| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 4, December 2018, pages 139-149
Effect of Phyllanthus emblica L. fruit on improving regulation of methylglyoxal-induced insulin resistance in 3T3-L1 cells
Hui-Chi Chena, †, Yu-Tang Tungb, †, Sheng-Yi Chena, Jer-An Linc, Gow-Chin Yena, c, *
aDepartment of Food Science and Biotechnology, National Chung Hsing University, 145 Xingda Road, Taichung 40227, Taiwan
bGraduate Institute of Metabolism and Obesity Sciences, Taipei Medical University, 250 Wu-Hsing Street, Taipei 110, Taiwan
cGraduate Institute of Food Safety, National Chung Hsing University, 145 Xingda Road, Taichung 40227, Taiwan
†These authors contributed equally to this work.
*Corresponding author: Gow-Chin Yen, Department of Food Science and Biotechnology, National Chung Hsing University, 145 Xingda Road, Taichung 40227, Taiwan
DOI: 10.31665/JFB.2018.4170
Received: December 4, 2018
Revised received & accepted: December 21, 2018
| Abstract | ▴Top |
The increasing methylglyoxal (MG) level of body has been found in people with obesity and insulin resistance, resulting from their dietary style and abnormal metabolic functions. MG promotes inflammation, oxidative stress, glycation, and all of which are closely related to insulin resistance and chronic diseases. Phyllanthus emblica L. fruit has various bioactivities such as anti-inflammation, anti-diabetes, anti-nonalcoholic fatty liver, and anti-dyslipidemia. Therefore, this study was aimed to investigate the effects of water extract of P. emblica (WEPE) and its enriched compound, ellagic acid, on MG-induced inflammation, insulin resistance, and lipogenesis in 3T3-L1 cells. The results showed that MG activated the peroxisome proliferator activated receptor-gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα), which can increase adipogenesis in adipocytes. In addition, MG enhanced pro-inflammatory cytokine IL-6 protein expression and release through the activation of MAPK and NF-κB signaling pathway, as well as increasing the monocyte chemoattractant protein-1 expression to cause macrophage infiltration. MG also significantly reduced glucose uptake, indicating that insulin resistance in obese patients may be related to MG generation. WEPE and ellagic acid effectively decreased IL-6 protein expression and cytokine release through inactivation of JNK and p65 pathways. WEPE and ellagic acid significantly increased glucose uptake and reduced insulin resistance by MG treatment. WEPE also decreased the protein-tyrosine phosphatase 1B to reduce insulin resistance and inhibited MG-induced fat accumulation related proteins such as PPARγ, C/EBPα, FAS, and p-ACC. Therefore, WEPE may have the potential to ameliorate MG-induced inflammation, increase glucose uptake, and decrease fat accumulation.
Keywords: Methylglyoxal; 3T3-L1 cells; Insulin resistance; Inflammation; Phyllanthus emblica
| 1. Introduction | ▴Top |
More than 1.5 billion people around the world are overweight, among which about 5 million are obese (Hammond and Levine, 2010). Obesity not only consumes much of the healthcare resources but also increases the probability of insulin resistance, type 2 diabetes, abnormal lipid metabolism, cardiovascular disease, and cancers (Han and Lean, 2016). Additionally, over nutrition and obesity activates the immune system resulting in inflammation, which has a significant relationship with chronic diseases (Gerriets and MacIver, 2014). The main cause of obesity is excessive energy intake and too much energy being stored in the adipose tissue, which is composed of pre-adipocytes and adipocytes. The hypertrophy adipocytes enhance pro-inflammatory cytokines, monocyte chemoattractant protein-1 (MCP-1), and free fatty acids that can promote macrophage infiltration to adipose tissue (Kamei et al., 2006). Inflammatory cytokines cause abnormal phosphorylation of IRS-1 resulting in excessive insulin secretion by the pancreas, which finally leads to insulin resistance. Excessive insulin cannot be utilized by the cells, which inhibit glucose uptake in adipose tissue and increase inflammatory cytokines (Qatanani and Lazar, 2007).
Previous studies have shown that the plasma concentrations of advanced glycation end products (AGEs) and methylglyoxal (MG) are increased in obesity and in patients with insulin resistance (Bo et al., 2016). Furthermore, long-term intake of AGEs and MG could increase AGEs and MG levels in the body, and circulating of AGEs in the body is not easily eliminated (Sims et al., 2010; Tikellis et al., 2014). In addition, AGEs and MG generate oxidative stress and promote the chronic inflammation, which are an important factor in insulin resistance (Kellow et al., 2014; Dong et al., 2016).
Phyllanthus emblica L. fruit is mainly grown in tropical and subtropical regions, and enriches in a variety of biologically active compounds such as flavonoids, polyphenols and vitamin C. Previous studies have shown that the extract of P. emblica fruit can improve blood pressure, fatty liver, nonalcoholic steatohepatitis, diabetes, inflammation, antioxidant, and cancer (Fugh-Berman, 2000; Nain et al., 2012; Muthuraman et al., 2011; Ansari et al., 2014; Zhao et al., 2015; Tung et al., 2018). However, the effect of P. emblica fruit on methylglyoxal-induced insulin resistance remains unclear. Therefore, the objective of this study was to investigate the effect of P. emblica fruit extract on MG-induced inflammation, lipid metabolism, and insulin resistance in 3T3-L1 cells.
| 2. Material and methods | ▴Top |
2.1. Materials
Glycine, methylglyoxal, isopropanol, gallic acid, ellagic acid, Oil Red O, N,N,N′,N′,-tetramethylethylenediamine (TEMED), TWEEN 20, bovine serum albumin (BSA), anti-β-actin antibody, and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich company (St. Louis, MO, USA). Anti-IL-6 antibody and anti-protein-tyrosine phosphatase 1B (PTP1B) antibody were purchased from Abcam (Cambridge, UK). Nitrocellulose (NC) membrane, anti-peroxisome proliferator activated receptor-gamma (PPARγ) antibody, and dimethyl sufoxide (DMSO) were purchased from Merck Millipore (Darmstadt, Germany). Mouse IL-6 ELISA Ready-SET-GO kit was purchased from eBioscience Company (San Diego, CA, USA). Anti-JNK antibody, anti-phospho-JNK antibody, anti-p65 antibody, anti-phospho-p65 antibody, and anti-CCAAT/enhancer-binding protein alpha (C/EBPα) antibody were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Glucose uptake Cell-Based assay kit was purchased from Cayman Chemicals Company (Ann Arbor, MI, USA). Penicillin-streptomycin solution (PS) and DMEM (High glucose) were purchased from the Thermo Fisher Scientific (Waltham, MA, USA). Anti-phospho-acetyl-CoA carboxylase (ACC) antibody and anti-ACC antibody were purchased from Cell Signaling Technology Corporation (Danvers, MA, USA). Anti-fatty acid synthase (FAS) antibody was purchased from Gene Tex Corporation (Irvine, CA, USA).
2.2. Cell differentiation
Mouse preadipocytes lines, 3T3-L1 (purchased from BCRC No. 60159), was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 4.5 g/L glucose, 4 mM L-glutamine, 1.5 g/L sodium bicarbonate and 1% PS (100 units/mL pencillin and 100 μg/mL streptomycin). Cells were incubated in a 5% CO2 incubator at 37 °C. Cells were cultured to about 70% confluence, and then cell differentiation was induced by changing the medium to differentiation medium I (DMEM containing 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, 1 μM insulin, 1.5 g/L sodium bicarbonate, 10% FBS, and 1% PS). After 48 h, the medium was changed to differentiation medium II (DMEM containing 1 μM insulin, 1.5 g/L sodium bicarbonate, 10% FBS, and 1% PS) for 4 days. The differentiation medium II was refreshed on days 8, 10, 12, and 14. Intracellular lipid droplets were increased in both number and size in the cell differentiation.
2.3. Quantification of methylglyoxal (MG)
Quantification of MG was carried out by HPLC according to the method of Arribas-Lorenzo and Morales (2010). O-phenylenediamine (OPD) was used to derivatize MG into stable 2-methylquinoxaline (2-MQ), and then 2-MQ was analyzed by HPLC. The cell culture medium of preadipocytes or adipocytes was mixed with 0.45 N perchloric acid for 5 min, and then added 10 mM OPD at 37 °C for 2 h following at 60 °C for 1 h. After derivatization with OPD, the sample was loaded onto the solid phase extraction (SPE) column and then the content of 2-MQ was determined by HPLC. A standard calibration curve of 2-MQ was constructed with a series of concentrations of standard compounds. In addition, different concentrations (1–100 μg/mL) of MG were prepared in accordance with the above-described reaction to obtain a standard calibration curve of 2-MQ in order to determine the concentration of MG. Samples were separated by chromatography with LichroCART® (250 mm × 4 mm, Merck) column. Elution was performed isocratically with a mixture of 0.5% (v/v) acetic acid in water and methanol (40:60, v/v) with a detection (Hitachi L-2400 ELITE LaChrom) of 315 nm, and flow rate of 0.7 mL/min.
2.4. Extraction of WEPE
P. emblica fruit was generously supplied by the Miaoli District Agricultural Research and Extension Station, Council of Agricultural, Executive Yuan (Miaoli, Taiwan). The dry powder of P. emblica fruit was added to double-distilled water on a rotary shaker (120 rpm) at 25 °C overnight. The water extract of P. emblica fruit (WEPE) was decanted, filtered under vacuum, concentrated in a rotary evaporator, and then lyophilized. Furthermore, the extract was stored at −20 °C for assay. The yield of the extract contains two compounds consist of gallic acid and ellagic acid.
2.5. Oil red O assay
The 3T3-L1 adipocytes were seeded in 6-well plate, and then 0, 10, 50, or 100 μM MG was added for 3 days or 6 days. At the end, the cells were washed twice with PBS and fixed in 10% neutral buffered formalin for 20 min. After the cells were covered with 100% propylene glycol for 3 min, the cells were stained with 5 mg/mL oil red O solution for 60 min. After the stain was removed, the cells were decolored with 60% propylene glycol and then washed with PBS. Representative photomicrographs were captured at a 200× magnification using a camera mounted onto a microscope (Olympus, Osaka, Japan) and intracellular lipid droplets were analyzed using ImagePro (Media Cybernetics, Bethesda, MD, USA). Oil red O-stained material (OROSM) was extracted using isopropanol, and the absorbance was measured at a wavelength of 520 nm using a spectrophotometer.
2.6. Analysis of the proteins
The 3T3-L1 adipocytes were pretreated with 0, 10, 50, or 100 μM MG for 48 h, or co-treated with 10 μM MG and test samples (different concentrations of WEPE or 5 mM AG) for 48 h. The expressions of IL-6, p-JNK, JNK, p-p65, p65, PPARγ, C/EBPα, MCP-1, PTP1B, p-ACC, ACC, and FAS in cell extract proteins were analyzed using western blot analysis. The western blot analysis was carried out following the method of Cheng et al. (2014).
2.7. Enzyme-linked immunosorbent assay
The 3T3-L1 adipocytes were pretreated with 0, 10, 50 or 100 μM MG for 48 h, or co-treated with 10 μM MG and test samples (different concentrations of WEPE or 5 mM AG) for 48 h. IL-6 was measured using a specific ELISA kit as indicated by the manufacturer’s instructions.
2.8. Glucose uptake assay
The 3T3-L1 adipocytes were exposed to 100 nM insulin for 1 h and continuously treated with 0, 10, 50 or 100 μM MG for 48 h, or co-treated with 10 μM MG and test samples (different concentrations of WEPE or 5 mM AG) for 48 h. Glucose uptake assay was measured using Glucose Uptake Cell-Based Assay Kit (Cayman, Ann Arbor, MI, USA). Finally, the cells were detected using a flow cytometer (Becton Dickson Immuno-cytometery System USA, San Jose, CA, USA) and then analyzed using Cell Quest analysis software.
2.9. Statistical analysis
Data were expressed as the means ± SD. The significance of difference was calculated by Duncan’s test, and results with p < 0.05 were considered to be statistically significant.
| 3. Results | ▴Top |
3.1. Effects of MG contents and Oil red O stained material (OROSM) in MG-induced insulin resistance
There is an increased accumulation of AGEs in obesity and diabetes compared to normal subjects (Vlassara and Striker, 2011). MG was the main precursor of AGEs (Brix et al., 2012), and therefore the MG contents of 3T3-L1 preadipocytes and adipocytes were determined in this study. Figure 1a showed that the MG contents of cells and the culture medium were significantly higher in 3T3-L1 adipocytes compared to those in preadipocytes (p < 0.05). The oil droplets are increased in differentiated adipocytes (Koopman et al., 2001), and thus OROSM is one of the most important indicators of the adipocyte differentiation. Figure 1b showed adipocytes treated with MG significantly increased the accumulation of oil droplets compared with the MG-untreated group (p < 0.05).
