| Lumbrokinase | Canada RNA biochemical Inc., Richmond, BC, Canada | In vivo | I-R injury in male Sprague-Dawley rats | 1,10 μg/kg | Inhibited TLR4 expression, the phosphorylation of JNK, NF-κB, IκBα and the protein expression of COX-2, iNOS and MMP-9 | TLR4, JNK, NF-Κb, IκBα, COX-2, iNOS, MMP9 | (Wang et al., 2016) |
| Lumbrokinase | Canada RNA biochemical Inc., Richmond, BC, Canada | In vivo | mice | 0.1, 1 mg/kg/7 days | Decreased the expression of IRE1 and inhibited the expression of transcription factors, XBP-1, caspase-12 and NF-κB, reduced NLRP3 inflammasome | IRE1, XBP-1, NF-κB, NLRP3, caspase-12 | (Wang et al., 2022) |
| Earthworm active protein | Xian Guanmao Biotechnology Co., Ltd. | In vivo | Hyperlipidemic SD rats | 50, 100, 200 mg/kg/28 days | Increased lipase activity and enhanced intestinal cholesterol metabolism transformation | – | (Yuan et al., 2018) |
| Polysaccharide-protein complex (PPC-DV) | Dendrobaena veneta | In vitro | Blood from peripheral vein | 25, 50, 100 μg/ml | Targeted the P2Y12 receptor, COX-1 and PAR-1 pathways | P2Y12R, COX-1, PAR-1 | (Poniedzialek et al., 2022) |
| Dilong extract | Pheretima vulgaris | In vivo and vitro | Collagen-induced arthritis in mice and murine RAW 264.7 macrophages | 5, 10 g/kg/28 days and 75, 150, 300 μg/kg/1 days | Inhibited the NF-κB p65 activation, regulated the ratio of Th1/Th2 cells and improved the abnormal levels of inflammatory cytokines | NF-κB p65 | (Bao et al., 2022) |
| Earthworm extract | El-Bagour, Menoufia, Egypt | In vivo | Acrylamide induced reproductive Injury in male rats | 300 mg/ml/15 days | Inhibited the expression of P53 and Ki67, restored testicular antioxidant balance | P53, Ki67 | (Ahmed et al., 2022) |
| Lumbrokinase | – | in vivo | I-R induced testicular injury in rats | 80mg/kg | Inhibited the expression of Bax | Bax | (Danarto et al., 2019) |
| Earthworm extract | – | In vivo | Mouse wound model | 0.1 ml | Reduced the ill-effects of inflammation, accelerated the secretion of hydroxyproline and TGF-β, increased the synthesis of collagen, and promoted the proliferation of blood capillary, and fibroblast. Accelerated the removal of dead tissue and foreign bodies by accelerating the production of IL- 6, white blood cells and platelets | TGF-β, IL- 6 | (Deng et al., 2018) |
| Earthworm active ingredients | Eisenia foetida | In vitro | Tunicamycin induced L-02 cells apoptosis | 0.1, 0.2, 0.4 mg/L/24 hours | Inhibited mRNA and protein expressions of GRP78, PERK, ATF4, Elf2α, CHOP, Bax, relieved ERS, he protein and mRNA expressions of Bcl-2, promoted the proliferation of L-02 cells. | GRP78, PERK, ATF4, Elf2α, CHOP, Bax, ERS, Bcl-2 | (Duan et al., 2018) |
| Earthworm extract | Allolobophora caliginosa | In vivo | SiNPs induced liver injury in male albino rats | 500, 1,000 mg/kg/30 days | Regulated the antioxidant balance of the liver | – | (Sadek et al., 2016) |
| Earthworm coelomic fluid | Allolobophora caliginosa | In vivo | Gentamicin induced hepatorenal toxicity in rats | 45 mg/kg/7 days | Regulated the balance of oxidative system in the body | – | (Mohamed et al., 2020) |
| Earthworm extract | Perionyx excavates | In vivo | HFD-induced non-alcoholic fatty liver in guinea pigs | 0.3, 1.4, 6.8 μg/kg | Inhibited the expression of TC, TG and LDL-C, alleviated liver injury in guinea pigs | TC, TG, LDL-C | (Deng et al., 2021) |
| Earthworm extract | – | In vivo | Silica-induced pulmonary fibrosis in mice | 600 U/7, 14, 28 days | Activated Nrf2 pathway, inhibited oxidative stress, alleviated silica-induced apoptosis of lung epithelial cells and EMT | Nrf2 pathway, EMT pathway | (Yang et al., 2016) |
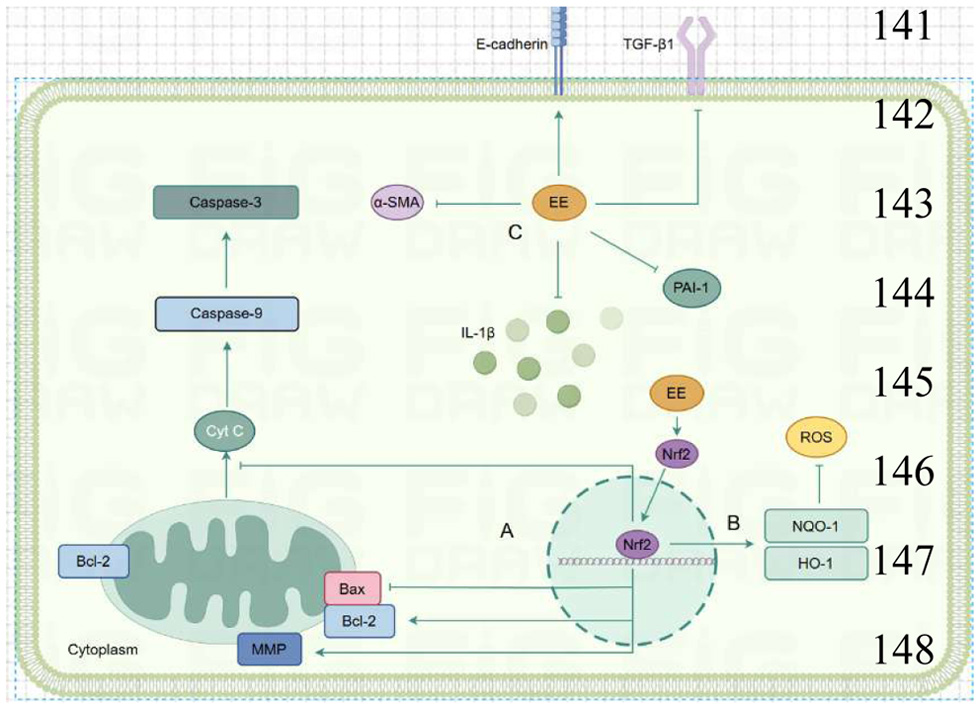
| Dilong extract | Guang dilong | In vivo | Bleomycin-induced pulmonary fibrosis mice | 1.175, 2.35, 4.7g/kg/14 days | Combined with TGF-β1, inhibited the expression of α-SAM | α-SAM | (Wang et al., 2019) |
| Pheretima protein P2 | Guang dilong | In vitro and vivo | TGF-β induced abnormal proliferation in MRC5 cells and bleomycin induced pulmonary fibrosis in mice | 13.125μg/ml/2h and 5mg/kg/22days | Inhibited the protein expression of smad2/3 and p-smad2/3, inhibited the expression of α-SMA, Vimentin and Collagen I and increased the expression of E-cadherin | smad2/3, p-smad2/3, α-SMA, Vimentin, Collagen I, E-cadherin | (Li et al., 2022) |
| Water extract of earthworms | Pheretima aspergillum | In vivo and vitro | CCL4- induced liver fibrosis in mouse and TGFβ1 treated LX-2 HSCs and AML-12 cells | 100,200 mg/kg/7 days and 30, 100 μg/ml/24 hours | Enhanced AMPK/GSK3β/Nrf2 cascade by activating LKB1, inhibited HSC activation, hepatocyte apoptosis and ferroptosis | LKB1, AMPK/GSK3β/Nrf2 cascade | (Zhang et al., 2024) |
| Earthworm granulation tissue extract | Eisenia fetida | In vivo | STZ induced diabetic rats | 50, 70%/21 days | Scavenged free radicals, inhibited inflammatory pathways, reduced the levels of TNF-α, IL-1β, LPO, IL-6, promoted the synthesis of connective tissue framework protein and the expression of growth promoters | TNF-α, IL-1β, LPO, IL-6 | (Elkhalifa et al., 2024) |
| Earthworm extract | – | In vivo | burn wound model on skin of mice | 0.1 ml/3, 7, 11, 15 days | decreased edema, suppressed fibrosis, activated angiogenesis and epithelial regeneration, inhibited scar formation, and reduced the risk of infection. | – | (He et al., 2021) |
| Earthworm extract | Pheretima aspergillum | In vitro | Osteoblast-like MG-63 cells and RAW 264.7 macrophage cells | 500 ng/ml – 12 mg/ml/2 days | increased osteoblast proliferation and differentiation, matrix calcium deposition and the expression levels of alkaline phosphatase (ALP), osteopontin (OPN) and osteocalcin (OCN), reduced the tartrate-resistant acid phosphatase (TRAP) activity of osteoclasts | ALP, OPN, OCN, TRAP | (Fu et al., 2014) |
| Earthworm active protein | – | In vitro | NIH3T4 cell line | 37.5, 75, 150 mg/ml | Activated the MEK/ERK signaling pathway, promoted the cell cycle from G1 phase to S phase, and promoted cell proliferation | MEK/ERK signaling pathway | (Song et al., 2016) |
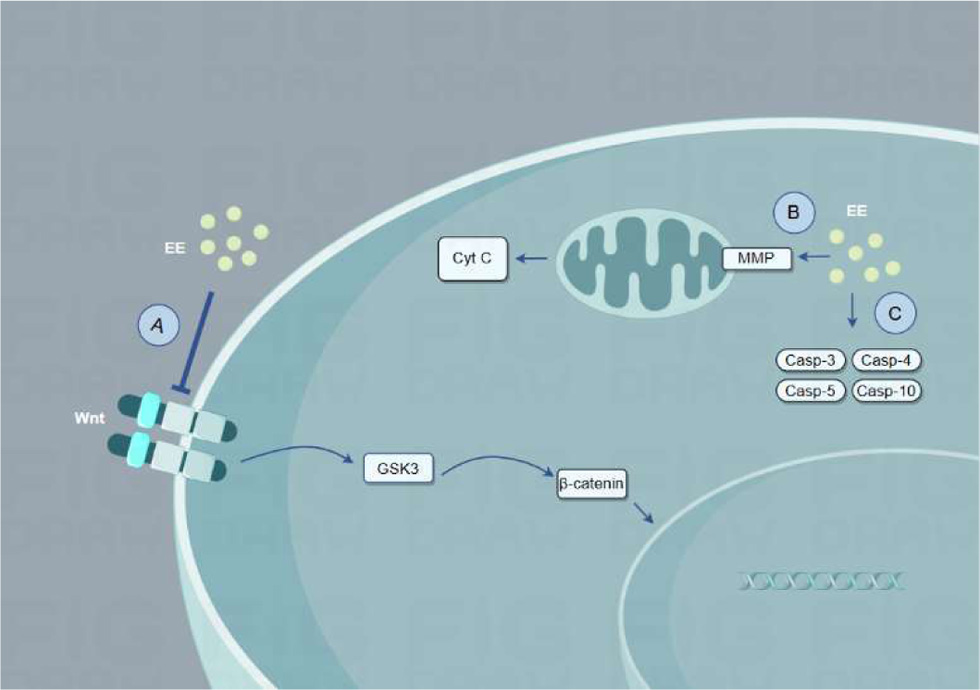
| Earthworm extract | – | In vitro | Human ovarian cancer cells A2780 | 400, 800 mg/ml/72 hours | Inhibited wnt / βcatenin pathway and promoted apoptosis | wnt / βcatenin pathway | (Chen et al., 2023) |
| Earthworm powder | Lumbricus terrestris | In vitro | Breast cancer cells (MCF-7) and prostate cancer cells (PC-3) | 100, 200, 400 μg/ml/2 4 hours | Changed the permeability of the mitochondrial membrane and released cytochrome c | – | (Shafi and Faleh, 2019) |
| Earthworm extract | Perionyx excavates | In vivo | S180 tumor-bearing mice | 30, 60, 90 mg/kg/25 days | Reduced the level of LDH, regulated the expression of Bax and Bcl2 protein | LDH, Bax, Bcl2 | (Deng et al., 2019) |
| Earthworm coelomic fluid | Dendrobaena veneta | In vitro | A549 cells | 125, 187.5, 250 μg/ml/24 hours | Increased expression of caspase3/4/5/10 | caspase3/4/5/10 | (Fiolka et al., 2019) |
| Protein-carbohydrate fraction (PE) | Dendrobaena veneta | In vitro | HT-29 cells | 10–200 μg/ml | Inhibited mitochondrial metabolism, up-regulated caspase-3 expression, blocked cell cycle, inhibited human 20S proteasome | caspase3/4/5/10, 20S proteasome | (Czerwonka et al., 2020) |
| Earthworm extract | – | In vivo | Epinephrine induced myocardial infarction in rats | 60 mg/kg/7 days | improved cardiac, liver and renal biomarkers such as creatine kinase (CK), albumin, creatinine, uric acid and repaired the antioxidant system | CK | (Oma et al., 2022) |
| Earthworm extract | Pheretima aspergillum | In vitro | LPS induced H9c2 cells apoptosis | 62.5, 125, 250 μg/ml/1 hour | Increased the expression of Bcl2 and Bcl-x, and inhibited the expression of TNF-α, caspase3/8/9, t-Bid and Bax | Bcl2, Bcl-x, TNF-α, caspase3/8/9, t-Bid, Bax | (Li et al., 2015) |
| Dilong extract | Pheretima aspergillum | In vitro | High-KCl cardioplegic solution induced H9c2 cells apoptosis | 31.25, 62.5, 125, 250 mg/ml/24 hours | Inhibitied the activation of IGF-I/IGF-IR/ERK pathway and the expression of uPA, Sp-1 and CTGF, inhibitied the expression of caspase-3 through MEK | uPA, Sp-1, CTGF, caspase-3, IGF-I/IGF-IR/ERK pathway, MEK pathway | (Han et al., 2014) |
| Earthworm extract | Pheretima aspergillum | In vitro | KCL induced H9c2 cells apoptosis | 31.25, 62.5, 125, 250mg/ml/24 hours | Inhibited the expression of t-Bid, p-ERK1/2, uPA, SP1, CTGF, promoted the expression of Bcl2 and Bcl-x, and activated the IGF1R/PI3K/Akt signaling pathway | t-Bid, p-ERK1/2, uPA, SP1, CTGF, Bcl2, Bcl-x, IGF1R/PI3K/Akt signaling pathway | (Huang et al., 2019) |
| Lumbrokinase | Canada RNA biochemical Inc., Richmond, BC, Canada | in vivo | I-R injury in male Sprague-Dawley rats | 10 μg/kg | Activated Sirt1, inhibited the protein expression of Bax and Cleaved Caspase-3, upregulated the protein expression of Bcl-2, alleviated myocardial I-R injury and decreased the induced expression of COX-2, iNOS and NF-κB | Sirt1, Bax, Cleaved Caspase-3, Bcl-2, COX-2, iNOS, NF-κB | (Wang et al., 2018) |
| Lumbrokinase | Harbin Jiuxin Science and Technology Industrial | in vivo | I-R injury in male Sprague-Dawley rats | 1.2mg/kg, twice/week, a month | Inhibited ERK1/2 mediated activation of uPA/MMP and SP/CTGF fibrotic signaling pathways | ERK1/2, uPA/MMP and SP/CTGF signaling pathways | (Lai et al., 2015) |
| Dilong extract | Pheretima aspergillum | In vitro | RSC96 Schwann cells | 125 μg/ml/24 hours | Mediated phosphorylation of PI3K/Akt pathway through the induction of IGF - I, stimulated the increase of PCNA, promoted cell proliferation | IGF-1, PI3K/Akt pathway | (Chang et al., 2011) |
| Earthworm extract | – | In vitro | PC12 cells | 31.5, 125, 500 g/ml | Increased the level of GAP-43 and synapsin I, promoted the nerve growth factor mediated neurite outgrowth | GAP-43, synapsin I | (Chen et al., 2010) |
| Earthworm extract | Ohira ii earthworms | In vivo | Mice | 100 ml (50%, 100%)/kg/7 days | Reduced the levels of NE, 5-HT and nitric oxide synthase (NOS) in serum and brain of mice and increased the pain threshold of mice | NE, 5-HT, NOS | (Luo et al., 2018) |
| Lumbrokinase | Boluoker, CRNA, Canada | in vivo | STZ induced diabetic rats | 300, 600, 1,200 g/kg/28 days | Stimulated the secretion of IL-1, NGF, PDGF and TGF-β and the expression of CGRP and the recruitment of macrophages | IL-1, NGF, PDGF, TGF-β, CGRP | (Lee et al., 2015) |
| Earthworm extract | Eisenia foetida | In vivo | Ova induced asthmatic mice | 50, 100, 200 mg/kg/56days | Regulated the balance of MMPs/TIMP-1, inhibited the infiltration of inflammatory cells in the lungs, and reduced the levels of Eot, IL-4, IL-5, IL-13 and plasma OVA-specific IgE | MMPs/TIMP-1, Eot, IL-4, IL-5, IL-13 | (Zhang et al., 2021) |
| Earthworm protein | – | In vivo | STZ induced diabetic rats | 0.045, 0.09, 0.18 g/kg/28 days | Inhibited oxidative stress, increased serum T level and activated NO receptor cGMP signal pathway | NO receptor cGMP signal pathway | (Zhang et al., 2023) |
| Earthworm protein | Baoji Fangsheng Biological Development Co., Ltd. | In vivo | STZ combined with high-fat diet induced diabetic rat. | 45, 90, 180 mg/kg/28 days | Inhibited the transformation of CCSMC from “contractile” to “synthetic (proliferation)” | – | (Ji et al., 2024) |
| Earthworm protein | Baoji Fangsheng Biological Development Co., Ltd. | In vivo | STZ induced diabetic rats | 45, 90, 180 mg/kg/28 days | Inhibited the expression of NF-κB, IL-1β and TNF-α, increased the expression of SOD and Nrf2 | NF-κB, IL-1β, TNF-α, SOD, Nrf2 | (Zhang et al., 2023) |
| Glycolipoprotein extract (G90) | Eisenia foetida | In vivo | Alloxan-induced diabetic rats | -/21 days | Promoted the formation of extracellular matrix, increased the fibroblast proliferation and neovascularization, collagen synthesis and early epithelial layer formation | – | (Goodarzi et al., 2016) |
| Glycoprotein extract (PvE-3) | Pheretima vulgaris | In vitro and vivo | NIH3T4 cell line and db/db mice, db/m mice | 40, 80, 160, μg/ml/and 100μl(160mg/ml)/3, 7, 14 days | Promoted cell cycle progression from G0 phase to G1/S/G2/M and preserved ΔΨm | – | (Wang et al., 2023) |