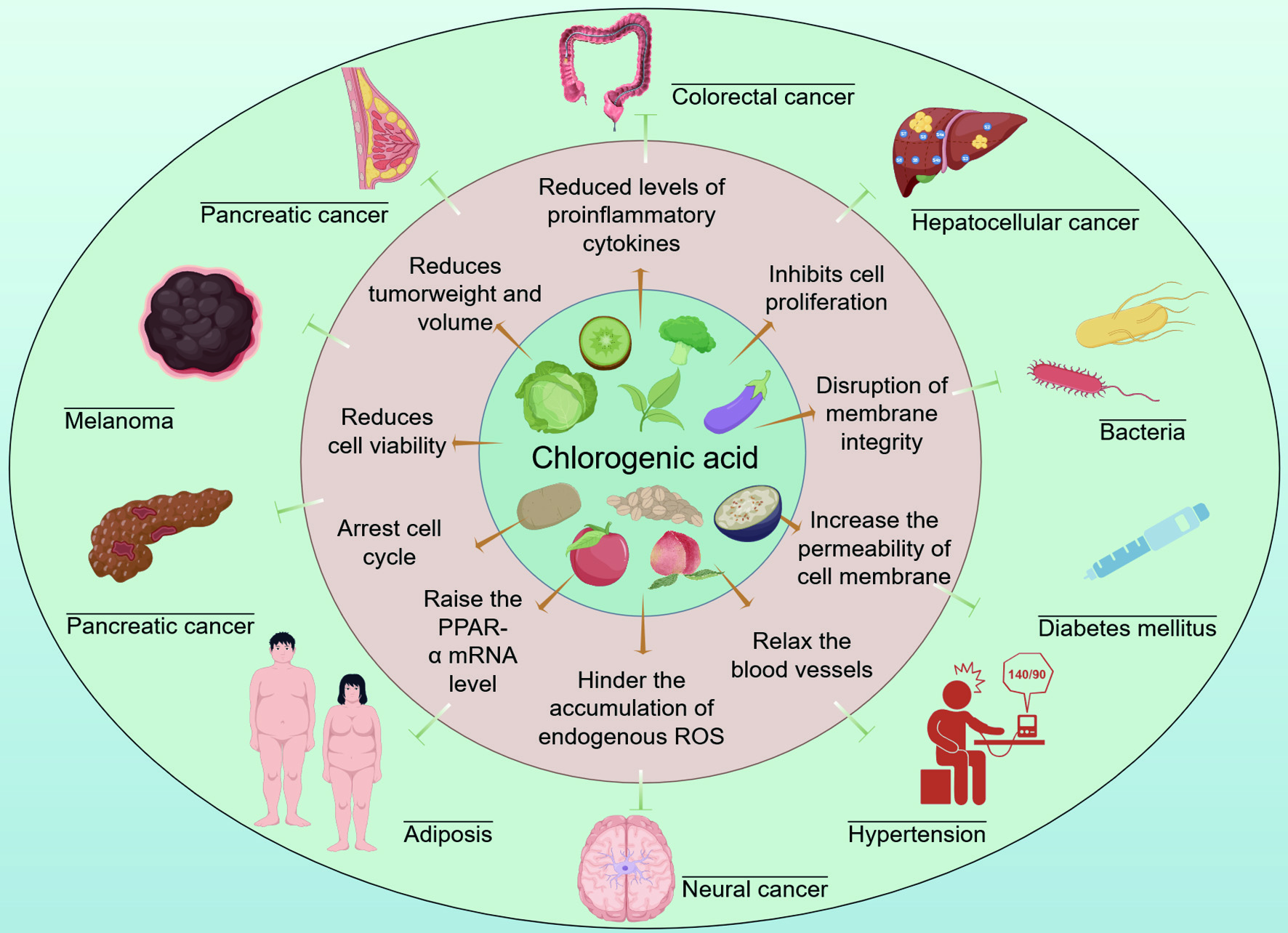
Figure 1. The bioactivity and potential application of CGA by Figdraw (ID: AUAYOc77fd).
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 31, September 2025, pages 31-40
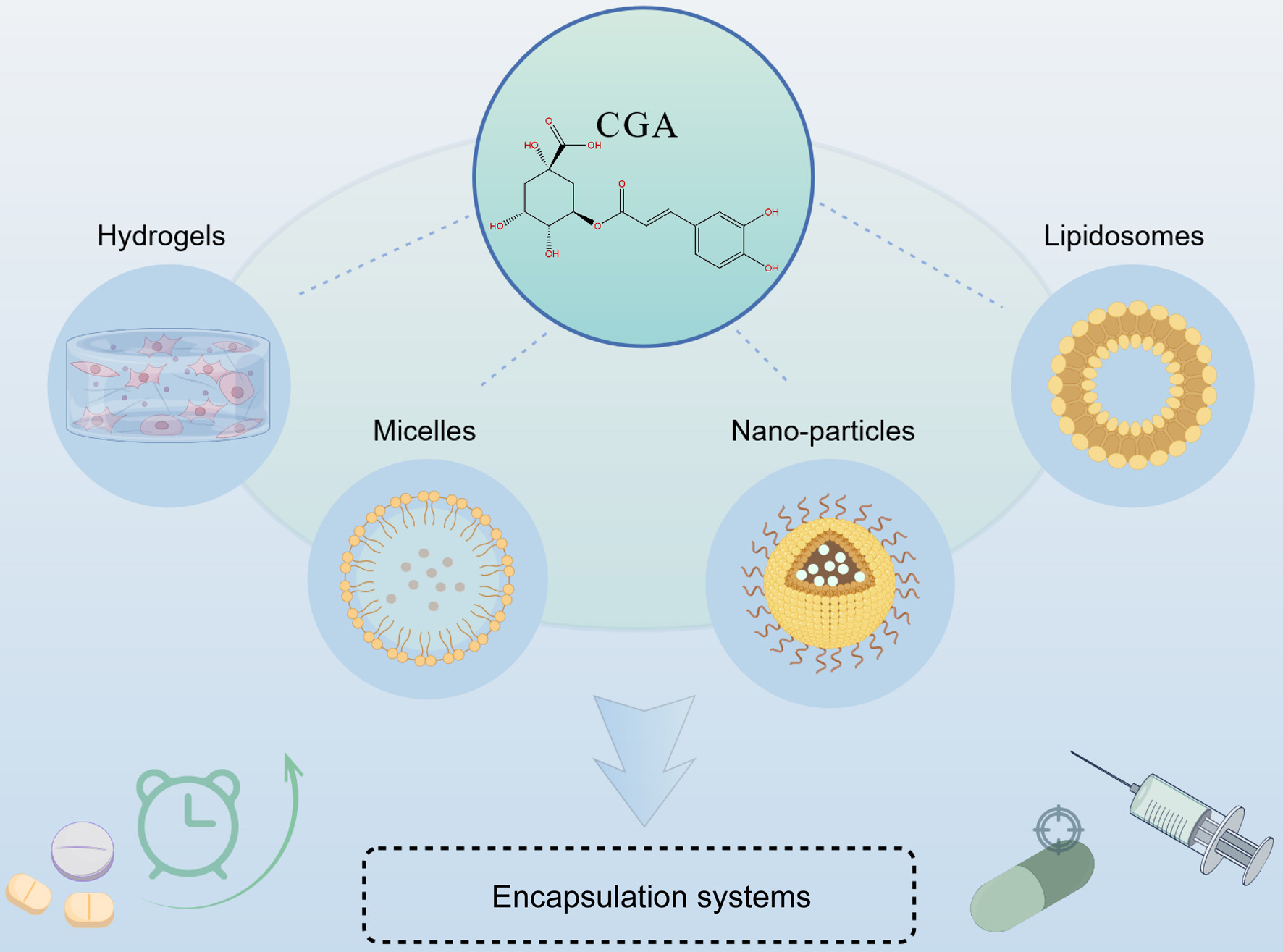
Encapsulation strategies for dietary chlorogenic acid: advances in delivery systems and functional applications
Figures

Tables
| Carrier System | Preparation Method | Surface Modification | Size | Effects | Advantages | Ref. |
|---|---|---|---|---|---|---|
| PVA/γ-PGA Nanofiber Mats | Electrospinning | / | 119 nm | Reduced burst & extended release | 82% vs 36% CGA release after 72 h (10% γ-PGA) | (Sandoval-Herrera et al., 2021) |
| PPCNPs | Nanoprecipitation | / | 211.62–429.79 nm | ∼7.75% higher in vitro bioaccessibility | Improved digestion stability and antioxidant capacity | (Liang et al., 2024) |
| CGA-SMEDDS | Self-microemulsification | / | 16.37 nm | 2.5× oral bioavailability | Oral bioavailability increased to 249.4% vs suspension | (Chen et al., 2017) |
| Q[7]@2CGA NPs | Host–guest inclusion | / | 100nm | Improved release stability | ∼70% CGA released steadily over 5 days | (Jiang et al., 2024) |
| BBR/CGA NPs | Self-assembly | / | 360.6 nm | Improved bioavailability | S. aureus inhibition 87.94% vs 59.05% (free); MRSA: 88.10% vs 46.28% | (Fu et al., 2024) |
| SA-Apo-CGA NPs | Co-encapsulation | / | 156.6 nm | Improved bioavailability and GI release control | CGA UV retention: 96.50% vs 77.79%; slower release in GI environment | (Zhang et al., 2023) |
| CS/RL/CGA NPs | Ionic crosslinking | / | 190 nm | CGA fixation in RL and CS | Lower CGA migration and slower release vs F/CGA indicate good fixation | (Ma et al., 2024) |
| CAGO | Intermolecular interactions | / | / | Prolonged CGA release | CGA release duration >103 h; beyond CGA’s pharmacokinetic half-life | (Barahuie et al., 2017) |
| TGN-CGA@SeNCs | Selenium nanoparticle base | TGN peptide surface modification | 58.06 nm | Improved GI stability | CGA degradation under pH 8: 47.83% vs 74.22% for free CGA | (Li et al., 2023) |
| AgNPs-CGA-BSA | Silver nanoparticle synthesis | BSA surface modification | 96 nm | Antibacterial, antioxidant, and anticancer effects | DPPH IC50: 91.72 µg/mL vs 94.03 µg/mL (free CGA); enhanced bioactivity | (Roy et al., 2022) |
| CGA@MIL-125-NH2 | MOF synthesis + adsorption | / | 226–653 nm | Improved aqueous stability | At pH 8, CGA@MOF retained >85% after 7 days vs <10% for free CGA; sustained release | (Rincón et al., 2024) |
| Carrier System | Composition/Structure | Size | Release Behavior | Enhancement on CGA | In Vivo/Therapeutic Effects | Ref. |
|---|---|---|---|---|---|---|
| PLGA-b-CGA/PLGA-b-CGA–MTX | Electrospinning | 90–110 nm | Stable at pH 7.4; fast dual release at pH 5.8 with esterase | Prevents premature CGA leakage; pH/enzyme-triggered release; improves inflammation targeting | Suppressed joint swelling, delayed cartilage erosion in arthritis model | (Vyawahare et al., 2024) |
| CGA-PLGA@PVP | CGA covalently linked to PLGA; surface PVP-modified | 154.4 ± 19.3 nm | Sustained CGA release: ∼56% at 72 h; ∼65% at 132 h | Enhanced solubility, colloidal stability, and prolonged CGA retention in tissue | Reduced gingival inflammation and bone loss in periodontitis model | (Li et al., 2022a) |
