| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 30, June 2025, pages 68-76
Allyl isothiocyanate confers resistance against low-pH stress conditions to RGM1 gastric normal epithelial cells
Shiho Kawaguchi, Akari Ishisaka, Akira Murakami*
Department of Food Science and Nutrition, School of Human Science and Environment, University of Hyogo, Hyogo, Japan
*Corresponding author: Akira Murakami, Department of Food Science and Nutrition, School of Human Science and Environment, University of Hyogo, Hyogo, Japan., +81-79-292-9325; E-mail: akira@shse.u-hyogo.ac.jp
DOI: 10.26599/JFB.2025.95030416
Received: June 2, 2025
Revised received & accepted: June 21, 2025
| Abstract | ▴Top |
In this study, we attempted to elucidate the effects of ally isothiocyanates (AITC) on stress resistance. RGM1 cells, derived from the normal gastric mucosa of rats, were pretreated with AITC (0-30 μM) 24 hr or 48 hr prior to posttreatment with AITC (0 or 20 μM), or both. While approximately 90% of the vehicle-pretreated cells died by a posttreatment with AITC, pretreatments with AITC (10-30 μM), especially 24 hr prior and double pretreatments, exhibited striking cytoprotective effects. AITC, as a xenobiotic, increased the amounts of reactive oxygen species and insoluble proteins. On the other hand, double pretreatments with AITC markedly upregulated the mRNA expression levels of anti-oxidative, detoxification, and molecular chaperone genes for homeostasis. Interestingly, pretreatments with AITC (10 and 15 μM) significantly mitigated low-pH, but not high-pH, stress conditions, which may involve the activation of phosphoinositide 3-kinase and Na+/H+ exchanger. Taken together, we show here that multiple exposures to AITC can confer a stress resistance phenotype, including adaptation to acidic pH, by upregulating the expressions of self-defensive enzymes. Therefore, this study implies the importance of continuous ingestion of phytochemicals for efficiently increasing the stress resistance capacity against harmful chemicals.
Keywords: Isothiocyanate; pH stress; Hormesis; Oxidative stress; Detoxification
| 1. Introduction | ▴Top |
Isothiocyanates (ITCs) are produced from glucosinolates by the function of myrosinase in many cruciferous vegetables. They have an electron-deficient carbon that is susceptible to the electrophilic addition by anti-oxidative glutathione (GSH), leading to its consumption and thus increased oxidative stress conditions. Based on this biochemical property, ITCs at high concentrations or excess doses have been shown to have harmful effects, as demonstrated by cellular and experimental rodent models. For instance, benzyl ITC (BITC) induces both chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary (CHO) cells (Musk et al., 1995). Both 0.1% phenethyl ITC (PEITC) and BITC in the diet of F344 rats lowered the urinary pH and aggravated the proinflammatory scores in the urinary bladder (Akagi et al., 2003). In addition, the fetal and placental weights of rats treated with 25 and 50 mg/kg BITC were significantly lower than those of the control (Adebiyi et al., 2004).
On the other hand, ITCs are known to have diverse biofunctions, including anti-cancer (Gupta et al., 2014), anti-oxidation (Li et al., 2015), and detoxification (Abdull Razis et al., 2018) activities. ITCs at moderate concentrations oxidize and/or react with the thiol moiety of Kelch-like ECH-associated protein 1 (Keap1) to liberate its partner transcription factor, nuclear factor-erythroid 2-related factor 2 (Nrf2), for up-regulating many adaptive enzymes (Baird and Yamamoto, 2020). This biochemical property of ITCs accounts for the mechanisms underlying their cancer preventive activities, in which activated procarcinogens are detoxified by the functions of the Nrf2-dependent enzymes (Kwak and Kensler, 2010). It is worth noting that sulforaphane, an ITC found in broccoli, at low concentrations increased the cell proliferation of astrocytes via the p38 mitogen-activated protein kinase pathway, whereas it showed inhibition at higher concentrations (Yang et al., 2022). In addition, Okulicz et al. treated diabetic rats with allyl ITC (AITC, 2.5, 5 and 25 mg/kg) for 2 weeks and found that AITC at the highest dose caused pancreatic amylase and lipase drops and thyroid gland hypertrophy, whereas blood glucose levels were significantly reduced at 2.5 and 5 mg/kg (Okulicz et al., 2021). These phenomena are consistent with the concept of hormesis, in which exogeneous and endogenous stressors at moderate doses show beneficial effects while high doses are harmful, along with a biphasic response (Calabrese et al, 2024).
Phytochemicals, including ITCs, are the secondary metabolites of plants that are substantially xenobiotics to animals. Therefore, ingestion of phytochemicals increases the expression of detoxification enzymes (Reuland et al., 2013). Additionally, some anti-oxidation enzymes are also up-regulated as many phytochemicals have been uncovered to be pro-oxidants (Fernando et al., 2019; Canedo-Santos et al., 2022; de Roos and et al., 2015). Therefore, treatments with phytochemicals at moderate concentrations may increase the stress resistance capacity by up-regulating self-defensive enzymes. To ascertain this hypothesis, in the present study, we examined whether repetitive treatments with AITC, a pungent in Japanese horseradish or Wasabi, can cause phenotypic changes in RGM1 rat gastric normal epithelial cells.
| 2. Materials and methods | ▴Top |
2.1. Cells and reagents
RGM1 rat gastric normal epithelial cells were kindly provided by Prof. Mitsugu Akagawa (University of Tokushima), who purchased them from RIKEN BioResource Research Center (Tsukuba, Japan). Dulbecco’s Modified Eagle’s Medium (DMEM)/Nutrient Mixture F-12 Ham was purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Thermo Fisher Scientific (Walthman, MA). All other reagents were purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan) and were of special grade unless specified otherwise.
2.2. Cytotoxicity test
RGM1 cells were maintained at 37°C in 50% DMEM/Ham’s F12 media with 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml), and dimethyl sulfoxide (DMSO) was used as a vehicle. For the cytotoxicity assay, RGM1 cells (1 x 106/mL) were preincubated in 200 μL of 50% DMEM/Ham’s F12 media on a 96-well plate overnight. After washing the cells twice with phosphate-buffered saline (PBS), they were exposed to the first AITC pretreatment (0–30 μM) for 24 hr and to the second one for another 24 hr, which was followed by posttreatment with AITC (0 or 20 μM). After 24 hr, cell viability was measured by using Cell Counting Kit-8 (DOJINDO Laboratories, Kumamoto, Japan).
2.3. Reactive oxygen species (ROS) detection
RGM1 cells (5 x 105/mL) were seeded on eight consecutive chamber slides (Thermo Fisher Scientific) and precultured in 200 mL of DMEM/Ham’s F12 medium containing 10% FBS overnight. After washing the cells twice with PBS, the well was replaced with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, DOJINDO Laboratories, 10 µM) dissolved in serum-free DMEM and incubated at 37°C for 30 min. