| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 3, September 2018, pages 1-7
Sugarcane rind: applications and health benefits: a review
Yue Luo, Shiming Li, Chi-Tang Ho*
Department of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
*Corresponding author: Chi-Tang Ho, Department of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
DOI: 10.31665/JFB.2018.3148
Received: July 13, 2018; Revised received & accepted: September 3, 2018
| Abstract | ▴Top |
Sugarcane rind is usually treated as an industrial waste. However, it contains valuable phytochemicals that can be extracted and utilized. Herein we provide a comprehensive review about application and health benefits of the phytochemicals in sugarcane rind, including polyphenols, flavonoids, especially anthocyanins, phenolic acids, long chain fatty alcohols particularly 1-octacosanol, phytosterols and fiber. Various bioactivities are associated with these phytochemicals, such as antioxidant, anticancer, antivirus, inhibition of inflammatory, and attenuation of the risk of cardiovascular and coronary disease. However, further studies are warranted to focus on health benefits of sugarcane rind and to elucidate their action mechanisms.
Keywords: Sugarcane; Phytochemicals; Flavonoids; Biological activity; Sugarcane rind
| 1. Introduction | ▴Top |
Sugarcane (Saccharum officinarum), known for its high content of sucrose in its stem, is a crop indigenous to India and common in tropical and subtropical countries (DAFFRSA, 2014). Therefore, sugarcane is largely used in sugar production. Global sugar production for marketing year 2017/18 was estimated to reach a new record of 185 million metric tons, and Brazil, India, European Union, Thailand and China are the top five sugar producers (USDA, 2017). Around 80% of the world sugar production is from sugarcane and the remaining 20% is from sugar beet and palm (Nair, 2009). One hundred tons of sugarcane can roughly produce raw sugar, bagasse, filter cake, molasses and waste water as 14.3, 27.2, 5.2, 2.6 and 50.7 tons, respectively (Allen et al., 1997).
The outer layer of sugarcane stem may be called skin, peel, rind, or epidermis, among others. In sugar industry, sugarcane is usually directly squeezed to extract sucrose juice without peeling (AG, 2011). High yields of sugar would be obtained and shorter process time is needed if sugarcane was peeled first by abrasive tools and then squeezed (Songsermpong & Ijttanit, 2010). The leftover sugarcane rind after sugar extraction is usually regarded as a waste material. However, plenty of phytochemicals exist in sugar rind such as flavonoids, phenolic acids, phytosterols and others that have not been investigated and utilized adequately for their potential values (Li et al., 2010).
Recent studies have shown that phytochemicals contained in sugarcane rind have significant health benefits such as antioxidant, anticancer, anti-tumor (Chu et al., 2016) and anti-fibrotic (Wang et al., 2018) properties. For instance, one study indicated the beneficial effects of sugarcane peel ethanolic extract on hematological and histopathological parameters of Wistar Albino rats. Decreased levels of white blood cells, packed cell volume, platelets and hemoglobin and degeneration of follicles were increased especially at high dose levels of the ethanolic extract of sugarcane peels (Ashade et al., 2014). The anti-fibrotic properties of sugarcane polyphenol extract have been proven by inhibition of p38 and JNK phosphorylation MAPK signaling pathways in CCl4 induced liver-fibrosis rats (Wang et al., 2018).
Thus far, there has been little investigation in reusing sugarcane rind, underlying the actual profound values of sugarcane rind both in nutrition and economy. In this review, we aim to provide a comprehensive summary of composition and health benefits of phytochemicals in sugarcane rind, to draw more attention to sugarcane rind research and usage.
| 2. Phytochemicals and bioactivity | ▴Top |
Sugarcane rind includes surface wax layer, thin colorful layer and inside thin pulp layer. Sugarcane wax can be easily and efficiently extracted from sugarcane rind (Partha & Sivasubramanian, 2006). The yield of crude wax can reach up to 0.95% (w/w) of dried sugarcane peels using supercritical CO2 extraction. Sugarcane wax is the major source of esters, alkanes, fatty acids and alcohols in sugarcane, from which the valuable ones include very long fatty alcohols and acids such as glycolic acid and policosanols, especially 1-octacosanol (1-OC) (Inarkar & Lele, 2012). Besides, polyphenols, including flavonoids (Pallavi et al., 2012), anthocyanins (Zhao et al., 2018) and phenolic acids (Feng et al., 2015b), dietary fibers (Zhuang et al., 2016) and phytosterols such as stigmasterol, campesterol and β-sitosterol (Georges et al., 2006) can be extracted from sugarcane rind.
