectory recession of cerebral structure in old age (Reddan et al., 2018), the roles in the growth of infant brain and gut (Ortega-Anaya and Jimenez-Flores, 2018), the effects of immune-mediated anti-carcinogenic effects and anti-inflammatory activity (Verardo et al., 2017), and the relevance to hepatoprotection and cardiovascular diseases (Castro-Gómez et al., 2015). Moreover, milk phospholipids consequently reduced the waist circumference of the participants in this trial, compared with soy lecithin in a clinical trial, although the blood lipid concentrations of the attendants in the trial was not altered (Weiland et al., 2016). In addition, the effects of Lacprodan® PL-20 on supporting infant intestinal maturation (Arla, 2019) and a healthy microbiota (Nejrup et al., 2017) have been clinically demonstrated. Furthermore, buttermilk and krill oil phospholipids were associated with the improvement of synaptic signalling in aged rats (Tomé-Carneiro et al., 2018).
5.1. Neurocognitive effects
The nutritional value of milk polar lipids includes gut development (SM), neurocognitive development (SM), liver protection (PC), bacteria inhabitation (lyso-phospholipids), maintaining homeostasis (PE), cell signalling (PI) and memory restoration (PS), as reviewed in previous reports (Gallier et al., 2014; Le et al., 2014; Le et al., 2015). It has been documented that milk phospholipids can enhance the neurocognitive development in the trials. For example, the research has shown that sphingolipid supplementation improved the myelination of central nervous system and was responsible for the normal brain weight of rat infants (Castro-Gómez et al., 2015). L-serine is essential for the synthesis of sphingolipids and phosphatidylserine (PS) in particular types of central nervous system neurons (Hirabayashi and Furuya, 2008). Additionally, the cognitive performance benefits of dietary milk phospholipid have been evidenced with the clinical trial (Boyle et al., 2019), the rats model (Schipper et al., 2016), and the piglet model as well (Liu et al., 2014).
Present results appertaining the cognitive functions of milk phospholipids, from either ex vivo models or in vivo models are illustrated in Table 5. Most tests conferred the benefits of milk phospholipids on brain function, however one examination showed that it might be due to the combined effects of membrane proteins and polar lipids (Timby et al., 2014). In terms of commercial application, milk phospholipids are well-recognized ingredients for infant milk formula (IMF), which represent the world’s fastest growing functional food in recent years (Ireland, 2014).
 Click to view | Table 5. Health effects of milk phospholipids |
5.2. Skin care
Skin parameter enhancement examination has been performed in both in vivo and ex vivo, yielding positive results except for a non-effectiveness report under the set conditions (Keller et al., 2014), as illustrated in Table 5. Some of these benefits appear to be related to phospholipids, altering the hydration of skin and therefore increasing elasticity and resilience.
5.3. Anti-inflammatory in gastrointestinal development
Milk phospholipids have proven to be able to modulate inflammatory reaction and to protect against gastrointestinal leakiness, as illustrated in Table 5. Animal models and cell models have shown that the polar lipids fraction from MFGM affects infant gastrointestinal development. Milk phospholipids diet decreased gut permeability (Snow et al., 2010), altered distal gut microbiota and reduced serum lipopolysaccharide (LPS) (Norris et al., 2016), inhibit infectivity of rotavirus (Fuller et al., 2012), and regulate the neonatal gut microbiome and promote intestinal development (Bhinder et al., 2017).
5.4. Antioxidant activity
Milk phospholipids act as both antioxidants and a pro-oxidants and sometimes are used to alleviate food oxidation. Anti-oxidative activity of phospholipids might be due to such mechanisms as metal-chelation, alteration of the location of other antioxidants, and regeneration of other primary antioxidants. However, phospholipids can also act as primary antioxidants and pose significant antioxidant activity to biological membranes (i.e. meats), owning to their unsaturated fatty acids and negative charge (Cui and Decker, 2016). Phospholipid supplementation to soybean oil significantly retarded the oxidative process, extending oxidative stability index (OSI) from 7.62 to 12.96 h. However, phosphatidylcholine addition caused trimethylamine (TMA, fishy off-odor) generation (Jiang et al., 2016). Marine lecithin (i.e. krill oil) consists of a natural antioxidant (astaxantin) and phospholipids bound LC-PUFA, which inhibits oil peroxidation during its shelf life (Ben-Dror et al., 2018). Αlpha-tocopherol enhanced the oxidative stability of marine phospholipid emulsions (Lu, 2013).
| 6. Conclusion | ▴Top |
In this review, milk vesicle properties and health impacts were addressed. As an emerging material of vesicles in nutraceutical and bio-pharmaceutical, milk phospholipids show advantages over lecithin of soy and egg yolk in encapsulation efficiency. Recently, various kinds of liposomes have been fabricated for enhancing the solubility and bioavailability of encapsulates. Phytosomes, more stable carriers than liposomes, should provide a further area to study. In recent reports, milk phospholipids have been proven to support cognitive development owning to their balanced distribution in phosphatidylserine and sphingomyelin, which was almost absent in soy and egg yolk lecithin. Apart from brain function, milk phospholipids have a role in skin care, due to their more saturated fatty acids, which lead to milky-white color and stability.
In conclusion, milk phospholipids have prospective applications in nutritional delivery, infant formula and cosmetic for their vesicle properties and biological functionalities. As potential alternatives to traditional polar lipids from egg yolk and soy, milk phospholipids need to be efficiently produced in large-scale. Ethanolic extraction remains the most used lipid extraction process in dairy industry. Defat with supercritical carbon dioxide or acetone are frequently used to further refine phospholipids from lipids.
| References | ▴Top |