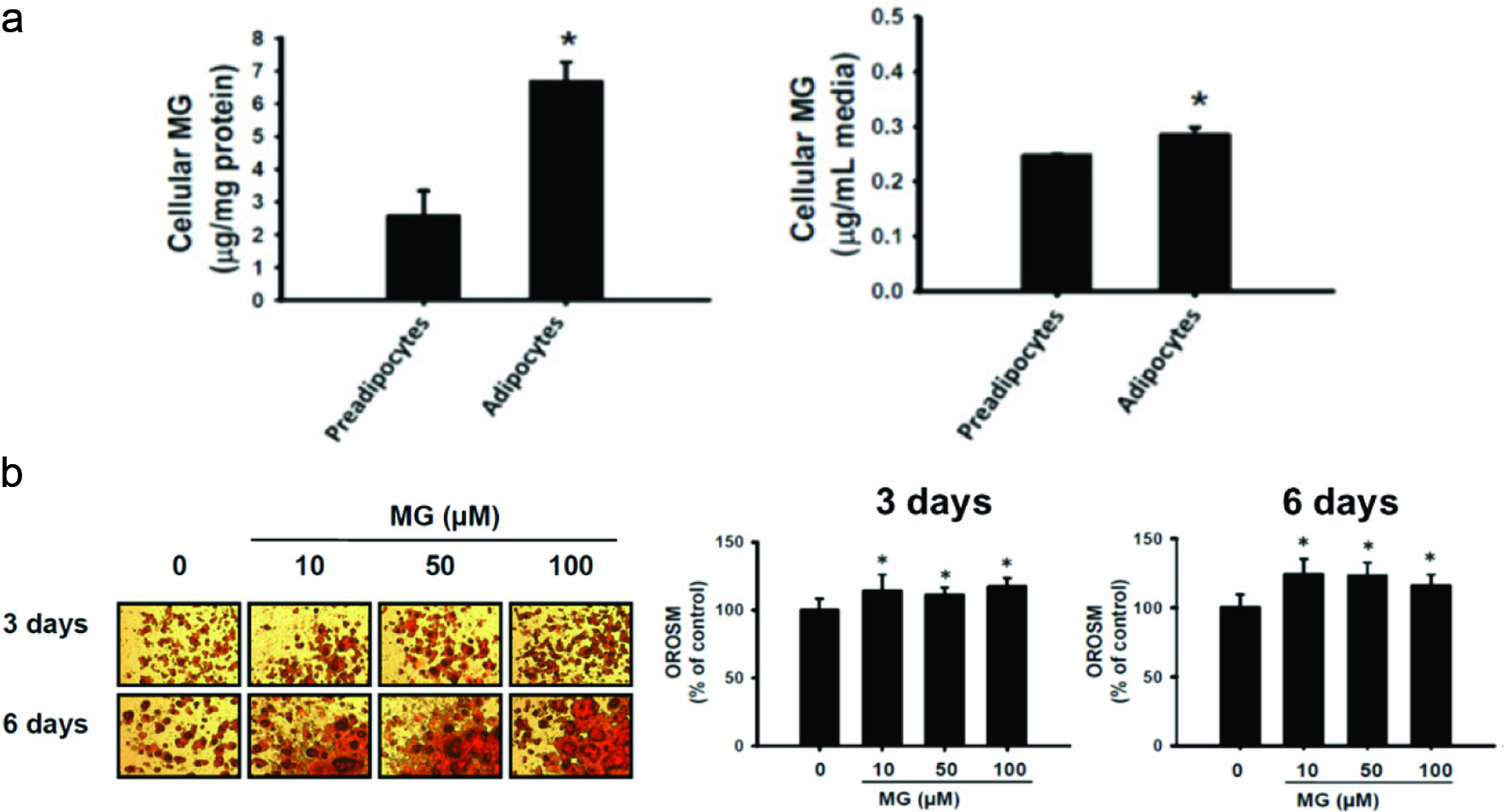 Click for large image | Figure 1. Oil droplets accumulation by methylglyoxal (MG) treatment. (a) Determination of both intracellular and extracellular MG as 2-methylquinoxaline by high-performance liquid chromatography (HPLC). The data were expressed as the mean ± SD (n = 3). * p < 0.05 compared to preadipocyte. (b) Effects of methylglyoxal (MG) on Oil red O stained material (OROSM) in 3T3-L1 adipocytes. Cells were treated with 10, 50, and 100 μM of MG for 3 days and 6 days. |
3.2. Expressions of p-JNK, p-p65, IL-6, and MCP-1 in MG-induced insulin resistance
Insulin resistance is caused by obesity through JNK pathway (Kaminska, 2005; Lee and Lee, 2014). In addition, JNK activation promotes the production of cytokines, such as IL-6, TNF-α, and IL-1β (Kaminska, 2005; Lee and Lee, 2014). As shown in Figure 2a, p-JNK expression was significantly increased after 48 h of MG induction (p < 0.05). Therefore, MG may promote the inflammatory response through JNK pathway. NF-κB consists of two subunits, p50 and p65. When NF-κB is activated, p-p65 translocates into the nucleus and activates the target gene transcription (Kopitar-Jerala, 2015). As shown in Figure 2b, the expression of p-p65 was significantly increased after 48 h of MG induction (p < 0.05). IL-6 protein is one of major pro-inflammatory cytokines secreted by adipocytes in insulin-resistant human (Rotter et al., 2003). These results showed that IL-6 protein expression and release were significantly increased after 48 h of MG induction (p < 0.05) (Figure 2c, d). Therefore, MG increased pro-inflammatory cytokines. Obese patients are often accompanied by chronic inflammation, which leads to the development of many chronic diseases such as insulin resistance, fatty liver, and high blood pressure (Ye, 2013). In addition, MCP-1 could induce macrophage infiltration (Deshmane et al., 2009; Panee, 2012). Figure 2e showed that MCP-1 was markedly increased after 48 h of MG induction (p < 0.05). Therefore, MG can increase the expression of MCP-1 expression, thereby enhancing the possibility of macrophage infiltration to generate inflammation.
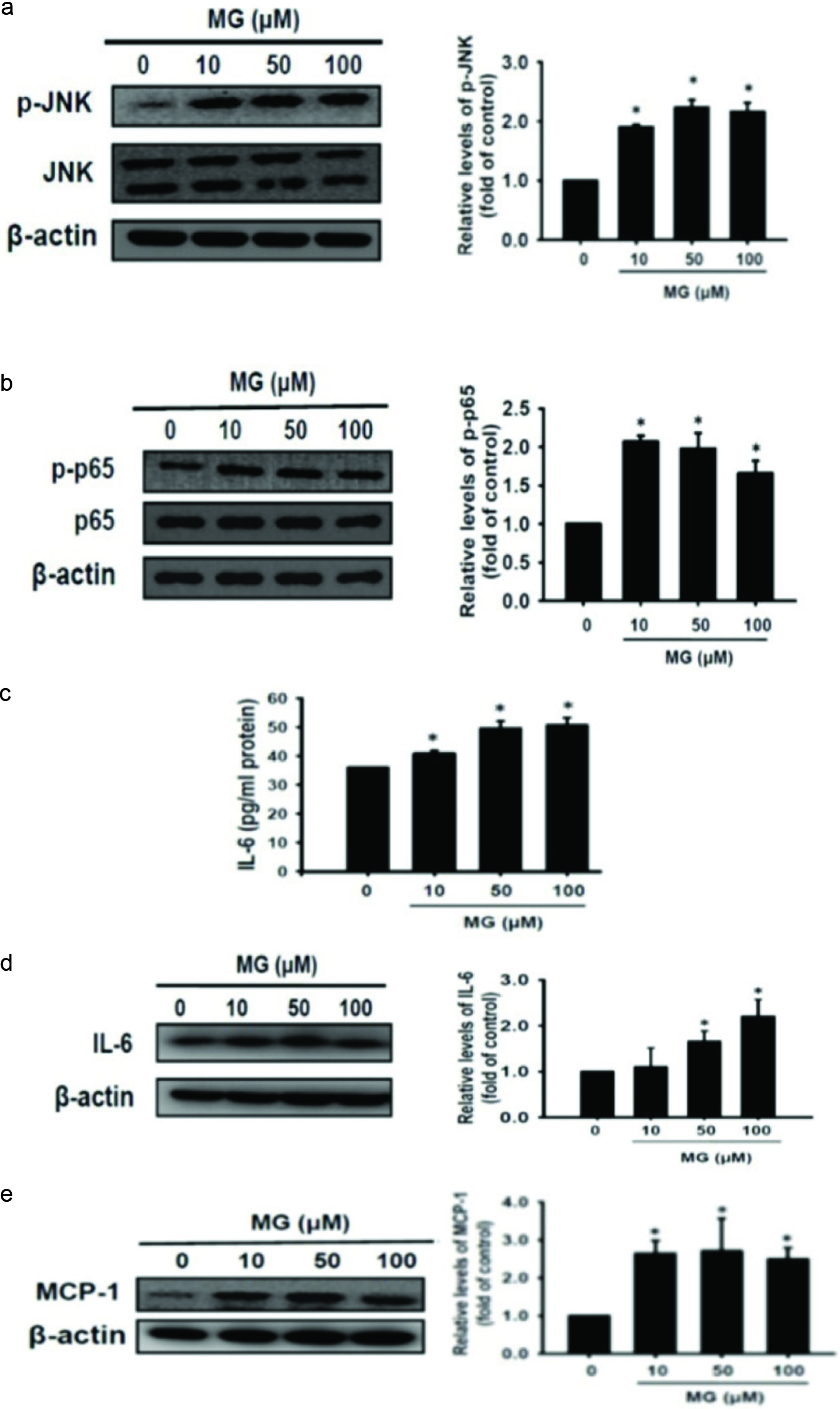 Click for large image | Figure 2. Inflammatory relative cytokine and chemokine production promoted by MG initiated signaling. 3T3-L1 adipocytes were treated with 10, 50, and 100 μM of MG for 48 h, respectively. (a) p-JNK protein, (b) p-p65 protein, (c) IL-6 release, (d) IL-6 protein, and (e) MCP-1 protein expression were measured using western blot and ELISA assay. The data were expressed as the mean ± SD (n = 3). * p < 0.05 compared to untreated control. |
3.3. Effect of MG on glucose uptake, PPARγ, and C/EBPα in 3T3-L1 adipocytes
MG reduces glucose uptake and causes insulin resistance through reduction of insulin signaling pathway (Jia and Wu, 2007). Figure 3a shows that glucose intake was significantly reduced after 48 h of MG induction (p < 0.05). Thus, MG decreased glucose uptake in adipocytes, which may cause insulin resistance. As shown in Figure 1b, MG enhances the oil droplet generation of 3T3-L1 adipocytes which may be regulated by PPARγ and C/EBPα. PPARγ is activated by C/EBPβ and C/EBPδ, and then activates C/EBPα. PPARγ and C/EBPα can furtherly activate ACC and FAS. ACC and FAS accelerate the synthesis of triacylglycerol and accumulate the oil droplets. Figure 3b and c shows that MG significantly increased the protein expressions of PPARγ and C/EBPα in adipocytes compared to the MG-untreated group (p < 0.05). Therefore, MG can promote the accumulation of oil droplets through upregulation of PPARγ and C/EBPα proteins.
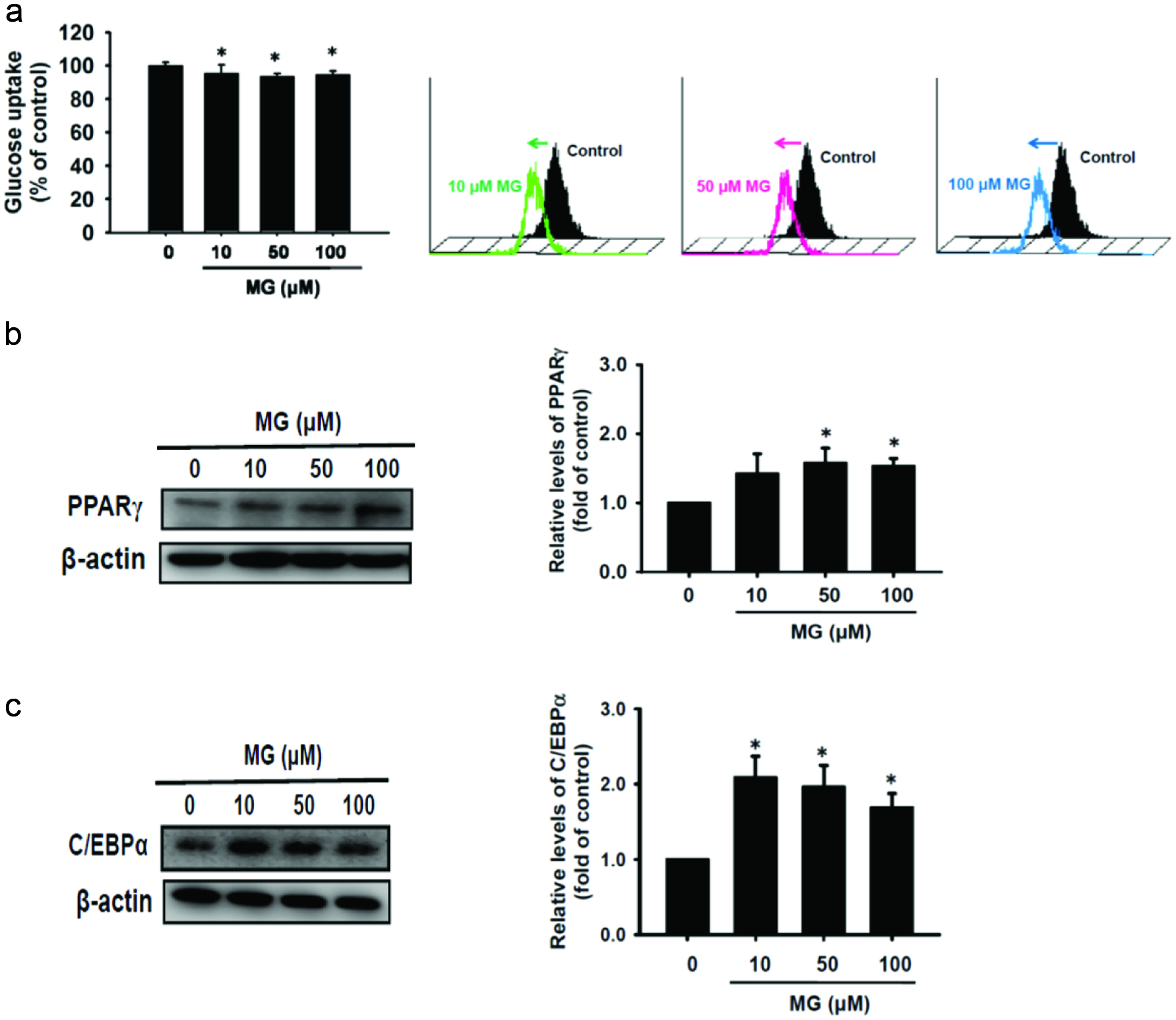 Click for large image | Figure 3. MG not only reduced glucose uptake also enhanced oil droplets accumulation-associated PPARγ and C/EBPα proteins expression. (a) Cells were treated with MG for 24 h. The glucose uptake assay was performed using flow cytometry. After MG treatment for 48 h, protein expressions of (b) PPARγ and (c) C/EBPα were assessed by western blot. The data were expressed as the mean ± SD (n = 3). * p < 0.05 compared to untreated control. |
3.4. Effect of WEPE on glucose uptake and the protein expressions of IL-6, p-JNK, p-p65, and MCP-1 in MG-induced insulin resistance
MG is a highly reactive metabolite of carbohydrates. When MG and MG-derivatives AGEs are increased, it may force development of insulin resistance (Vidal et al., 2014). MG levels are also higher in diabetic patient (Zhu et al., 2015). Aminoguanidine (AG), an investigational drug, acts to reduce the level of advanced glycation end products (AGEs) through interacting with 3-deoxyglucosone (Matsui et al., 2017). In here, we intended to compare the therapeutic effects between AG and WEPE on MG-induced insulin resistance. As shown in Figure 4a, WEPE effectively improved the glucose uptake of MG-induced adipocytes (p < 0.05). Obesity can be divided into adipocyte hyperplasia and hypertrophy. Adipocyte hyperplasia occurs in early childhood and pregnancy, and adipocyte hypertrophy occurs mainly in the adults. Adipocyte hypertrophy is usually accompanied with IL-6 and TNF-α, which induces insulin resistance (Abranches et al., 2015). Figure 4b and c show pro-inflammatory cytokine IL-6 protein expression and release were both significantly decreased in MG-adipocytes treated with WEPE (p < 0.05). Figure 4d indicates that p-p65 protein expression was significantly reduced by treatment with WEPE after 48 h of MG induction of 3T3-L1 adipocytes (p < 0.05). Thus, WEPE could significantly inhibit IL-6 and p-p65 protein expression induced by MG and thus reduce inflammation. MCP-1 is one of the inflammatory markers, mainly in adipose tissue, which can induce macrophage infiltration. Serum MCP-1 levels are higher in obese patients and patients with insulin resistance (Kamei et al., 2006). As shown in Figure 4e, WEPE significantly reduced MCP-1 protein expression (p < 0.05) induced by MG, and therefore WEPE can alleviate the MG-induced macrophage infiltration. When cells are stimulated by oxidative stress or proinflammatory cytokines, the MAPK signaling cascade is activated by ERK, JNK, and p38 (Johnson and Lapadat, 2002) that is involved in cell growth, differentiation, inflammation, and apoptosis (Singh et al., 2014). Figure 4f shows that p-JNK protein expression was significantly increased after treatment of MG in adipocytes (p < 0.05). However, WEPE effectively attenuated p-JNK (p < 0.05). Therefore, WEPE improves inflammation by down-regulating the expression of MAPK-related proteins.
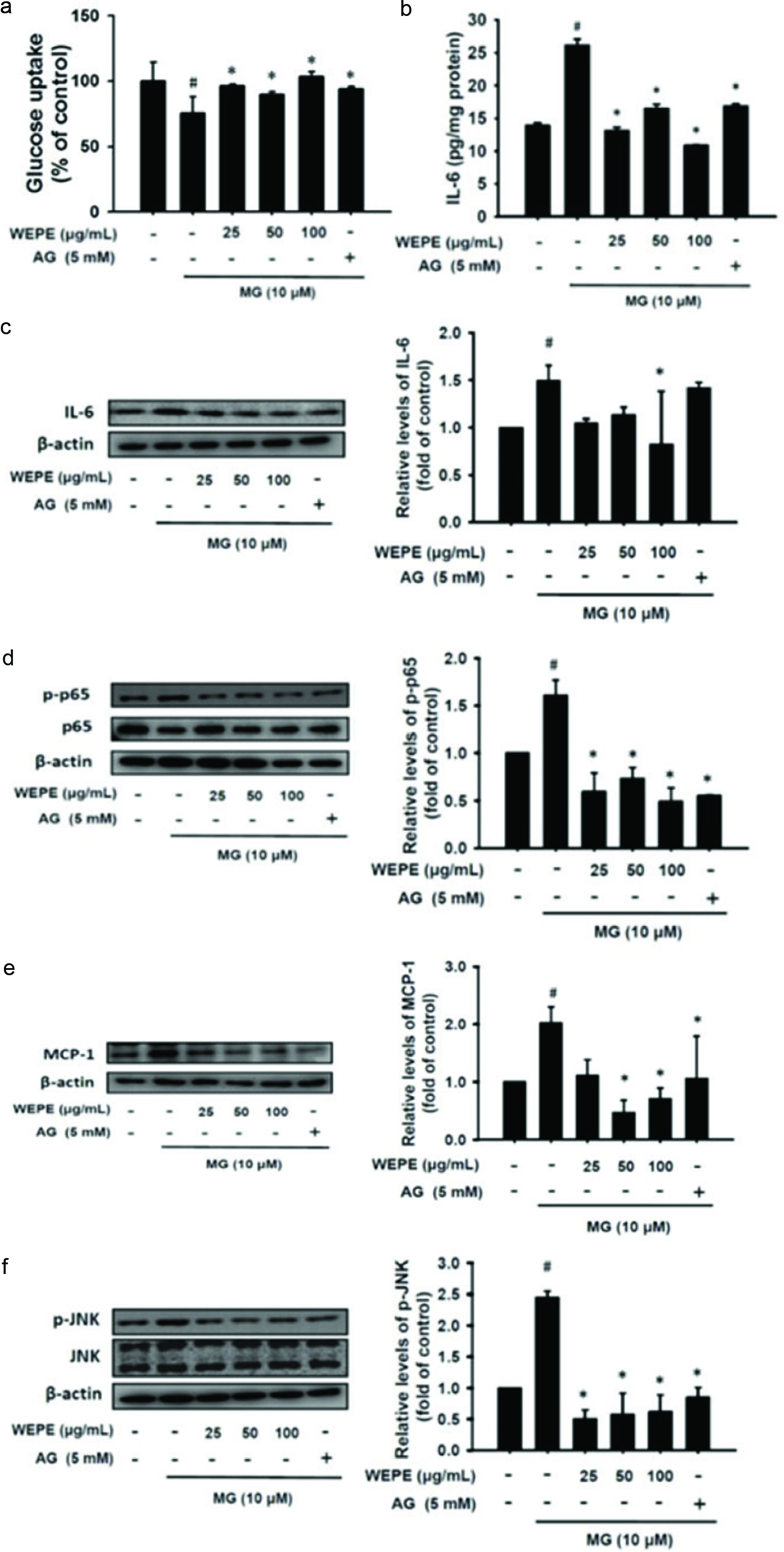 Click for large image | Figure 4. WEPE effectively inhibited glucose uptake and attenuated inflammatory relative signaling initiated by MG treatment. (a) MG treatment combined with or without WEPE and 5 mM AG treatment in 3T3-L1 adipocytes for 24 h. The glucose uptake assay was performed using flow cytometry. (b) IL-6 secretion and (C-F) protein expressions of IL-6, p-p65, MCP-1, and p-JNK in MG-treated 3T3-L1 adipocytes combined with or without WEPE and 5 mM AG treatment for 48 h. The data were expressed as the mean ± SD (n = 3). # p < 0.05 compared to untreated control; * p < 0.05 compared to MG-induced adipocytes. |
3.5. Effect of WEPE on the protein expressions of PTP1B, PPARγ, C/EBPα, p-ACC, and FAS in MG-induced insulin resistance
MG causes ROS and oxidative stress, in which endoplasmic reticulum stress plays an important regulatory function. Protein tyrosine phosphatase 1B (PTP1B) belongs to the family of protein tyrosine phosphatase (PTP). Endoplasmic reticulum stress reduces the activity of the tyrosine kinase on the insulin receptor, leading to the blockage of insulin pathway to cause insulin resistance (Cheng et al., 2012). Figure 5a indicates that MG-induced adipocytes show PTP1B protein expression decreasings significantly after treatment with WEPE (p < 0.05). PPARγ and C/EBPα are the major indicators of differentiated adipocytes (Kim et al., 2012; Ilavenil et al., 2014). As shown in Figure 5b, WEPE significantly decreased the protein expressions of PPARγ and C/EBPα induced by MG (p < 0.05). Acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) are the key proteins in the development of obesity (Tan et al., 2011). ACC is the first step of the enzyme for the synthesis of fatty acids. Thus, the inhibition of p-ACC can reduce triacylglycerol synthesis, and then decrease fat accumulation. Figure 5c shows that WEPE significantly reduced the expression of p-ACC in the adipocytes after 48 h of MG induction (p < 0.05), and thus WEPE can alleviate the activation of ACC to reduce lipid accumulation. The FAS is an important enzyme in lipid synthesis. Figure 5c showed that WEPE significantly reduced the protein expression of FAS (p < 0.05). Therefore, WEPE can regulate fat synthesis related proteins induced by MG, and thus may decrease the deposition of oil droplets in adipose.
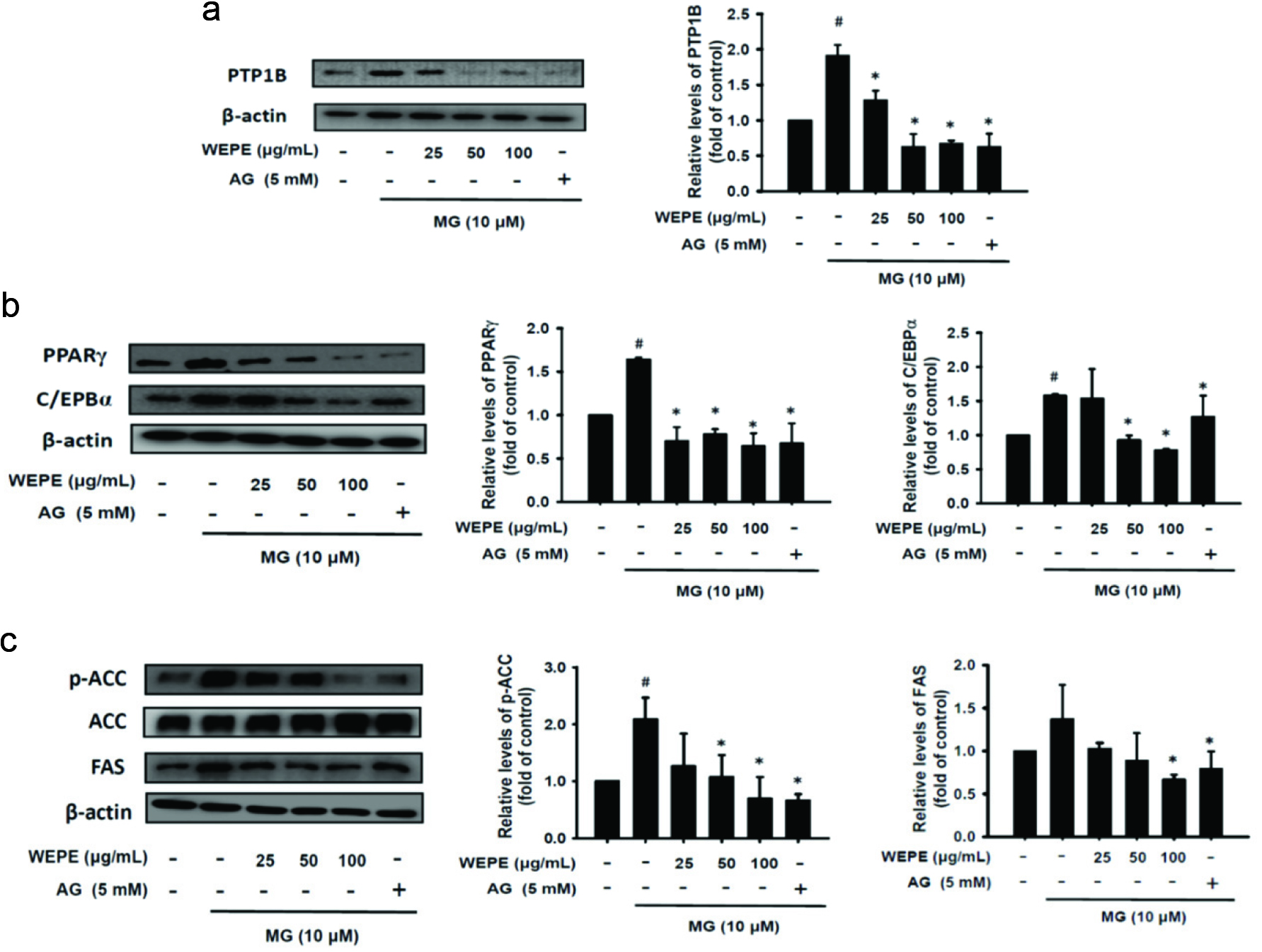 Click for large image | Figure 5. WEPE decreased fat synthesis related proteins expression induced by MG treatment. MG-treated 3T3-L1 adipocytes combined with or without WEPE and 5 mM AG treatment for 48 h. Protein expressions of (a) PTP1B, (b) PPARγ and C/EBPα, and (c) p-ACC and FAS were measured by western blot, respectively. The data were expressed as the mean ± SD (n = 3). # p < 0.05 compared to untreated control; * p < 0.05 compared to MG-induced adipocytes. |
3.6. Effect of ellagic acid on glucose uptake and IL-6 level in MG-induced insulin resistance
P. emblica is a rich source of various phytochemicals such as ellagic acid, gallic acid, kaempferol, and quercetin, all of which are excellent for capturing free radical and controlling lipid damage and DNA oxidation (Singh et al., 2015). Among them, the main constituents of WEPE are ellagic acid (4.8 mg/g of crude extract) and gallic acid (1.8 mg/g of crude extract). As shown in Figure 6a, IL-6 was elevated by treatment of MG with adipocytes for 48 h. However, ellagic acid alone or co-treatment with ellagic acid and gallic acid both showed a significant reduction in MG-induced IL-6 secretion (p < 0.05), and their inhibitory activities were similar to that of AG. On the other hand, ellagic acid could improve the release of IL-6 in a dose-dependent manner (p < 0.05) (Figure 6b). In addition, ellagic acid may also effectively improve the glucose uptake of MG-induced insulin resistance (p < 0.05) (Figure 6c).