2.1. Overall polyphenols and associated bioactivity
Polyphenols are natural phytochemicals in whole plant foods. Different categories are classified by the number of phenol rings and structural elements (Pietta et al., 2003). Flavonoids are the most abundant dietary polyphenols consisting of six subclasses, namely anthocyanins, flavonols, flavanols, flavanones, flavones and isoflavones. Phenolic acids in the diet include two main classes of hydroxybenzoic acid derivatives (protocatechuic acid, gallic acid, p-hydroxybenzoic acid) and hydroxycinnamic acid derivatives (caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, sinapic acid) (Bahadoran et al., 2013). Polyphenols have been studied for decades, and enormous evidences showed that polyphenols had nutraceutical properties like antioxidant, anti-radical activity, anticarcinogenic, anti-atherosclerotic, anti-inflammatory, antimicrobial, antihyperglycemic, spasmolytic, antiviral, hepatoprotective, oestrogenic activity and inhibition of histamine release (Hanamura et al., 2006; Iwai, 2008; Pietta et al., 2003).
A recent study in colon cancer cell model showed that whole dried sugarcane (WDS) ethanol extract had large quantity of polyphenols, strong antioxidant and anti-inflammatory activity. WDS altered protein expression in NFκB pathway, suppressing the phosphorylation of NFκB and inhibiting secretion of the pro-inflammatory cytokine IL-8. Kinase enrichment analysis showed that C-Raf played a role in controlling WDS activity in this mechanism (Bucio-Noble et al., 2018).
Sugarcane polyphenol extract (Table 1) showed potential anti-fibrotic activity by inhibiting p38 and JNK phosphorylation in MAPK signaling pathways in CCl4 induced liver-fibrosis rats. Prominent endogenous profibrotic growth factor TGF-β1 contributes to liver fibrosis by activating hepatic stellate cells into myofibroblast like cells via p38, ERK and JNK signaling pathways, and activated myofibroblast like cells will successively secrete excess extracellular matrix proteins like α-SMA which is responsible for developing hepatic fibrosis. The expression of TGF-β1 induced by α-SMA is reduced once the p38 and JNK are inhibited by sugarcane polyphenol extract, therefore, sugarcane polyphenol extract can be considered as being effective in prevention of liver fibrosis (Wang et al., 2018).
 Click to view | Table 1. Major phenolic compounds in the sugarcane polyphenol extracta |
2.2. Flavonoids and associated bioactivity
Studies have shown that sugarcane is a promising source of flavonoids such as quercitrin, epicatechin, quercetin, catechin, isoquercitrin, rutin, naringenin, tricin, apigenin and luteolin derivatives (Figure 1 for structures) (Abbas et al., 2014; Joaquim et al., 2006; Pallavi et al., 2012). The highest flavonoid content was found in rind rather than other parts of sugarcane. Two various kinds of sugarcane with red and green rind were investigated for their flavonoid contents; the values were 408.62 ± 15.92 and 263.76 ± 1.38 mg rutin equivalents/100 g dry weight, respectively (Feng et al., 2014). One study investigated the antioxidant potential and DNA damage protective activity of thirteen sugarcane cultivars, showing low concentration of sugarcane extract can significantly reduce DNA damage induced by hydroxyl radicals since flavonoids act as hydrogen donators and antioxidant agents. A flavonoid, tricin-7-O-β-(6″-methoxycinnamic)-glucoside, was extracted from sugarcane juice and was proved to have a better antioxidant activity than trolox in the DPPH assay. It also had antiproliferative activity against several human cancer cell lines, especially in the multidrug resistant breast (NCI-ADR) cancer cell line (Duarte-Almeida et al., 2007).
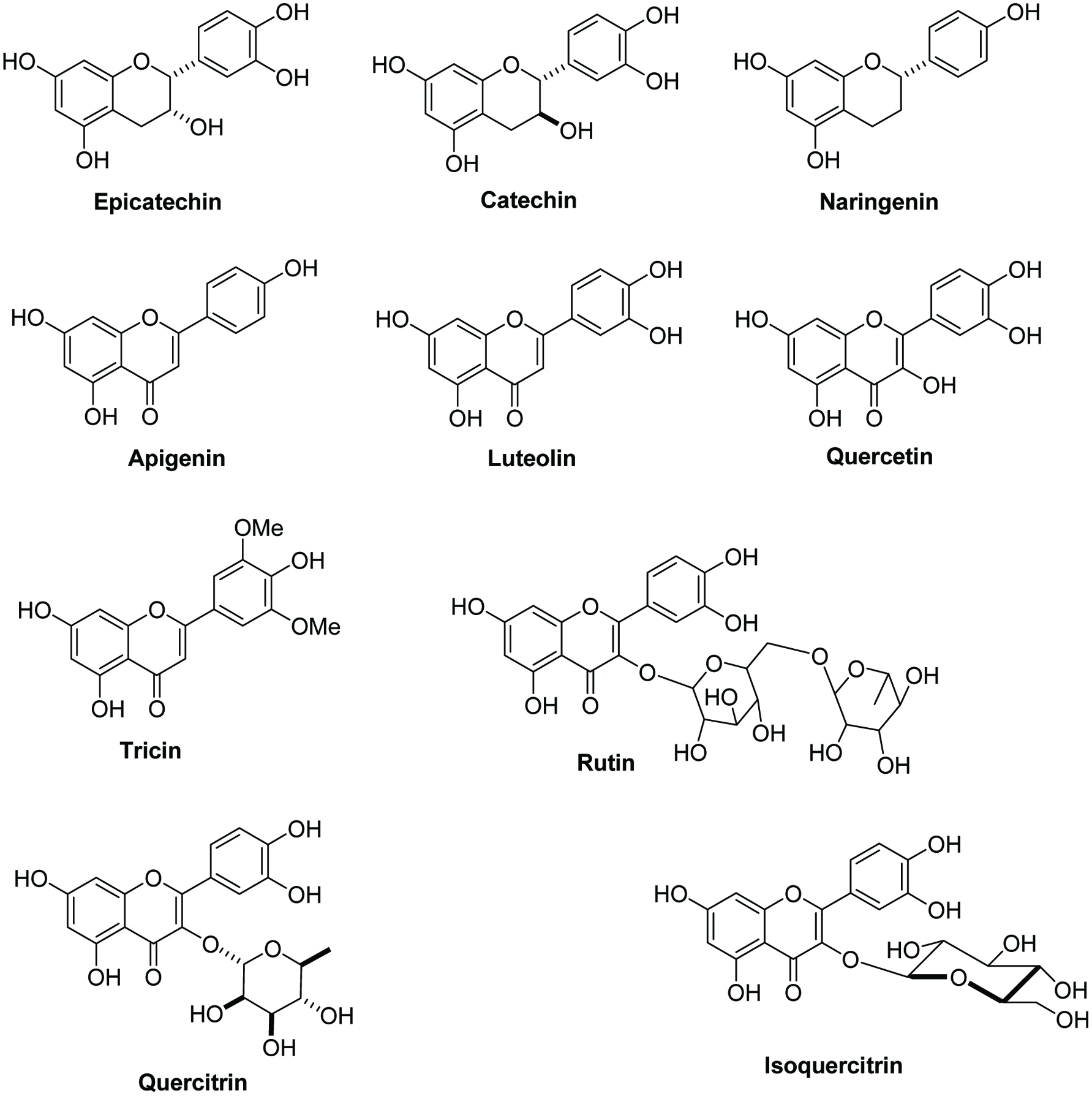 Click for large image | Figure 1. Chemical structures of flavonoids from sugarcane rind. |
Anthocyanins are another subclass of flavonoids that are always associated with colorful appearance and antioxidant activity in flowers, fruits and vegetables (Moyer et al., 2002). Intake of flavonoids/anthocyanins is associated with prevention of cardiovascular diseases, cancers and degenerative disorders (Kozlowska & Szostak-Wegierek, 2014). Anthocyanins possess prominent antioxidant capacities such as superoxide anion scavenging activity and inhibition of lipoperoxidation in human erythrocytes (Ramirez et al., 2015). In addition to their anticancer activities (Bontempo et al., 2015; Zhao et al., 2004), anthocyanins showed anxiolytic and neuroprotective effects in an animal model (Gutierres et al., 2014) and exhibited potential in preventing Parkinson’s and Alzheimer’s diseases in animal and human models (Kim et al., 2010; Krikorian et al., 2010; Shih et al., 2010). In one study, Alzheimer’s disease was induced in rats by scopolamine by lowering nitrite/nitrate (NOx) levels and Na+, K+-ATPase and Ca2+-ATPase and increasing acetylcholinesterase (AChE) activities in the cerebral cortex and hippocampus. Anthocyanins were able to prevent these altered effects significantly, showing cholinergic neurotransmission regulation and restore abnormal effects capabilities and prevention of memory impairment (Gutierres et al., 2014). Anthocyanins also showed stimulating insulin secretion, outstanding antihyperglycemic, HbA1c-decreasing activity and β-cell protective activity in pancreatic β-cell culture and db/db mice (Hong et al., 2013).
While peonidin-3-monogalactoside was found in sugarcane peel of Saccharum Officinarum in 1935, two anthocyanins, namely petunidin-3-O-(6″-succinyl)-rhamnoside and cyanidin-3-O-glucoside were first found in the peels of Chinese sugarcane S. sinensis using HPLC -UV/DAD and HPLC-MS/MS (Li et al., 2010). Researchers have also developed and used methods for extraction and determination of the total anthocyanins content (Li et al., 2011). One study used acidified methanol as a solvent to analyze anthocyanin, total flavonoid and total phenol contents of sugarcane peel as 0.00253, 28.5 and 136.12 mg/g, respectively (Pallavi et al., 2012). Besides, for the first time, researchers concluded that sugarcane peel extracts were able to exert in vitro anti-proliferative activity against HT29 colon cancer cell line. The latest study of anthocyanins of sugarcane rind was done by Zhao et al. (2018). In the study, thirteen kinds of anthocyanins from three sugarcane cultivars were identified and quantified using ultra performance liquid chromatography (UPLC) with electrospray ionization quadrupole-time-of-flight tandem mass spectrometry (ESI-QTOF-MS/MS). Twelve anthocyanins were identified from sugarcane for the first time except cyandin-3-glucoside. The total anthocyanin content varied significantly from 10.8 to 132.0 μg/g dry rind weight. Antioxidant activities were determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC) and rapid peroxyl radical scavenging capacity (PSC) analysis, showing that red sugarcane rind had higher antioxidant activity than green sugarcane rind.
2.3. Phenolic acids and associated bioactivities
So far, there has been limited reports on the phenolic acid profiles of sugarcane rind. One study used a rapid ultrasonic-assisted extraction assay with HPLC to extract and quantify phenolic compounds from sugarcane rind, concluding that gallic acid, chlorogenic acid and ferulic acid were the three main phenolic components with high antioxidant ability (Feng et al., 2015b). The total phenolic content from rind extract was 302.50 ± 19.50 and 670.00 ± 17.00 mg/g using macro-porous resin adsorption and solvent extraction methods, respectively. The highest total phenolic content was found in the rind rather than the other parts.
As important components in human diet, phenolic acids have been researched for decades for their potential health benefits such as antioxidant, anti-inflammatory, antitumor, and antihistamine activities and reduced risk of cardiovascular diseases (Goleniowski et al., 2013). Caffeic acid as one of the most predominant natural cinnamic acids is known to possess antitumor ability against colon cancer, to impede biosynthesis of leukotrienes involved in asthma and immunoregulation diseases, and to act as an antihistamine against allergic reactions (Krupka & Pelster, 1984; Olthof et al., 2001). Ferulic acid has low toxicity and can be absorbed and metabolized easily. Currently, ferulic acid is widely used in food and cosmetic industries for its physiological functions like antioxidant, antimicrobial, anti-inflammatory, anti-thrombosis and anticancer activities (Fujisawa, 2002; Hosoda et al., 2010). In addition, ferulic acid has potential benefits in the prevention of bone degeneration, hot flash symptoms in menopause, protection of skin from UV damage, and reducing serum LDL level (Lee et al., 1998). Gallic acid has impressive anticancer, anti-melanogenic and antivirus (HSV-2, herpes simplex virus) properties (Goleniowski et al., 2013; Kratz et al., 2008). Gallic acid exhibited ROS (reactive oxygen species)-mediated anticancer activity in human prostate cancer cells and induced death of HeLa cervical cancer cells by apoptosis and/or necrosis. Moreover, it had been reported to be involved in the treatment of brain tumor for its suppression of cell viability, proliferation, invasion and angiogenesis in human glioma cells (Luy et al., 2010; Wu et al., 2009).
2.4. Long-chain fatty alcohols and associated bioactivities
Policosanols, including 1-octacosanol (Figure 2), are generally extracted from sugarcane wax and have potential health benefits in lowering cholesterol, antiaggregatory properties, ergogenic properties and cytoprotective use (Taylor et al., 2003). Policosanol has been proven to lower hepatic and serum cholesterol levels by increasing HDL and decreasing LDL levels effectively in different animal studies (rats and monkeys) and clinical studies (Hargrove et al., 2004; Taylor et al., 2003). Policosanol is a more acceptable and effective substitute for statins as a normolipidemic agent without statin’s typical side effects including muscle effects like myositis, headache, gastrointestinal effects, rash, angio-edema, altered liver function, and hepatitis (Castaño et al., 2000; Fernandez et al., 2001). Recent studies have further shown that dietary octacosanol can reduce plasma triacylglycerol levels in apoliproprotwin E-knockout mice (Xu et al., 2007). For anti-tumor activity, 1-octacosanol can control angiogenesis and metastasis via inhibition of matrix metalloproteinases (MMPs) and translocation of transcription factor nuclear factor NF-κB and then restraining of VEGF gene expression (Thippeswamy et al., 2008). Based on previous anti-tumor activity, lately, 1-octacosanol has been successfully incorporated in pharmaceuticals to improve the anti-tumor activity and eliminate toxicity of paclitaxel by acting as micellar carrier (Chu et al., 2016). Besides, studies have demonstrated that 1-octacosanol has anti-inflammatory and cytoprotective effects related to prostaglandin, thus, preventing ulceration and irritation (Carbajal et al., 1995; Carbajal et al., 1996). Since secretion of prostaglandin can protect gastric mucosa and further prevent ulceration and irritation, policosanol, unlike aspirin, may replace aspirin to protect against ischemic and thrombolytic events without interfering with prostaglandin production (Taylor et al., 2003).
 Click for large image | Figure 2. Chemical structures of policosanol and 1-octacosanol. |
Until now, different research efforts have extracted 1-OC from bagasse and press mud (Hou et al., 2007), but it is more convenient to directly extract it from sugarcane rind. Corresponding studies are shown in Table 2.
 Click to view | Table 2. Method of 1-OC extraction from sugarcane rind |
2.5. Phytosterols and associated bioactivities
Phytosterols have been extracted from sugarcane rind. Feng et al. (2014) demonstrated that the highest total sterol content was present in the rind rather than other parts. Red rind and green rind had total sterol content (TSC) of 438.71 ± 28.62 and 457.22 ± 22.20 mg phytosterol/100 g DW, respectively. Another study improved traditional solvent extraction with a combination of direct acid, alkaline saponification and HPLC analysis, successfully quantifying sterol content including steryl glycosides (SGs) and acylated steryl glycosides (ASGs) in sugarcane rind (Feng et al., 2015a). As a reference, traditional solvent extraction method failed to quantify SGs and ASGs easily. In this study, stigmasterol and β-sitosterol contents of green sugarcane rind cultivar were 848.3 ± 29.7 and 487.6 ± 5.30 μg/g dry weight, while that of red sugarcane rind cultivar were 883.3 ± 23.5 and 663.4 ± 32.4 μg/g dry weight, respectively (Feng et al., 2015a).
In general, phytosterols have two basic forms in plant tissues as free sterol and three conjugated sterols including steryl esters, steryl glycosides (SGs) and acylated steryl glycosides (ASGs) (Moreau et al., 2002). Phytosterols have been added in various conventional and functional foods and supplements marketed in many countries safely. No harmful side effects were reported among extensive toxicological studies and side-effects were comparable to placebo in clinical studies (Plat & Mensink, 2001). Phytosterols are known to reduce cholesterol absorption and plasma LDL cholesterol level efficiently, so that phytosterols have potential in reducing the risk of coronary heart disease (Lin et al., 2011; Srigley & Haile, 2015). Daily intake of 2–3 g of phytosterols can reduce LDL-cholesterol level by 10–15% (Plat & Mensink, 2001). ASG has been proven to reduce plasma and liver cholesterol levels in mice by 86% or more four hours after gavage and to eventually reduce cholesterol absorption efficiency by 45 ± 6 % in a three-day fecal recovery study (Lin et al., 2011). One 2-weeks human study selected 11 healthy subjects to receive 300 mg of added phytosterols (phytosterol glycosides, phytosterol esters or placebo) daily, concluding that phytosterol glycosides and phytosterol esters reduced cholesterol absorption by 37.6 ± 4.8 and 30.6 ± 3.9%, respectively, when compared with placebo test (Lin et al., 2009).
Besides, phytosterols are known to display anti-inflammation, anti-cancer and immunomodulatory properties (Awad & Fink, 2000). Various studies have demonstrated that phytosterols were toxic to breast cancer, colon cancer and prostate cells (Feng et al., 2015a). An in vitro study showed that campesterol and β-sitosterol inhibited the growth of human prostate cancer PC-3 cells by 14 and 70%, respectively; they also impeded PC-3 cells from disseminating effectively. In following in vivo study, diet containing 2% of phytosterols mixture resulted in 40–43% smaller tumors than that fed instead with cholesterol diet (Awad et al., 2001). Studies demonstrated that phytosterols had anti colon cancer effect (Awad et al., 1998; Rao & Janezic, 1992), in which β-sitosterol at a dosage as 16 μM for five days inhibited growth of HT-29 human colon cancer cells by activating the sphingomyelin cycle.
2.6. Fibers and associated bioactivities
Dietary fibers consist of two types of soluble and insoluble. Soluble fibers such as β-glucan can attenuate postprandial glycaemia/insulinaemia and lower serum LDL cholesterol level (Jones, 2013; Mudgil & Barak, 2013), reduce body weight gain, suppress excessive accumulation of white adipose tissue (WAT) and increase energy expenditure (Wang et al., 2018), while insoluble fibers can increase fecal bulk/laxation and stimulate colonic fermentation (Fernandez & Borroto, 1996; Fuller et al., 2016). In a 60,000 persons 4-year follow up study, higher long-term intake of dietary fiber (25 g/day) was associated with decreased risk of fecal incontinence in older women with mean age 73 and also liquid stool FI by 18 and 31% than that of lower quantity fiber intake (13.5 g/day) (Staller et al., 2018).
Additionally, dietary fibers have potential in lowering the risk of ovarian cancer (Xu et al., 2018), coronary heart disease and cardiovascular aliment (Mcrae, 2017). Sugarcane bagasse acts as a lignocellulosic material and consists of soluble fiber (20–25% hemicellulose) and insoluble fibers (45–55% cellulose and 18–24% lignin) (Reddy & Yang, 2016).
A multicenter, randomized, double blind and placebo-controlled trial was conducted to investigate sugarcane bagasse dietary fiber (SBDF) as an adjuvant therapy for stable chronic obstructive pulmonary disease. In total, 196 participants were treated with 6 g of SBDF daily for 30 days and a 6-month follow-up. Post-treatment pulmonary clinical symptoms and severity of dyspnea were significantly relieved in trail group, besides, better results showed in St. George’s Respiratory Questionnaire for trial group compared to the control group (Miao et al., 2016).
Sugarcane dietary fiber (SDF) has been researched in various food products for different reasons. SDF was added to replace fat in meat products like frankfurter containing 30% animal fat with high content of cholesterol and saturated fat unfavorable by customers (Zhuang et al., 2016). In this way, actual usage of SDF indirectly lowered the risk of obesity, hypertension, coronary heart and cardiovascular diseases. SDF has been added in baked food contributed to its neutral in taste and odor, in which SDF was treated with alkaline hydrogen peroxide (AHP) to improve its physical and chemical properties. AHP treatment removed 53% of lignin and increased water capacity and oil-binding capacity by 96 and 55%, respectively. As a result, addition of 10% AHP-treated SDF and 1.5% sucrose ester in bread enriched nutraceutical components and maintained good customer acceptance (Sangnark & Noomhorm, 2003).
| 3. Summary | ▴Top |
Sugarcane rind has valuable phytochemicals including various flavonoids, phenolic acids, octacosanol, phytosterols and dietary fiber. These components can provide vital health benefits such as antioxidant, anti-inflammatory, anticancer, antivirus and further attenuation of the risk of cardiovascular and coronary heart disease. As a disposed part in sugarcane industry, effective extraction of these phytochemicals from sugarcane rind is a double-win strategy. However, optimum extraction method of each phytochemical or a thoughtful entire extraction process of several or all phytochemicals still needs to be investigated. Furthermore, relatively few research has focused on health benefits of sugarcane rind and further studies are required to confirm the actual mechanism behind the associated health benefits.
| References | ▴Top |
doi