| CGA-loaded PVA/PLGA NPs (F2) | Electrosprayed PVA/PLGA loaded with CGA | 454.36 ± 36.74 nm | Initial burst: 29.46%; Cumulative: 97.42% | Enhanced antiviral potency (↓IC 50), prolonged release, higher tissue accumulation | Effective against HCoV-229E, MERS-CoV, and P. aeruginosa in lung infection model | (Saleh et al., 2023) |
| Hydrogel System | Key Mechanism | Enhancement vs. Free CGA | Target Application | Ref. |
|---|---|---|---|---|
| Dual pH/ROS-responsive hydrogel with ZIF-8@CGA | ZIF-8 stabilization; oxidative and acidic stimulus-triggered release | ↑ Thermal stability, ↑ on-demand release, ↑ wound healing via M1 polarization | Diabetic wound healing | (Wang et al., 2024) |
| Enteric-coated chitosan–carrageenan CGA hydrogel beads | Colon-specific pH-triggered release; mucoadhesion | ↑ GI stability, ↑ mucosal retention, ∼95% sustained release at colonic pH | Ulcerative colitis | (Harwansh et al., 2025) |
| Nanophytovesicle-loaded hydrogel | Nanoencapsulation improves skin permeation and antioxidant retention | ↑ Skin permeation (2.2×), ↑ antioxidant persistence, ↑ in vivo wound healing | Topical wound therapy | (Trivedi and Puranik, 2023) |
| Self-assembling CGA hydrogel dressing | Self-assembly enables structural integrity and controlled release | ↑ Anti-inflammatory effect, ↑ collagen deposition, 69% sustained release at 24h | Skin wound healing | (Huang et al., 2023) |
| Formulation Name | Composition & Features | Target/Model | CGA Enhancement | Therapeutic Outcome | Ref. |
|---|---|---|---|---|---|
| Man-PEG-Lipo | PEGylated liposomes functionalized with mannose for TAM targeting | Tumor-associated macrophages (TAMs), glioblastoma | Increased TAM targeting; reduced clearance; immune activation | Inhibited GBM growth via M2→M1 polarization | (Ye et al., 2020) |
| CPPL | PEGylated liposomes based on CGA–phospholipid complex | Systemic tumor model | Sustained plasma CGA levels (>200 ng/mL at 24h); 4-day dosing interval; improved stability | Reduced injection frequency; maintained anti-tumor efficacy | (Zhang et al., 2021a) |
| CGA-DOX-SAL | CGA + Doxorubicin co-loaded liposomes with SA-ODA surface modification | Melanoma | Sustained CGA release (60.56% at 24h); controlled dual drug release | Enhanced tumor accumulation and anti-melanoma effect | (Zhu et al., 2024) |
| CAL | CGA-loaded liposomes using cholesterol and phosphatidylcholine | Oral delivery (pharmacokinetics) | Extended circulation; 1.29× increase in oral bioavailability | Improved antioxidant activity and systemic exposure | (Feng et al., 2016) |
| CGA–SPL (Soy-liposomes) | Liposomes loaded with sea fennel extract (CGA-rich) using soy phosphatidylcholine | Simulated GI digestion | Protected CGA from pH/enzymatic degradation; retained structural integrity during digestion | Enhanced GI stability → potential for nutraceutical or oral formulations | (Alemán et al., 2022) |