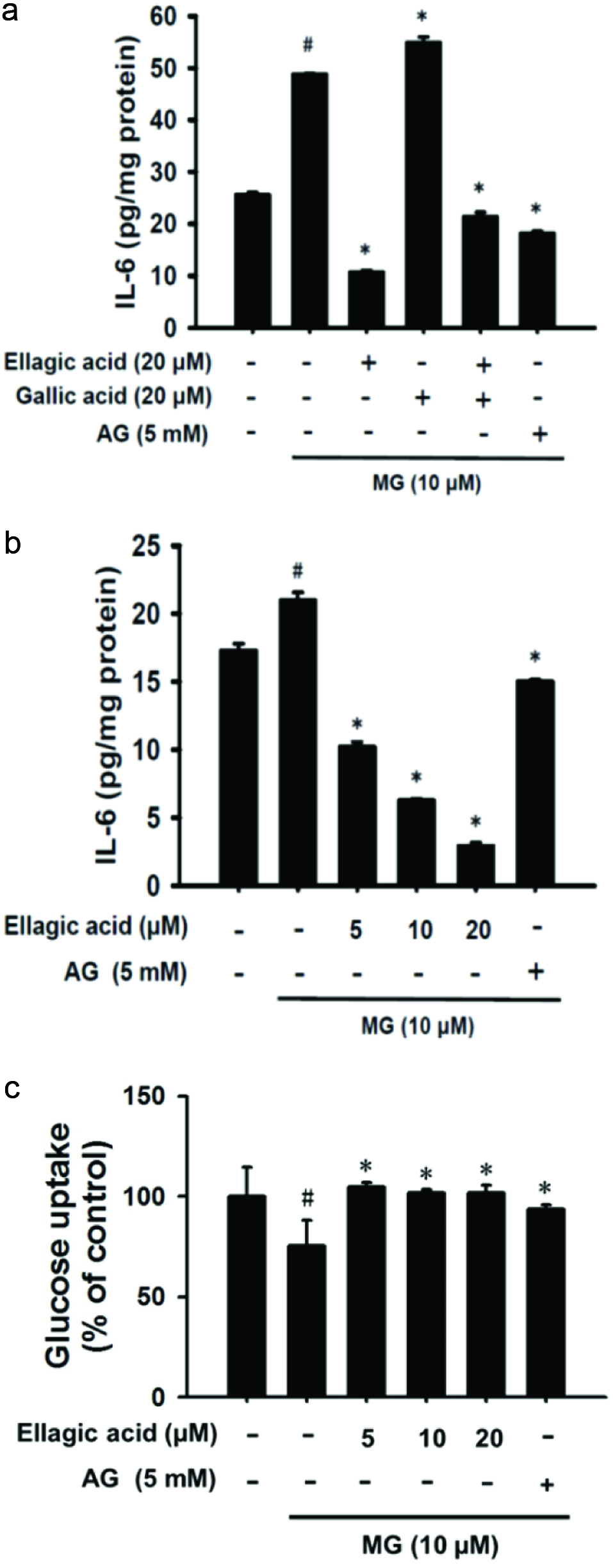 Click for large image | Figure 6. Ellagic acid dramatically inhibited IL-6 secretion and raised glucose uptake in MG-induced insulin resistance. (a) MG-treated 3T3-L1 adipocytes combined with or without 20 μM ellagic acid and 20 μM gallic acid treatment for 48 h. IL-6 production was accessed using ELIAS assay. (b) MG-treated 3T3-L1 adipocytes combined with or without ellagic acid (5, 10, and 20 μM) treatment for 48 h. IL-6 production was measured by ELIAS assay. (c) Cell treatment condition of glucose uptake was the same as in (b) but treatment for 24 h. Glucose uptake was measured by flow cytometry. The data were expressed as the mean ± SD (n = 3). # p < 0.05 compared to untreated control; * p < 0.05 compared to MG-induced adipocytes. |
| 4. Discussion | ▴Top |
Metabolic syndrome patients generally have a higher amount of MG (Vidal et al., 2014). MG enhances free radicals, thus resulting in oxidative stress and inflammation (Matafome et al., 2013). The use of botanical plants is gaining renewed interest in the treatment of some clinical disorders. In the present study, MG was used to induce adipocytes, which establishes the cell model of inflammation and insulin resistance. The results so obtained showed that WEPE effectively improved MG-induced IL-6 secretion in adipocytes (Figure 4b, c). Dang et al. (2011) showed that WEPE has a good anti-inflammatory effect in acetic acid-induced peritonitis in mice. Muthuraman et al. (2011) showed that phenolic compounds of P. emblica can reduce acute inflammation induced by carrageenan in rats. P. emblica has a high content of ellagic acid and gallic acid (D’Souza et al., 2014). Therefore, we investigated whether treatment withellagic acid and gallic acid would improve pro-inflammatory effect. These results showed that ellagic acid significantly reduced IL-6 protein expression and cytokine release in MG-induced adipocytes, whereas gallic acid did not (Figure 6a, b). Therefore, ellagic acid plays a pivotal role in anti-inflammatory effect belongs to WEPE. Seo et al. (2016) showed that ellagic acid can inhibit the production of PGE2, TNF-α, and IL-6 induced by LPS. Therefore, ellagic acid can effectively reduce IL-6 to alleviate inflammation.
External stimulus causes inflammation through MAPK pathway (Kaminska, 2005). Yamawaki et al. (2008) pointed out that acute induction of MG activates p-JNK to cause inflammation. Barnes and Karin (1997) showed that NF-κB activation may be mediated by phosphorylation of IκBα, which is positively associated with chronic inflammation. As shown in Figure 2a and b, MG induced inflammation through the activation of JNK and p65 pathway. However, WEPE can decrease p-p65 protein (Figure 4d) and p-JNK (Figure 4f) after 48 h of MG induction.
MCP-1 plays an important role in the migration of macrophages, which promotes the infiltration of macrophages in the adipose tissue resulting in chronic inflammation. As shown in Figure 2e, MG increased MCP-1 protein expression in adipocytes, indicating that MG promoted macrophage infiltration to result in inflammation. In addition, WEPE significantly decreased MCP-1 protein expression (Figure 4e). When inflammation is increased, adipocytes can easily lead to insulin resistance (de Luca and Olefsky, 2008). Figure 3a shows that MG significantly reduced glucose uptake to cause insulin resistance. Jia and Wu (2007) also demonstrated that MG may reduce glucose uptake and lead to insulin resistance through reducing the activation of insulin pathway such as IR, IRS-1 and PI3K. However, WEPE and its major compound, ellagic acid, could increase the glucose uptake ability in adipocytes (Figures 4a, 6c). Kalekar et al. (2013) showed that 200 μg/mL of P. emblica could increase the glucose uptake of adipocytes. Akhtar et al. (2011) showed that diabetic patients receiving 1, 2 or 3 g of Emblica officinalis powder per day for 21 days significantly decreased the fasting and 2-h post-prandial blood glucose levels. Therefore, WEPE can improve obesity-induced insulin resistance and the risk of developing type 2 diabetes, in which ellagic acid may play an important role.
PTP1B is one of the targets for the development of new drugs for diabetes (Teng et al., 2011). Cheng et al. (2012) showed that MG results in an increase in PTP1B expression; however, resveratrol may attenuate PTP1B protein expression. The results of this study also showed that the expression of PTP1B was increased after MG-induced adipocytes (Figure 5a); however, WEPE could significantly reduce PTP1B protein. PPARγ and C/EBPα play an important role in the differentiation of adipocytes (Song et al., 2013). In this study, we found that MG could significantly promote the expressions of PPARγ and C/EBPα in adipocytes (Figure 3b, c); however, WEPE significantly inhibited the protein expressions of PPARγ and C/EBPα (Figure 5b). Obesity is caused by imbalances in energy intake and energy expenditure, and excessive energy tends to form triacylglycerols that accumulate in cells to make cell hypertrophy (Fruhbeck et al., 2001). PPARγ and C/EBPα can activate ACC and FAS to increase the efficiency of lipid synthesis (Oh et al., 2016). The results showed that WEPE can reduce the protein expressions of PPARγ and C/EBPα (Figure 5b), and thus inhibit the p-ACC and FAS proteins (Figure 5c) to reduce fat accumulation in adipocytes. Berndt et al. (2007) suggested that FAS is associated with lipid accumulation, which leads to a decrease in insulin sensitivity and an increase in fasting serum insulin, IL-6, leptin and the retinol-retinol- binding protein (RBP4). Therefore, the lipid synthesis pathway plays an important role in the disorders of energy metabolism, obesity and type 2 diabetes. Previous studies have shown that ellagic acid can reduce triacylglycerol and fat accumulation by reducing PPARγ and C/EBPα (Wang et al., 2013; Woo et al., 2015). Akhtar et al. (2011) showed that P. emblica can also reduce triacylglycerols in normal human beings and diabetic patients. Therefore, WEPE may offer a potential opportunity for the development of new dietary supplement for inflammation, obesity, and type 2 diabetes.
| 5. Conclusion | ▴Top |
MG enhances the expression and release of IL-6 by activating JNK and p65 in inflammatory pathways, and increases PPARγ and C/EBPα in MG-induced insulin resistance. In addition, MG also promotes MCP-1 protein and decreases glucose uptake, which leads to the occurrence of insulin resistance and chronic diseases. P. emblica fruit has various bioactivities, such as anti-inflammatory, lipid-lowering, anti-cancer, antioxidant, and treatment of diabetes. In this study, the results showed that WEPE improved inflammation, glucose uptake, and lipid accumulation, thereby it might play an important role in regulating obesity, inflammation, and insulin resistance. In the further, P. emblica fruit has the potential to develop healthy food for regulating blood lipids and blood sugar.
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |