| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 31, September 2025, pages 31-40
Encapsulation strategies for dietary chlorogenic acid: advances in delivery systems and functional applications
Xin Guoa, #, Qian-Lan Wua, #, Tian-Le Maoa, Jing Lia, Kiran Thakura, b, Ya-Fang Shanga, b, Shao-Hua Yanga, Yi-Long Maa, b, *, Zhao-Jun Weia, b, *
aSchool of Food and Biological Engineering, Hefei University of Technology, Hefei 230009, China
bSchool of Biological Science and Engineering, North Minzu University, Yinchuan 750021, China
#These authors contributed equally to this work.
*Corresponding author: Yi-Long Ma and Zhao-Jun Wei, School of Food and Biological Engineering, Hefei University of Technology, Hefei 230009, China., E-mail: yilong.ma@hfut.edu.cn (YLM) and zjwei@hfut.edu.cn (ZJW)
DOI: 10.26599/JFB.2025.95031419
Received: August 19, 2025
Revised received & accepted: September 5, 2025
| Abstract | ▴Top |
Chlorogenic acid (CGA) is a naturally occurring dietary polyphenol that has attracted significant attention due to its anti-oxidant, anti-inflammatory, anti-microbial, neuroprotective, and anti-cancer properties. These bioactivities highlight CGA’s potential applications across food, nutraceutical, pharmaceutical, and cosmetic industries. However, its application remains currently limited by low stability, poor physiological stability, and insufficient bioavailability. To address these challenges, diverse encapsulation systems including nanoparticles, micelles, gels, liposomes, and metal-organic frameworks have been developed to protect CGA and from degradation and improve its bioactive efficacy. This review systematically summarizes the state of art encapsulation system for CGA, highlighting their design principles, release characteristics, and therapeutic applications. We aim to compare the strengths and limitations of each system, and provide a comprehensive reference to guide future research and promote the industrial translation of CGA-based formulations.
Keywords: Chlorogenic acid; Encapsulation; Nanocarriers; Bioavailability; Controlled release; Application
| 1. Introduction | ▴Top |
Chlorogenic acid (CGA) is one of the most abundant dietary phenolic acids, widely distributed in coffee, fruits, and vegetables (Gupta et al., 2022; Lu et al., 2020). Structurally characterized by its multiple hydroxyl groups, CGA exhibits strong antioxidant capacity through free radical scavenging, metal chelation, and inhibition of lipid peroxidation, which further contributes to its diverse biological activities, including anti-inflammatory, antimicrobial, neuroprotective, and anticancer effects (Kumar and Goel, 2019). These antioxidant properties are fundamental to CGA’s therapeutic mechanisms, as oxidative stress underlies many pathological conditions. Owing to these properties, CGA has emerged as a promising candidate for therapeutic and functional food applications in the pharmaceutical, nutraceutical, and cosmetic industries.
Despite its broad bioactivity, the application of CGA is severely restricted by its poor physicochemical stability and low bioavailability. CGA undergoes rapid degradation through oxidation, isomerization, and enzymatic hydrolysis during storage and under gastrointestinal (GI) conditions, resulting in diminished efficacy (Kiokias et al., 2020). Furthermore, its low aqueous solubility, limited intestinal absorption, and extensive metabolism significantly reduce systemic bioavailability. These limitations necessitate the development of advanced stabilization and delivery strategies to fully harness CGA’s therapeutic potential.
Encapsulation technologies have recently emerged as a promising solution, providing structural protection, controlled release, and targeted delivery of bioactive molecules (Zhang et al., 2021b). Nanostructured carriers such as liposomes, polymeric nanoparticles, micelles, emulsions, and metal–organic frameworks can be engineered to enhance solubility, improve permeability, and extend circulation time (Ge et al., 2022; Bae and Park, 2020; Miao et al., 2025). By tuning the internal architecture and surface functionality, these systems can achieve controlled release kinetics and enhanced mucosal penetration, thereby overcoming physiological barriers such as the gastrointestinal tract (Laffleur and Bauer, 2021; Wang et al., 2022). These features collectively render encapsulation systems particularly promising candidates for improving the application of unstable bioactive compounds like CGA.
While numerous studies have reported the encapsulation strategies for CGA, a comprehensive comparative analysis of their mechanisms, efficacy, and translational potential is still lacking. This review therefore aims to summarize the current advancements in CGA encapsulation strategies, highlight their biological and technological advantages, and discuss future research directions to support the development of scalable, clinically relevant formulations.
| 2. Biological functions of CGA | ▴Top |
CGA, one of the most abundant dietary polyphenols, has attracted substantial interest due to its broad spectrum of pharmacological activities, including anti-inflammatory, antimicrobial, and anticancer effects. Beyond its well-established antioxidant properties, CGA exerts additional pharmacological functions, including cardioprotective, antidiabetic, anti-obesity, and neuroprotective properties, highlighting its potential as a multifunctional therapeutic agent (Figure 1).
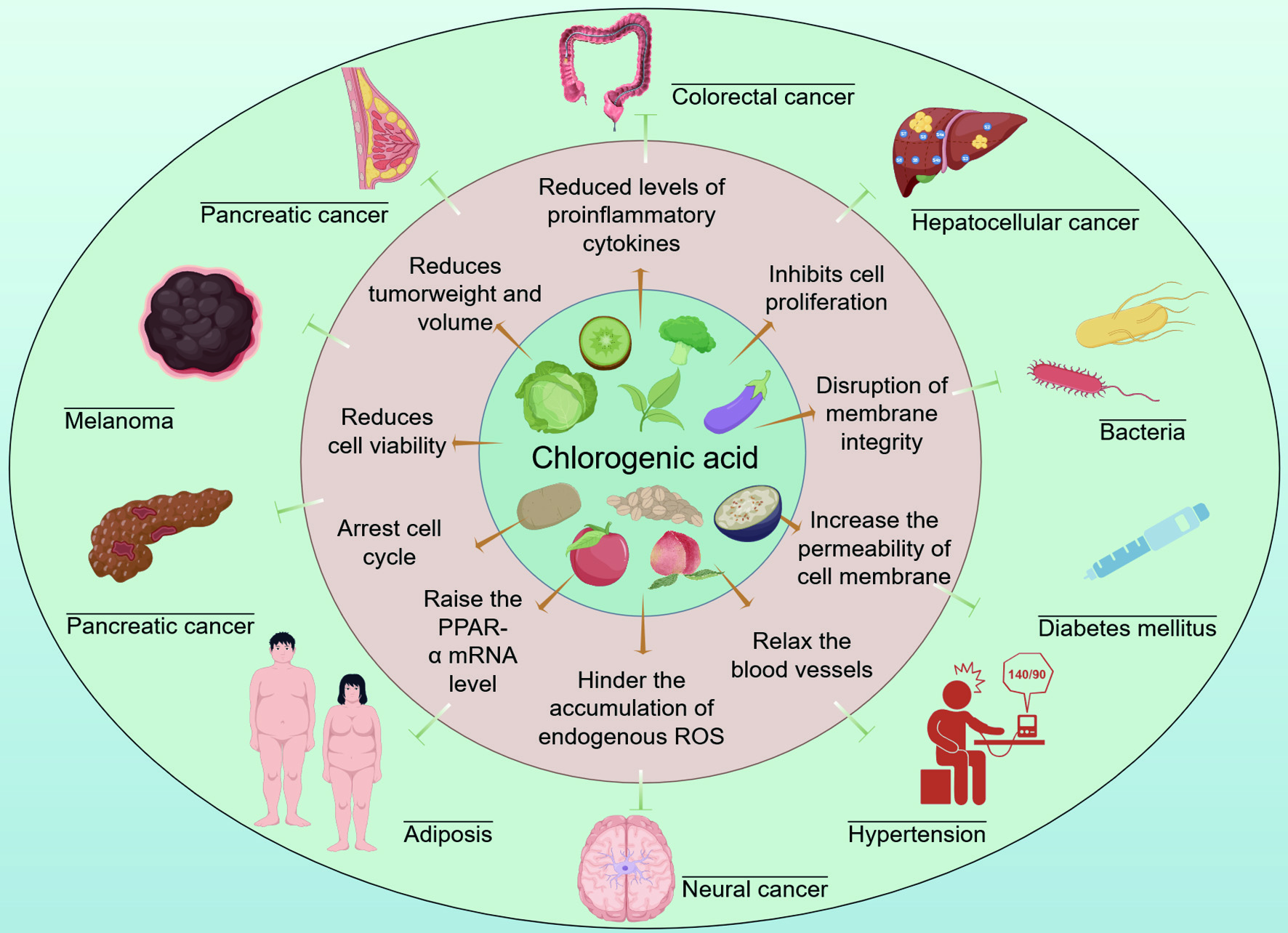 Click for large image | Figure 1. The bioactivity and potential application of CGA by Figdraw (ID: AUAYOc77fd). |
2.1. Anti-inflammatory activity
CGA modulates inflammatory responses primarily by suppressing the expression of pro-inflammatory cytokines. In Caco-2 cells stimulated with tumor necrosis factor-alpha (TNF-α) and hydrogen peroxide (H2O2), CGA treatment significantly reduced the expression of interleukin-8 (IL-8), a key inflammatory mediator (Shin et al., 2015). Feng et al. (2023) demonstrated that CGA treatment in a murine mastitis model downregulated serum IL-1β, IL-6, cyclooxygenase-2 (COX-2), and soluble adhesion molecules (SSA). Notably, CGA showed superior suppression of IL-1β and SSA compared with conventional antibiotics, underscoring its potential as a safer alternative in inflammatory disease management. Mechanistically, CGA’s anti-inflammatory effects are linked to inhibition of NF-κB and MAPK signaling pathways, which regulate cytokine expression and immune cell activation.
2.2. Antimicrobial activity
Beyond its anti-inflammatory properties, CGA exhibits broad-spectrum antimicrobial activity against both bacterial and fungal pathogens. Its mode of action involves disrupting the formation and structural integrity of microbial biofilms, thereby enhancing the susceptibility of pathogens to environmental stress. CGA has been shown inhibitory activity against a wide range of microorganisms, including Candida albicans (C. albicans) (Sung and Lee, 2010), Staphylococcus aureus (S. aureus) (Sun et al., 2021), Yersinia enterocolitica (Y. enterocolitica) (Chen et al., 2022), Fusarium fujikuroi (F. fujikuroi) (Kai et al., 2021), and Bacillus subtilis (B. subtilis)(Wu et al., 2020b). Importantly, CGA showed minimal inhibitory effects on beneficial probiotic strains, supporting its potential in food preservation and biomedical formulations as well as anti-microbial coatings (Bondam et al., 2022).
2.3. Anticancer activity
Extensive studies support the antineoplastic potential of CGA across multiple cancer types, including colorectal, breast, hepatic, melanoma, and pancreatic cancers. In colorectal cancer models, CGA inhibited the proliferation and migration of HCT116, HCT15, and CT26 cell lines in vitro, and significantly reduced tumor volume and weight in corresponding xenografted mice (Li et al. 2021b). Similar effects have been observed in breast cancer model (via p53 and caspase-3 activation), hepatocellular carcinoma, melanoma, and pancreatic cancer. Changizi et al. (2021) demonstrated that CGA suppressed tumor progression and mitigated cancer-induced weight loss in mice bearing 4T1 tumors. Similar inhibitory effects have also been observed in hepatocellular carcinoma (Yan et al., 2017), melanoma (Li et al., 2021a), and pancreatic cancer models (Yang et al., 2021), further validating CGA’s robust antitumor efficacy. Mechanistic studies suggest that CGA induces apoptosis through modulation of p53, Bax/Bcl-2, and caspase pathways, while also interfering with oncogenic signaling such as c-Myc–TFR1 axis in pancreatic carcinoma. These findings highlight CGA as a promising candidate for integrative cancer therapy.
2.4. Metabolic and neurological benefits
In addition to its well-documented anti-inflammatory, antibacterial, and anticancer properties, CGA demonstrates protective effects in metabolic and neurological disorders. It has been reported to lower blood pressure in hypertensive models by modulating endothelial nitric oxide production (Tom et al., 2016), reduce adiposity and improve lipid metabolism through upregulation of PPAR-α (Wan et al., 2013), regulate glucose metabolism in diabetes mellitus by enhancing insulin sensitivity and modulating hepatic glucose metabolism (Flanagan et al., 2014), and protect against neurodegenerative conditions (excitotoxicity and oxidative stress) by attenuating glutamate-induced apoptosis (Rebai et al., 2017). Collectively, these studies confirm CGA’s role as a multifunctional bioactive compound, with therapeutic potential extending from chronic inflammation and infectious diseases to cancer and metabolic syndromes. However, its clinical application remains hindered by limited stability and systemic bioavailability, emphasizing the need for advanced delivery strategies.
| 3. CGA stability and bioavailability | ▴Top |
Despite its diverse biological functions and considerable therapeutic potential, the clinical and industrial applications of CGA remain limited due to its poor physicochemical stability and low bioavailability (Liu et al., 2022; Abrankó and Clifford, 2017). These limitations are attributed to several factors: (1) its poor aqueous solubility due to strong intermolecular hydrogen bonding and hydrophobic interactions, which promote molecular aggregation in solution and reduce its dispersion, absorption, and utilization efficiency (Liu et al., 2022); (2) its low intestinal permeability due to multiple hydroxyl groups, unsaturated double bonds, and rigid ring configurations, reduce its ability to cross epithelial membranes via passive diffusion (Lv et al., 2025); (3) its chemical instability under physiological and storage conditions. Its ester bond is prone to enzymatic hydrolysis during digestion, generating caffeic and quinic acids. Additionally, CGA can undergo oxidation, or isomerization, and polymerization, leading to substantial degradation even after absorption (Kumar and Goel, 2019; Abrankó and Clifford, 2017); (4) its extensive metabolism through hydrolysis, conjugation (e.g., sulfation, glucuronidation, methylation), oxidation, and transformation by intestinal microbiota, further decrease its systemic availability (Clifford et al., 2020). Even when absorbed, CGA demonstrates short half-life and rapid renal clearance, which limit its therapeutic persistence and systemic exposure. Collectively, these barriers significantly compromise the therapeutic effectiveness of CGA. Therefore, the development of effective stabilization strategies, encapsulation systems such as nanoparticles, micelles, hydrogels, liposomes, and metal–organic frameworks offer promising solutions by (i) improving solubility, (ii) shielding CGA from chemical and enzymatic degradation, (iii) enabling controlled release, and (iv) enhancing absorption across biological barriers (Laffleur and Keckeis, 2020).
| 4. CGA encapsulation strategies and applications | ▴Top |
Encapsulation systems are nanostructured platforms composed of organic or inorganic hybrid materials designed to improve stability, solubility, and bioavailability of bioactive compounds. These systems not only protect sensitive molecules such as CGA from premature degradation, but also enhance their absorption and therapeutic efficacy. Furthermore, encapsulation systems can be engineered to achieve sustained release and site-specific delivery of active ingredients by incorporating specific functional components, including ligands that target cell surface receptors or other relevant proteins. In recent years, multiple nanotechnology-based systems including nanoparticles, micelles, hydrogels, liposomes, and metal–organic frameworks have been engineered for CGA delivery (Figure 2).
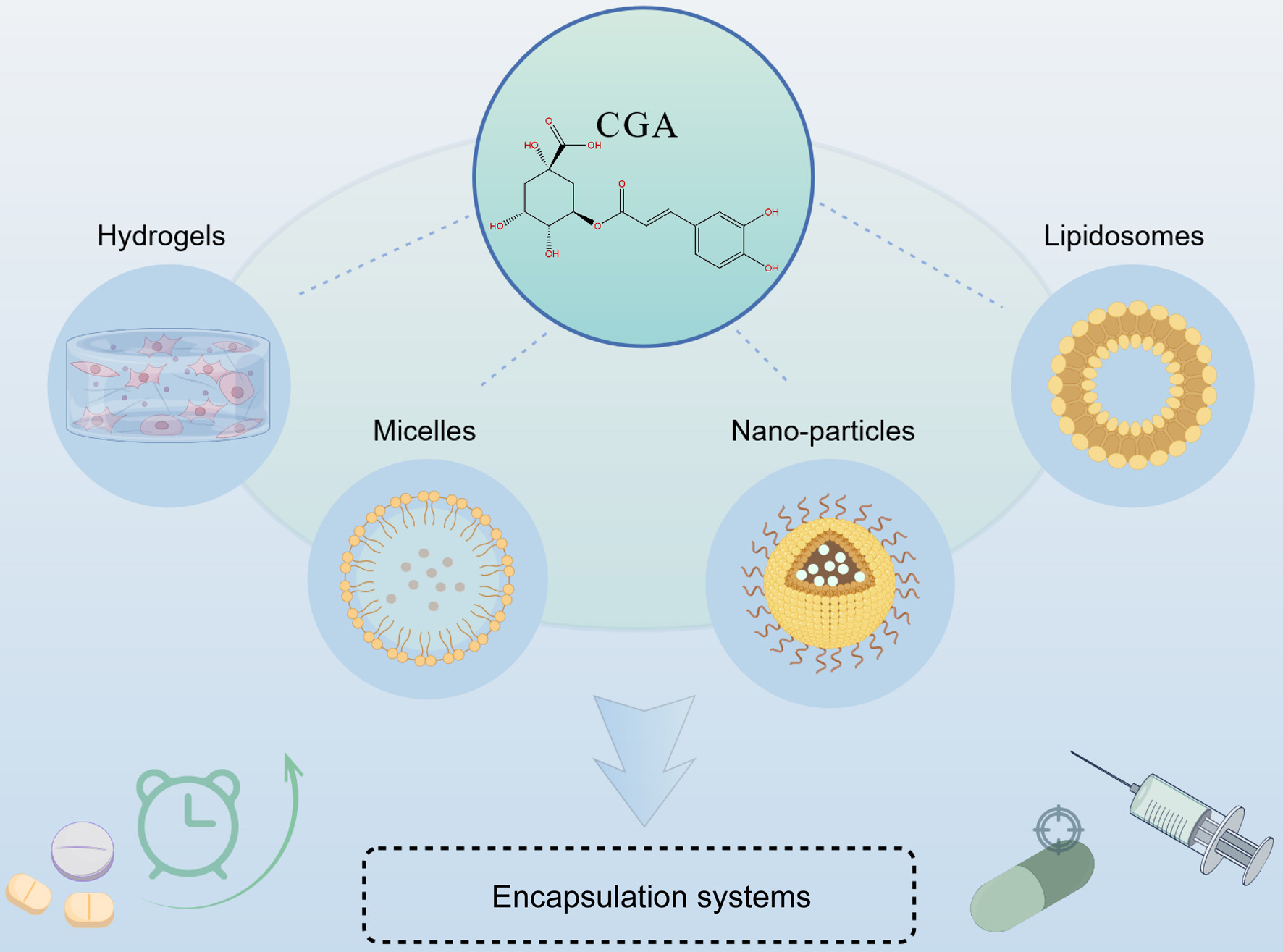 Click for large image | Figure 2. Encapsulation strategies of CGA by Figdraw (ID: AYRWY85a6f). |
4.1. Nanoparticles
Nanoparticles are versatile nanostructured carriers composed of organic, inorganic, or hybrid materials, typically formed through techniques such as self-assembly or layer-by-layer deposition. These systems are capable of efficiently encapsulating bioactive molecules, thereby significantly enhancing the stability, solubility, and bioavailability of these compounds (Ma and Moulton, 2011; Awad et al., 2023). To date, a variety of nanoparticles have been developed for CGA encapsulation, utilizing diverse materials such as proteins, polysaccharides, graphene, selenium, and other nanomaterials (Table 1).
 Click to view | Table 1. Nanoparticles for CGA encapsulation and application |
Encapsulation of CGA significantly enhances its stability, release profile, and bioavailability compared to free CGA. For example, in polyvinyl alcohol (PVA)/γ-polyglutamic acid (γ PGA) electrospun nanofiber mats, free CGA released only ∼36% after 72 h, whereas encapsulated CGA in mats containing 5% and 10% γ-PGA released ∼65% and ∼82%, respectively, demonstrating both reduced burst release and higher sustained release (Sandoval-Herrera et al., 2021). Similarly, pea protein nanoparticles (PPCNPs) achieved ∼61% encapsulation efficiency and increased in vitro ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging capacity, resulting in approximately 7.75% higher bioaccessibility versus free CGA. Additionally, Chen et al. (2017) demonstrated that oral administration of CGA-self-microemulsifying drug delivery system (CGA-SMEDDS) led to a 2.5-fold increase in bioavailability (249.4%) relative to conventional CGA suspensions, further supporting the bioavailability enhancement effect of nanocarrier systems. Jiang et al. (2024) reported that cucurbit[7]uril (Q[7]) @2CGA nanocomposites achieved a more stable release profile, with approximately 70% CGA released steadily over 5 days, offering enhanced structural protection compared to free CGA. These quantitative data support the claim the nanocarrier-based encapsulation yields superior protection, release control, and systemic exposure of CGA relative to its unencapsulated form.
In addition to conventional nanocarrier systems, co-assembly and macromolecular encapsulation strategies have also demonstrated significant potential in enhancing CGA functionality. Fu et al. (2024) developed supramolecular nanoparticles through the self-assembly of berberine (BBR) and CGA, which exhibited strong antibacterial activity against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). The co-assembled nanoparticles effectively disrupted bacterial membranes, downregulated resistance genes, and enhanced CGA utilization, leading to improved anti-inflammatory efficacy and accelerated wound healing in a murine skin infection model.
Similarly, Zhang et al. (2023) utilized the reversible assembly properties of apoferritin (Apo) to load CGA, either alone or with sodium alginate (SA). The resulting Apo/SA system improved CGA’s stability under heat and UV exposure and enabled controlled release in both gastric and intestinal environments, thereby enhancing oral bioavailability. In addition, Ma et al. (2024) formulated chitosan-based nanoparticles incorporating rhamnolipids (RL) and CGA via ionic crosslinking. The composite films (F/CRC) displayed excellent antioxidant activity, ultraviolet A (UVA) shielding, and biocompatibility, while also allowing for sustained CGA release, making them suitable for active packaging applications. These findings collectively demonstrate that both small molecule co-assembly and macromolecular carriers (e.g., proteins, polysaccharides) can significantly improve CGA’s stability, bioavailability, and therapeutic efficacy in diverse applications.
Inorganic nanomaterials have also shown great promise as delivery platforms for CGA due to their structural stability and functional versatility. Barahuie et al. (2017) developed a graphene oxide-based nanocomposite (CAGO) via hydrogen bonding and π–π stacking, which significantly enhanced CGA stability. Compared to free CGA, the CAGO system retained over 70% of its content after 72 h, while free CGA degraded by more than 50%, offering a more sustained release profile and reducing therapeutic fluctuations. While the > 103 h in vitro release suggests depot potential, clinical translation requires pharmacokinetic validation of absorption, metabolism, and clearance profiles.
Metal-based carriers have been explored for both protection and targeted delivery. Li et al. (2023) constructed selenium nanoclusters modified with a brain-targeting peptide (TGN-CGA@SeNCs), which improved CGA stability under alkaline gastrointestinal conditions and increased oral bioavailability by 2.3-fold in vivo. The system also enhanced neuroprotective effects and positively modulated gut microbiota composition. Likewise, Roy et al. (2022) synthesized CGA-loaded silver nanoparticles (AgNPs-CGA), further stabilized with bovine serum albumin (BSA). These hybrid nanoparticles showed enhanced cellular uptake and cytotoxicity against cancer cell lines, demonstrating potent antioxidant and antitumor effects compared to free CGA.
Metal–organic frameworks (MOFs) are crystalline, porous materials formed through the self-assembly of metal ions or clusters with organic ligands. These structures exhibit high surface areas, tunable pore sizes, and excellent stability, making them ideal candidates for controlled drug delivery (Li et al., 2017). Rincón et al. (2024) encapsulated CGA into MIL-125-NH2, a titanium-based MOF, using an impregnation method. While free CGA rapidly degraded in pH 8 buffer, the encapsulated form retained over 85% of its content after seven days, with sustained release, indicating superior long-term stability.
Collectively, these inorganic nanocarriers including carbon-based, metal-based, and MOF systems are next generation platform which offer enhanced physicochemical protection, bioavailability, and controlled release of CGA, supporting their application in advanced therapeutic delivery. However, challenges in toxicity, large-scale production, and regulatory acceptance must be addressed before widespread adoption.
4.2. Micelles
Micelles are nanoscale colloidal systems with a distinct core–shell architecture. They typically consist of a hydrophobic core enclosed by a hydrophilic outer shell, which enables them to encapsulate lipophilic active compounds and protect them from degradation in aqueous environments (Li et al., 2022b). This unique structural configuration enhances the solubility, stability, and bioavailability of encapsulated agents (Bui et al., 2012; Jo et al., 2020). In recent years, micelles have become a widely explored platform in drug delivery systems, particularly for combination therapies aimed at achieving synergistic pharmacological effects while minimizing toxicity. Micelle-based delivery systems (Table 2), particularly those formed from biodegradable amphiphilic copolymers, offer a promising strategy to overcome these challenges by stabilizing CGA, improving its solubility, and enabling targeted, sustained release. Poly(lactic-co-glycolic acid) (PLGA)-based polymeric micelles have become the benchmark carriers for CGA, combining FDA-approved biocompatibility, predictable hydrolytic degradation, and versatile surface chemistries that enable active-targeting ligand conjugation (Ge et al., 2022).
 Click to view | Table 2. Micelles for CGA encapsulation and application |
Vyawahare et al. (2024) developed PLGA-based micelles with a particle size ranging from 90 to 110 nm, which were designed to co-deliver CGA and methotrexate (MTX). These micelles maintained structural stability in neutral physiological conditions, effectively preventing premature CGA leakage or degradation. Under acidic and enzyme-rich inflammatory environments, however, the micelles underwent triggered destabilization, resulting in synchronized and accelerated release of CGA. PLGA micelles markedly amplified CGA’s anti-arthritic efficacy, curbing joint swelling, cartilage loss, and inflammation, while simultaneously prolonging its targeted action. Based on these findings, Li et al. (2022a) developed a refined micellar system-CGA-PLGA@PVP-where CGA was covalently bound to PLGA and further modified with polyvinylpyrrolidone (PVP) to improve hydrophilicity and colloidal stability. The resulting micelles (∼154.4 ± 19.3 nm, zeta potential: –19.6 mV) demonstrated excellent aqueous dispersion and controlled CGA release, reaching 56% in 72 h and ∼65% by 132 h. This formulation ensured CGA’s prolonged retention in target tissues after local administration. In a mouse model of periodontitis, the micelles achieved localized, long-acting delivery of CGA, which led to significant reductions in inflammation and alveolar bone resorption, underscoring their role in preserving CGA’s integrity and enabling therapeutic retention.
In addition to anti-inflammatory applications, micelle and nanoparticle-based delivery systems also significantly enhance CGA’s antimicrobial and antiviral activities. In a recent study, CGA was encapsulated within PVA/PLGA nanoparticles using electrospraying, achieving an optimal formulation (F2) with high loading efficiency (80.23%), sustained release (97.42% cumulative release), and a particle size of ∼454 nm (Saleh et al., 2023). Notably, the nanoparticle-loaded CGA exhibited significantly lower IC50 values against human coronavirus 229E (HCoV-229E) and Middle East respiratory syndrome coronavirus (MERS-CoV) (170 µg/mL and 223 µg/mL, respectively) compared to free CGA (p < 0.05). Moreover, in a murine model of Pseudomonas aeruginosa lung infection, the CGA nanoparticles showed superior antibacterial effects. These findings demonstrate that nanocarriers not only protect CGA from premature degradation but also amplify its pharmacological action across multiple infection models, further expanding its therapeutic potential beyond inflammatory diseases.
4.3. Hydrogels
Hydrogels are cross-linked polymeric systems characterized by a three-dimensional network structure composed of hydrophilic polymer chains and a substantial proportion of water molecules. These systems typically exist in a solid or semi-solid state at the macroscopic level and exhibit excellent water retention capacity. Due to their highly hydrated nature, biocompatibility, and tunable physicochemical properties, hydrogels have gained considerable attention as advanced wound treatment matrices (Toyoshima et al., 2022). Hydrogel-based delivery systems have shown considerable promise in addressing the key limitations of CGA, including its chemical instability, low oral bioavailability, and insufficient accumulation at target sites. A subset of advanced hydrogel formulations demonstrates clear advantages over free CGA, as evidenced by improved stability, controlled release, and enhanced bioactivity across various administration routes (Table 3).
 Click to view | Table 3. Hydrogels for CGA encapsulation and application |
Wang et al. (2024) developed a dual pH- and reactive oxygen species (ROS)-responsive hydrogel incorporating CGA encapsulated within zeolitic imidazolate framework-8 (ZIF-8). This system significantly enhanced the thermal stability of CGA, protected it from degradation, and enabled on-demand release under oxidative diabetic wound microenvironments. These features facilitated macrophage polarization and angiogenesis, resulting in accelerated wound repair.
In the context of oral delivery, Harwansh et al. (2025) formulated enteric-coated chitosan–carrageenan hydrogel beads loaded with CGA for the treatment of ulcerative colitis. The beads showed ∼95% sustained release of CGA at colonic pH over 24 h, with high encapsulation efficiency (∼84%) and targeted delivery to inflamed intestinal sites. This formulation significantly improved CGA’s gastrointestinal stability and mucosal retention, which are critical for its therapeutic effect in the colon.
For topical applications, Trivedi and Puranik (2023) designed a nanophytovesicle-loaded hydrogel containing CGA, which achieved a 2.2-fold increase in skin permeation compared to free CGA. The hydrogel also prolonged CGA’s antioxidant activity and accelerated wound closure in vivo, confirming its role in enhancing transdermal bioavailability and therapeutic effectiveness.
Huang et al. (2023) developed a novel CGA-based hydrogel dressing that self-assembles into a nearly uniform linear microstructure. In vitro drug release experiments showed a cumulative release of CGA reaching 69% within 24 h, demonstrating sustained release behavior. Further in vitro assessments confirmed the hydrogel’s pronounced anti-inflammatory activity. Its therapeutic effects in wound healing were attributed to enhanced wound closure, increased collagen deposition, and re-epithelialization, accompanied by suppression of pro-inflammatory cytokine expression and upregulation of growth factor expression. CGA-loaded hydrogels excel in oral, topical, and wound-care uses, offering biocompatibility, on-demand release, and sustained antioxidant/antimicrobial action; yet their industrial adoption hinges on stronger mechanics and scalable, reproducible manufacturing.
4.4. Liposomes
Liposomes are spherical vesicles primarily composed of phospholipids and fatty acids, closely mimicking the structure of biological membranes. This bilayer structure allows liposomes to encapsulate both hydrophilic and hydrophobic drugs, offering enhanced protection and stability for therapeutic agents. Compared with other delivery systems such as polymeric nanoparticles, micelles, and hydrogels, liposomes exhibit a more intricate architecture that enables superior drug loading and retention. Furthermore, the ease of surface functionalization and biocompatibility of liposomes have contributed to their widespread application in drug delivery through various administration routes (Waghule et al., 2022). In particular, liposomes have shown great promise in delivering low molecular weight active pharmaceutical ingredients (APIs) (Wu et al., 2020a).
CGA repolarizes M2 tumor-associated macrophages (TAMs) to the antitumor M1 phenotype, positioning it as a potent immunomodulator in cancer therapy. This modulation of the tumor microenvironment contributes to the inhibition of glioblastoma (GBM) growth. However, the clinical application of CGA remains limited owing to its rapid systemic clearance and insufficient accumulation at tumor sites (Xue et al., 2017). To address these challenges, Ye et al. (2020) developed mannosylated PEGylated liposomes (Man-PEG-Lipo) for the encapsulation and delivery of CGA. These liposomes effectively enhance CGA delivery to TAMs, reduce rapid clearance, and strengthen the anti-tumor immune response induced by CGA, while demonstrating minimal toxicity. As a result, the bioavailability and therapeutic potential of CGA in anti-tumor applications were significantly improved (Table 4).
 Click to view | Table 4. Liposomes for CGA encapsulation and application |
In clinical cancer immunotherapy, the inherent instability of CGA in vivo necessitates daily intramuscular injections, which presents considerable challenges for patient compliance. To overcome this limitation, Zhang et al. (2021a) designed a PEGylated liposomal system (CPPL) based on a CGA-phospholipid complex as the intermediate. In animal studies, CPPL administration resulted in plasma CGA concentrations exceeding 200 ng/mL at 24 h, markedly higher than those achieved by CGA solution. Furthermore, CPPL improved the stability of CGA in systemic circulation and sustained its antitumor efficacy, even when the dosing interval was extended to four days. This formulation offers a more convenient and effective strategy for the clinical use of CGA.
In the treatment of aggressive skin tumors such as melanoma, Zhu et al. (2024) proposed an innovative liposomal formulation to enhance therapeutic efficacy. They developed a liposome co-loaded with CGA and doxorubicin (DOX) and functionalized it with a sialic acid–octadecylamine conjugate (SA-ODA), referred to as CGA-DOX-SAL. In vitro release studies demonstrated that CGA-DOX-SAL liposomes provided sustained CGA release, achieving a cumulative release of 60.56% over 24 h, which was substantially lower than the rapid 95.97% release observed for free CGA within four hours. In vivo studies confirmed that CGA-DOX-SAL liposomes effectively accumulated in tumor tissues and enhanced anti-tumor activity. These findings highlight the potential of this liposomal system for melanoma therapy and suggest its applicability in the treatment of other tumor types.
In addition to their established role in cancer therapy and in enhancing drug potency and targeting, liposomes have shown significant promise in improving the oral absorption and bioavailability of CGA. Feng et al. (2016) reported that chlorogenic acid-loaded liposomes (CAL), formulated with cholesterol and phosphatidylcholine, markedly increased both the oral bioavailability and antioxidant activity of CGA. Compared with free CGA, CAL extended the circulation duration of CGA and achieved a 1.29-fold relative increase in bioavailability.
Based on these findings, Alemán et al. (2022) further explored the protective effects of liposomes on CGA stability within the gastrointestinal tract. They encapsulated an aqueous extract of sea fennel (Crithmum maritimum), which is rich in CGA, into soy phosphatidylcholine liposomes. In vitro gastrointestinal digestion simulations demonstrated that CGA encapsulated in liposomes remained stable throughout the digestion process, whereas the CGA content in the free extract decreased significantly by 40%. The liposomal encapsulation effectively protected CGA from degradation and morphological alteration, thereby substantially enhancing its stability during gastrointestinal digestion.
The application of liposome technology in CGA delivery not only broadens the scope of CGA in cancer immunotherapy but also provides innovative strategies for the development of potent and safe anti-tumor therapies by improving its stability and bioavailability during oral administration.
| 5. Conclusion and future perspectives | ▴Top |
CGA is an exceptionally versatile polyphenol, yet its susceptibility to oxidation, hydrolysis, and photodegradation severely limits its translational potential. Encapsulation technologies ranging from lipid-based nanocarriers and polymeric micelles to metal–organic frameworks and supramolecular hydrogels have emerged as powerful tools to enhance CGA’s physicochemical stability, control its release kinetics, and broaden its formulation space. Comparative analyses indicate that no single platform is universally optimal; instead, carrier selection must be tailored to the intended route of administration, target tissue, and desired release profile. Our review emphasizes the key gaps: (i) poorly characterized CGA–carrier interactions with limited understanding of binding mechanisms and their effects on release performance; (ii) low drug loading efficiency (<15% w/w of CGA relative to total carrier weight) combined with rapid burst release (>40% of encapsulated CGA released within 2–4 hours in PBS at pH 7.4, 37 °C, 100 rpm); (iii) unproven stability under industrial processing and storage conditions that could compromise CGA integrity and delivery performance. Future work should integrate multi-scale modelling (atomistic to population balance) with continuous-flow microfluidics for scalable, size-controlled carriers; extend CGA from nutraceuticals to other production processes.
Acknowledgments
This study was supported by the National Key R&D Program of China (2022YFF1100300), Fundamental Research Funds for the Central Universities (JZ2024HGTG0287), National Natural Science Foundation of China (32272312), Natural Science Foundation of Ningxia province (2023AAC05043).
Conflict of interest
The authors affirm that they have no conflicts of interest.
| References | ▴Top |