| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 31, September 2025, pages 63-75
An update on therapeutic potential of earthworm extract
Jie Pan, Hui Zhao*, Xiao-Jing Ding*
Tianjin Key Laboratory of Food and Biotechnology, College of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
*Corresponding author: Hui Zhao and Xiao-Jing Ding, Tianjin Key Laboratory of Food and Biotechnology, College of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China. E-mail: zhaohui@tjcu.edi.cn (HZ), tjmudxj@163.com (XJD)
DOI: 10.26599/JFB.2025.95031421
Received: July 10, 2025
Revised received & accepted: September 26, 2025
| Abstract | ▴Top |
Earthworms have been used in traditional medicine for thousands of years. Current studies have revealed that they may offer therapeutic benefits for various diseases, including hypertension, diabetes, and cancer. Current research primarily focuses on investigating the bioactivity of earthworm extracts and analyzing earthworm proteins, and earthworm peptides. This study summarized the bioactivities and mechanisms of these earthworm-derived substances in diseases treatment over the past decades, aiming to provide directions for further in-depth research and the application of earthworm-derived bioactive substances in the biological and pharmaceutical fields.
Keywords: Earthworm extract; Protein; Peptide; Bioactive substances
| 1. Introduction | ▴Top |
Earthworms, referred to as “Dilong” in Chinese, are invertebrates belonging to the phylum Annelida, class Oligochaeta, and family Lumbricidae. They naturally inhabit moist soil, where they contribute to soil promoting healthy and promote plant growth. Earthworms are rich in nutrients including proteins and and various amino acids. In recent years, they have been taken into account as emerging alternatives in the traditional poultry and fishing industry.
Earthworms have been broadly used in Traditional Chinese Medicine, Ayurvedic Medicine, and Islamic Medicine for thousands of years as an important medicine. The 2015 edition of the Chinese Pharmacopoeia classifies earthworms into four species: Pheretima aspergillum, P. vulgaris, P. guillelmi, and P. pectinifera (Gu et al., 2021). The earliest records of earthworms being used for medicinal purposes can be date back to the ancient Chinese medical classic Shennong Bencao Jing, where they were noted for their blood circulation, heat-clearing, and cough-relieving effects ( Ma et al., 2022; Shen et al., 2024).
With advancements in modern biological and pharmaceutical techniques, studies on the bioactivity of earthworms have increased. For instance, compounds from earthworm such as lumbrokinase have been shown to possess thrombolytic effects and have long been used for a long time as anti-thrombotic drugs (Wang et al., 2013). An increasing number of active ingredients in earthworms have been found to exert protective effects against neurodegenerative diseases and ischemia-reperfusion injury, as well as to display the anti-inflammatory, antioxidant, and antimicrobial activities. Consequently, the molecular mechanisms underlying these health-protective effects have been progressively elucidated through experimental and clinical investigations (Mustafa et al., 2022).
However, in-depth reviews on extraction methods, bioactive compounds and bioactivities of earthworms remain insufficient. Herein we collected reports on extracts, bioactive compounds, and bioactivities of earthworms on the basis of the PubMed, Web of Science, and China National Knowledge Infrastructure to provide a comprehensive review for researchers interested in the field.
| 2. The composition of earthworm extracts | ▴Top |
Earthworm extract (EE), widely studied for their diverse biological activities, contain a complex mixture of bioactive components. These components can be classified into several major categories including enzymes, proteins, peptides, organic compounds, and antimicrobial peptides (Zhu et al., 2024).
Enzymes comprise a range of substances that hydrolyze proteins and modify the functions of the affected proteins. For instance, earthworm fibrinolytic enzyme (EFE) and lumbrokinase are capable of dissolving fibrin, thereby demonstrating thrombolytic properties (Wang et al., 2019). Moreover, Earthworm fibronectinase (EFNase) is able to hydrolyze fibronectin, hydrolyze the extracellular matrix, and exert antifibrotic effects (Wang et al., 2008).
Proteins and peptides encompass a range of biologically active components. For example, hemolysin binds to sphingolipids and alters cell membrane permeability (Zhu et al., 2024). G-90 exhibits anti-tumor, anti-thrombotic, and anti-oxidant effects, promotes cell proliferation, and accelerates wound healing (Zhu et al., 2024).
Antimicrobial peptides include a series of peptides with antimicrobial activity and their derivatives, such as EWAMP-R, lumbritoxin-1, lumbritoxin PG, OEP3121, PP-1, Perinerin and Hedistin (Venkatachalam et al., 2025).
2.1. Extraction methods of earthworm
The primary active components and functions of EE vary depending on the extraction methods employed. The main approaches for EE include aqueous extraction (Yoshii et al., 2018) and ethanol extraction, which involve immersing cleaned earthworms in water or ethanol, followed by centrifugation, freeze-drying, and collection of the precipitate (Chang et al., 2011a). In some studies, researchers directly homogenize cleaned earthworms in phosphate-buffered saline (PBS) or Tris-HCl buffer, followed by centrifugation to collect the supernatant as the extract (Deng et al., 2018; Duan et al., 2018). Other methods including chloroform-methanol extraction and ammonium sulfate precipitation are also used for extracting earthworm proteins (Balamurugan et al., 2008 and 2009; Liu et al., 2012). Apart from isolating active components from earthworm bodies, some researchers collect coelomic fluid by placing earthworms in a warm water bag, a protocol known as the heat-shock method (Hussain et al., 2023). Furthermore, Liu et al. successfully isolated and purified an antimicrobial peptide (amino acid sequence ACSAG) through a combination of ammonium sulfate precipitation, ion-exchange chromatography, Sephadex G-10 column chromatography, and C-18 reverse-phase high-performance liquid chromatography (RP-HPLC) (Liu et al., 2004).
Notably, hydrolyzing earthworm proteins with proteases enables the production of protein hydrolysate with enhanced activity and small-molecule peptides. For example, Liu et al. (2004) used earthworms’ endogenous proteases to hydrolyze their organs, obtaining hydrolysates capable of regulating antioxidant balance and gut microbial composition. Similarly, Gaviria et al. hydrolyzed earthworm proteins with alkaline protease, resulting in protein hydrolysates with antioxidant activity (Gaviria and Zapata, 2023). In a related study, Luo et al. employed proteomics and peptidomics techniques to identify naturally active peptides (Luo et al., 2023).
The aforementioned content outlines the primary methods for EE extraction. This review is combined with a previous review to summarize the varieties, bioactivities, experimental models, molecular mechanisms, as well as related targets and signaling pathways of EE (Table 1), and also while lists the sequences, sources, bioactivities, molecular mechanisms, as well as related targets and signaling pathways of earthworm-derived peptides (Table 2) (Zhu et al., 2024).
 Click to view | Table 1. Bioactivity of EE and mechanism |
 Click to view | Table 2. Earthworm peptides, their sequence, bioactivity and molecular mechanism |
| 3. Bioactivities of EE | ▴Top |
3.1. Protective effects of EE on drug-induced liver and kidney damage
Recent studies have demonstrated that EE confers hepatoprotective and renoprotective effects, with antioxidant and anti-inflammatory mechanisms constituting the principal basis of its activity. Sadek et al. (2016) demonstrated that EE exhibits significant DPPH radical scavenging activity in vitro. In an in vivo study using male albino rats, the researchers showed that EE mitigates significant biochemical abnormalities induced by silica nanoparticles (SiNPs), such as serum transaminase, malondialdehyde (MDA), and catalase. Specifically, after oral administration of EE at doses of 500 mg/kg and 1,000 mg/kg, all aforementioned biochemical markers were significantly improved.
Histopathological analysis further revealed that EE alleviates structural liver abnormalities induced by SiNPs, which is likely mediated by its antioxidant properties, thereby improving SiNP-induced liver toxicity (Sadek et al., 2016). Another study found that a 45 mg/kg injection of earthworm coelomic fluid significantly ameliorated histopathological abnormalities in liver and kidney tissues caused by gentamicin, suggesting that earthworm coelomic fluid might regulate oxidative system balance and alleviate gentamicin-induced toxicity (Mohamed et al., 2020). In addition, biogenic silver nanoparticles (Ag NPs) synthesized from earthworm extracts could improve liver and kidney lesions triggered by sepsis through its antioxidant activity (Ali et al., 2024).
Taken together, these studies indicated that the inherent antioxidant activity contribute to EE in alleviation of drug-induced liver and kidney toxicity.
3.2. Inhibition of fibrosis by EE
Fibrosis represents a pathological feature of a wide range of chronic inflammatory diseases, which often leads to organ dysfunction or even failure and thereby affecting nearly all bodily systems (Wynn and Ramalingam, 2012). Fibrosis development is a dynamic process associated with chronic inflammation, metabolic homeostasis, and transforming growth factor-beta 1 (TGF-β1) signaling, with antioxidant system balance playing a crucial role (Antar et al., 2023). Recent studies have suggested that EE can mitigate both pulmonary and hepatic fibrosis (Figure 1). Yang et al. (2016) investigated the protective effects and underlying mechanisms of EE against silica-induced fibrosis at both the animal and cellular levels (HBE and A549 cell lines). In an in vivo, oral administration of EE reduced inflammatory cell infiltration, alleviated silica-induced pulmonary lesions, and decreased fibrosis scores in silicosis mice. Additionally, EE inhibited the release of cytochrome c, increased caspase 9 and 3 levels, downregulated Bax expression, and upregulated Bcl2 expression. These findings indicate that the antifibrotic effect of EE may be due to the suppression of mitochondrial-dependent apoptosis and inflammation. They also found that EE significantly inhibited silica-induced increases in TGF-β1, PAI-1, and IL-1β in HBE and A549 cells, with this suppression linked to the activation of the Nrf2 pathway. These results indicate that EE may alleviate silica-induced lung fibrosis by inhibiting oxidative stress through activating Nrf2 pathway and alleviating lung epithelial cell apoptosis and epithelial-mesenchymal transition (EMT) (Yang et al., 2016). In a comparable study, EE reduced bleomycin-induced lung fibrosis in mice by inhibiting fibrosis factors TGF-β1 and α-SMA expression (Wang et al., 2019). Separately, Li et al. isolated and purified earthworm protein P2, and both in vivo and in vitro studies have demonstrated that protein P2 regulates the TGF-β/Smad signaling pathway, thereby providing therapeutic benefits for lung fibrosis (Li et al., 2022).
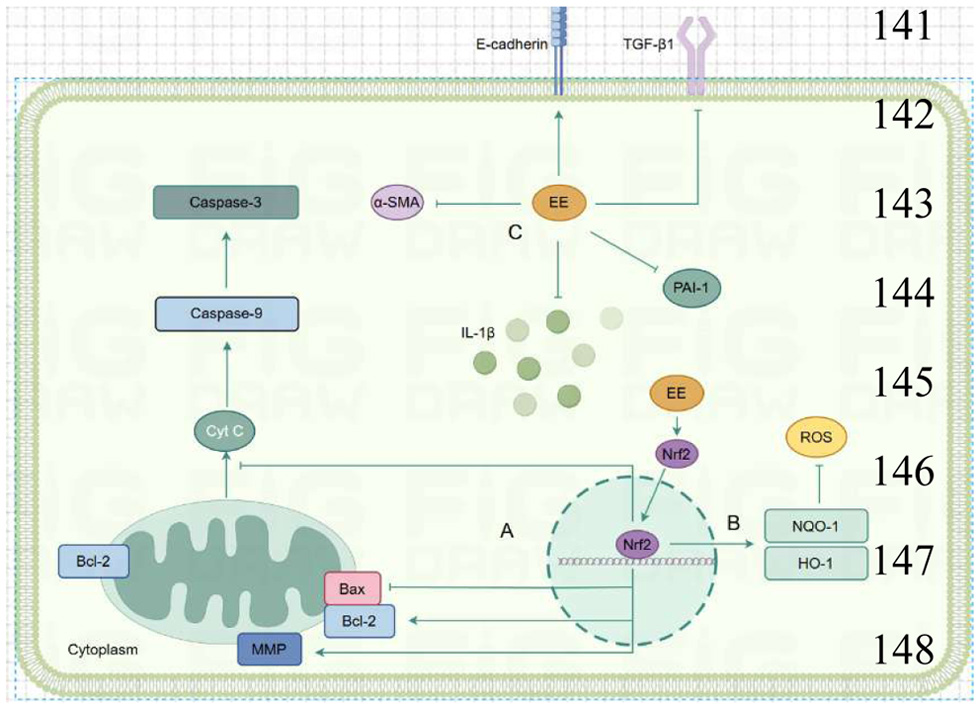 Click for large image | Figure 1. Mechanisms by which EE alleviates pulmonary fibrosis and liver fibrosis. (a) EE inhibits Bax expression, promoted Bcl2 expression, alters mitochondrial membrane potential, and inhibits cytochrome c release and Caspase-3/9 expression. (b) EE promotes Nrf2 nuclear translocation, promoted downstream NQO-1/HO-1 expression, and inhibits ROS levels. (c) EE inhibits the expression of TGF-β1, α-SMA, PAI-1, IL-1β and promoted the expression of E-cadherin. |
Additionally, water-extracted EE was found to significantly improve CCl4-induced hepatic fibrosis in mice, presumably by activating LKB1, enhancing the hepatic AMPK/GSK3β/Nrf2 antioxidant cascade, and inhibiting both hepatic stellate cell activation and hepatocyte apoptosis (Li et al., 2022). Another study found that active components derived from Perionyx excavatus reduced tunicamycin-induced apoptosis in L-02 hepatocytes, primarily by downregulating the expression of endoplasmic reticulum stress (ERS)-related markers (GRP78, PERK, ATF4, eIF2α, CHOP, Bax), and upregulating the expression of anti-apoptotic protein Bcl-2 (Duan et al., 2018). These findings indicate that EE mainly inhibits fibrosis progression by suppressing apoptosis and inflammation.
Collectively, strong evidence suggests that EE may serve as a safe and effective antifibrotic agent by targeting oxidative stress, inflammation, and apoptosis-related pathways, alleviating lung and liver fibrosis. However, further exploration of its specific active components is warranted.
3.3. EE in promoting wound healing and tissue regeneration
Inflammation and oxidative stress exert a significant impact on wound healing and tissue regeneration. Studies have shown that earthworm granule extracts can promote wound healing in diabetic rabbits by enhancing the synthesis of connective tissue protein and the expression of growth factor (Elkhalifa et al., 2024). EE quickly responds to inflammation, accelerates coagulation, clears necrotic tissue, promotes granulation, and enhances epithelial coverage and angiogenesis in mouse wound models. It normalizes abnormal serum IL-6 and TGF-β levels, which together underscores its ability to promote wound healing (Deng et al., 2018). Additionally, EE inhibits fibrosis, scar formation, and infection risk, further supporting skin wound healing (He et al., 2021). These findings indicate that earthworms have potential as wound healing agents.
Beyond skin wound repair, research has also explored earthworms’ roles in other tissue regeneration processes. For instance, Du et al. identified a collagen-like peptide, col4a1, from amputated earthworms, which enhances cell migration in a scratch model and accelerates skin wound healing in mice in a dose-dependent manner (Du et al., 2021). In the context of bone tissue regeneration, Fu et al. reported that 0.5–6 mg/mL EE significantly promotes osteoblast proliferation, with the optimal effect at 3 mg/mL, while simultaneously inhibiting osteoclast proliferation. This balanced modulation suggests EE holds potential for supporting bone tissue regeneration (Fu et al., 2014).
Fibroblasts play a critical role in wound healing and tissue repair (Darby et al., 2014). Song et al. investigated the regulatory effect of earthworm active protein (EAP) on the proliferation of fibroblast cell line NIH3T3 and demonstrated that EAP upregulates the level of phosphorylated ERK and AKT, and increases the expression of cyclin D1, suggesting that EAP may promote cell cycle progression through the activation of PI3K-Rac-PAK-MEK pathway, thereby promoting NIH3T3 cell proliferation (Song et al., 2016). Recently, Wang et al. integrated network pharmacology with metabolomics to reveal that earthworm extracts promote wound healing mainly by targeting key pathways and molecules such as the AGE-RAGE axis and MAPK signaling pathway (Wang et al., 2024).
In summary, EE promotes wound healing and tissue regeneration, likely mediated through its anti-inflammatory, antioxidant, and antifibrotic properties. Further studies are warranted to elucidate its roles in regulating cellular proliferation and inflammatory responses.
3.4. EE in inhibiting tumor development
Cancer is a highly complex disease with an immense global impact, leading to millions of deaths annually (de Visser and Joyce, 2023; Gilbertson, 2011). Studies have indicated that EE may promote apoptosis in cancer cells (Figure 2). For example, EE at concentrations of 400–800 mg/L can induce apoptosis in human ovarian cancer cell line A2780 by inhibiting the Wnt/β-catenin pathway, with the most notable effect observed at 800 mg/L (Chen et al., 2023). Shafi and Faleh (2019) proved that EE, at concentrations of 12.5–400 μg/mL, inhibits cell viability in a dose-dependent manner in breast cancer (MCF) and prostate cancer (PC-3) cells, with respective IC50 values of 265.5 μg/mL and 965.9 μg/mL. Notably, EE exhibited no cytotoxicity toward normal cells (WRL-68). Moreover, treating PC-3 cells with 100–400 μg/mL EE at 37°C for 24 h increased mitochondrial membrane permeability, causing cytochrome c release and apoptosis (Shafi and Faleh, 2019). Furthermore, Deng et al. (2019) treated mice implanted with S180 tumor cells with different doses of EE via gastric perfusion. EE inhibited tumor growth (in size and weight) in a dose-dependent manner and reduced lactate dehydrogenase (LDH) levels, a biomarker of tumor aggressiveness, proving that EE can promote tumor cell apoptosis and reduce tumor size in vivo (Deng et al., 2019). Fiolka et al. showed that earthworm coelomic fluid significantly reduced A549 lung cancer cell viability and increased caspase 3/4/5/10 expression (Fiolka et al., 2019). Hua et al. demonstrated that lumbrokinase has the potential to inhibit non-small cell lung cancer (Hua et al., 2024). Additionally, Czerwonka et al. (2020) extracted a protein-carbohydrate complex (PE) from earthworms, found that PE at concentrations of 10–200 μg/mL significantly inhibited mitochondrial metabolism in HT-29 cancer cells, increased caspase-3 expression, blocked the cell cycle, inhibited the human 20S proteasome, and promoted apoptosis (Czerwonka et al., 2020).
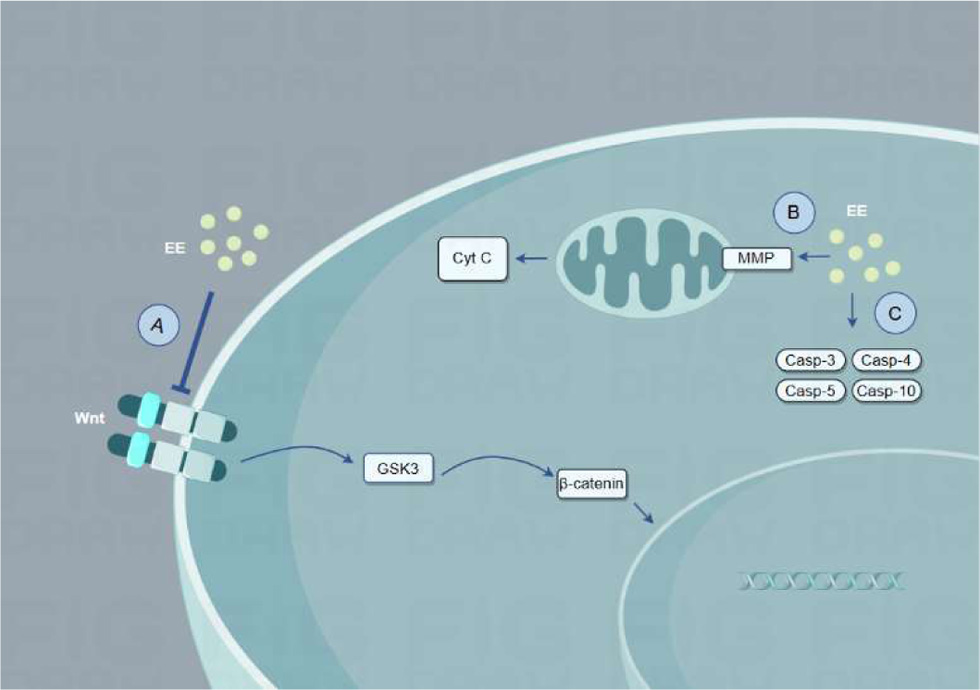 Click for large image | Figure 2. The mechanisms by which EE promotes apoptosis of cancer cells. (a) EE inhibits wnt/β-catenin pathway. (b) EE alters mitochondrial membrane permeability and releases cytochrome c. (c) EE promotes the expression of caspase3/4/5/10 in the cells. |
EE shows promise for cancer prevention and treatment by inducing cancer cell apoptosis through caspase upregulation. Its antitumor effects may stem from its protein components, though further research is required to identify the specific active components.
3.5. Cardioprotective effects of EE
Cardiovascular diseases are a major cause of morbidity and mortality worldwide. Heart failure, which frequently arises from improper repair following myocardial infarction (MI), is a significant outcome of many cardiovascular conditions (Bacmeister et al., 2019; Lien et al., 2012; Soppert et al., 2020). Omar et al. (2022) found that high-dose epinephrine injection caused significant abnormalities in biochemical markers, including troponin T, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alkaline phosphatase (ALP) total protein, creatinine, and urea, and oxidative markers like malondialdehyde (MDA) as well as superoxide dismutase (SOD) levels. A 7-day oral treatment with EE (60 mg/kg) significantly reversed these abnormalities, reducing inflammation in cardiac, hepatic, and renal tissues, indicating that EE can exert cardioprotective effects through its antioxidant and anti-inflammatory properties (Omar et al., 2022). Excessive cardiomyocyte apoptosis impairs cardiac pumping function, potentially leading to heart failure, highlighting the importance of ihibiting apoptosis in these cells (Steinhauser and Lee, 2011; Laflamme and Murry, 2011). Li et al. demonstrated that EE at 60–250 μg/mL inhibited lipopolysaccharide (LPS)-induced apoptosis in H9c2 cardiomyocytes by upregulating Bcl2 and Bcl-x, and downregulating TNF-α, caspase 3/8/9, t-Bid, and Bax expression (Li et al., 2015).
High-KCl cardioplegic solution (High-KCS) is commonly used in cardiac surgery, but preoperative injection often induces cardiomyocyte apoptosis. A study found that EE at 31.25–250 μg/mL reduced apoptosis in High-KCS-treated cardiomyocytes by inhibiting MEK-mediated caspase-3 expression, suggesting that EE may counteract the side effects of High-KCS (Han et al., 2014). Another study showed that EE preonditioning mitigated apoptotic protein t-Bid expression while enhancing anti-apoptotic proteins Bcl2 and Bcl-x in potassium chloride-stimulated H9c2 cells. Additionally, EE activated the IGF1R/PI3K/Akt pathway, thereby inhibiting ERK activation-induced fibrosis (Huang et al., 2019).
I-R injury frequently precipitates several diseases. Studies demonstrated that lumbrokinase reduces I-R-induced myocardial damage by inhibiting TLR4-mediated MAPK-JNK and NF-κB pathways, decreasing COX-2, iNOS, and MMP9 expression, and ameliorating associated cardiac damage (Wang et al., 2016). Lumbrokinase further alleviates I-R-induced arrhythmias and cardiac injury by upregulating silent information regulator 1 (SIRT1) expression, enhancing autophagy, and reducing oxidative stress, inflammation, and apoptosis (Wang et al., 2018). Another study indicated that lumbrokinase protects against secondhand smoke-induced cardiac fibrosis by inhibiting ERK1/2 phosphorylation and blocking uPA/MMP and SP/CTGF-mediated fibrotic pathways (Lai et al., 2015).
Overall, EE can mitigate cardiomyocyte apoptosis, prevent heart failure, and reduce I-R-induced diseases, positioning EE as a promising natural cardioprotective agent.
3.6. Neuroprotective effects of EE
Schwann cell proliferation is essential for nerve regeneration and functional recovery. Study results have demonstrated that EE promotes Schwann cells (RSC96) proliferation by increasing insulin-like growth factor-I (IGF-I)-mediated PI3K/Akt pathway phosphorylation, which in turn upregulates proliferating cell nuclear antigen (PCNA) expression and ultimately supports neuronal regeneration (Chang et al., 2011). Consistently, another study reported that water-extracted EE significantly elevated the expression of growth-associated protein 43 (GAP-43) and synapsin I in PC12 cells, two key molecular markers of neuronal growth and synaptic plasticity. This upregulation was associated with enhanced neurite outgrowth, providing further evidence that EE contributes to nerve repair and regeneration through the modulation of neurotrophic signaling pathways (Chen et al., 2010).
Kim et al. isolated an antimicrobial peptide, Lumbricusin, from earthworms. Lumbricusin specifically promotes neuron proliferation in a dose-dependent manner (Kim et al., 2014). In subsequent research, Kim’s team found that Lumbricusin reduces 6-OHDA-induced apoptosis in MNSCs cells, alleviating motor dysfunction in Parkinson’s disease (PD) model mice (Kim et al., 2015). Seo et al. (2017) further explored the neuroprotective effects of Lumbricusin Analogue 5 (LumA5) on neuroinflammation. They found that LumA5 suppressed the production of nitric oxide (NO) and the expression of iNOS, COX-2, and proinflammatory cytokines in LPS-stimulated BV-2 cells, while inhibiting the activation of the MAPK and AKT/NF-κB signaling pathways. Animal studies corroborated these cellular findings, showing that LumA5 reduces neuroinflammation by suppressing inflammatory pathways and cytokine expression (Seo et al., 2017). Additionally, Li et al. extracted two peptides, VQ-5 and AQ-5, from earthworm coelomic fluid, both of which alleviate persistent nerve pain in mice. AQ-5 was shown to inhibit NF-κB IKKα/β, JNK, and Erk phosphorylation levels, thus reducing inflammation (Li et al., 2017). EE decreases levels of nitric oxide synthase (NOS), a neurotransmitter associated with pain, in the serum and brain of mice, raising pain thresholds and indicating peripheral analgesic effects (Luo et al., 2018). Lumbrokinase also stimulates diabetic nerve segments to release IL-1, NGF, and other neurotrophic factors, promotes nerve fiber regeneration, and enhances the expression of calcitonin gene-related peptide (CGRP), supporting nerve regeneration in diabetic rats (Lee et al., 2015).
In summary, EE promotes nerve repair, reduces neuroinflammation, and relieves chronic neuropathic pain. This activity appears to stem from its bioactive peptides and lumbrokinase, making earthworm-derived neuroprotective peptides a valuable area for future research.
3.7. Protective effects of EE on ischemic stroke
Ischemic stroke frequently leads to nervous system damage. Wang et al. (2022) demonstrated that post-stroke EE administration reduces inositol-requiring enzyme-1 (IRE1) expression, downregulates transcription factor NF-κB, and suppresses downstream targets. This mechanism ultimately inhibits apoptosis and autophagy, thereby highlighting EE as a promising therapeutic strategy for ischemic stroke. A randomized controlled trial revealed that combining earthworm-derived protein DLBS1033 with standard drugs such as aspirin, atorvastatin, and vitamin B12 yielded greater efficacy in treating acute ischemic stroke compared to conventional drug therapy alone. This finding indicated the potential of DLBS1033 to serve as an adjunct therapy (Pinzon et al., 2021).
These studies suggested that EE is an effective therapeutic strategy for ischemic stroke, though further research is needed to identify specific bioactive components responsible for these effects.
3.8. Therapeutic effects of EE on asthma
In an ovalbumin (OVA)-induced asthma mouse model, EE significantly inhibited the total cell count in bronchoalveolar lavage fluid (BALF) and reduced inflammatory cell infiltration in the lungs (Zhang et al., 2021). Additionally, EE notably decreased levels of eosinophil chemotactic factor (Eot), cytokines such as IL-4 and IL-5, and OVA-specific IgE in plasma. EE at a dose of 200 mg/kg also decreased the ratio of matrix metalloproteinase (MMP)-2 to tissue inhibitor of metalloproteinases (TIMP)-1, with this effect being significantly stronger than that of dexamethasone. This observation demonstrates that EE may mitigate airway remodeling in asthmatic mice through modulation of the MMP-2/TIMP-1 balance (Zhang et al., 2021).
3.9. EE alleviates diabetes-induced erectile dysfunction and promotes wound healing in diabetic animals
The prevalence of diabetes continues to escalate globally, and its associated complications impose a substantial health and economic burden on affected patients (Jin and Ma, 2021). Recently, studies on the protective effects of earthworm proteins and protein complexes on diabetes-induced diseases have gained widespread attention. One study demonstrated that earthworm proteins could alleviate oxidative damage and activate the NO-cGMP signaling pathway, thus relieving streptozotocin (STZ)-induced erectile dysfunction in diabetic rats (Zhang et al., 2023). Diabetic erectile dysfunction primarily results from high-glucose-induced arterial and endothelial dysfunction (Castela and Costa, 2016). Another study showed that α-SMA, SMMHC, and Desmin expression was downregulated, while OPN and Collagen I expression was upregulated in corporal cavernosum smooth muscle cells (CCSMCs) in diabetic rats, indicating a shift from a “contractile” to “synthetic” phenotype. Earthworm proteins could prevent this shift, protecting against erectile dysfunction induced by STZ and a high-fat diet (Ji et al., 2024). Zhang et al. (2023) showed that in a diabetic erectile dysfunction model in rats, earthworm proteins significantly inhibited the expression of NF-κB, IL-1β, and TNF-α in penile tissues, while increasing the expression of SOD and Nrf2, suggesting that earthworm proteins may improve erectile function by modulating oxidative stress and inflammation-related pathways (Zhang et al., 2023a). Additionally, protein complexes derived from earthworms have attracted scientific attention. Specifically, the glycoprotein extract G-90 from earthworms was shown to promote wound healing in alloxan-induced diabetic rats (Goodarzi et al., 2016). Wang et al. (2023) isolated a glycoprotein, designated as PvE-3, from earthworms. This glycoprotein not only protected NIH3T3 cells from methylglyoxal (MGO)-induced damage but also promoted wound healing in diabetic mice (Wang et al., 2023).
These findings indicate that earthworm-derived protein components exhibit therapeutic efficacy for diabetes-induced complications, providing valuable insights into potential treatments for diabetes-related conditions.
3.10. Antibacterial effects of EE
In recent years, research into the antibacterial activity of earthworm-derived peptides has experienced a significant surge. Li et al. isolated an antimicrobial peptide, named lumbricin-PG, from the skin secretions of earthworm, demonstrating its broad-spectrum antibacterial activity against Escherichia coli, Staphylococcus aureus and other bacteria, as well as hemolytic activity (Li et al., 2011). Wu et al. (2023) optimized the sequences of potential antimicrobial peptides in the earthworm genome and discovered a new peptide, EWAMP-R, which demonstrated good antibacterial activity against E. coli and Staphylococcus aureus, including antibiotic-resistant strains. However, the molecular mechanisms underlying its antibacterial activity vary by dose and bacterial strain, requiring further investigation (Wu et al., 2023). In addition to the antimicrobial peptides, Venetin-1, a proteoglycan complex from earthworm coelomic fluid, also exhibited promising antifungal activity (Wojcik-Mieszawska et al., 2023).
In conclusion, earthworm-derived peptides exhibit significant antibacterial activity, although further research is needed to elucidate the molecular mechanisms responsible for this effect.
| 4. Conclusion and prospects | ▴Top |
In summary, earthworm exacts exhibit the therapeutic potentials a wide range of conditions, including various inflammatory diseases, redox balance regulation, and organ protection; However, the underlying molecular mechanisms remain to be fully elucidated. In particular, extract methods have a profound impact on the compounds and their bioactivities of earthworm extracts. Future research should focus on developing efficient and environmentally sustainable extraction techniques aimed at obtaining targeted active ingredients, particularly proteins and peptides, to better support their pharmacological applications.
Acknowledgments
The graphical abstract was created using the Figuredraw platform. We thank Figuredraw for providing a convenient platform for scientific research illustration.
Conflict of interest
All authors declare that there is no conflict of interest.
| References | ▴Top |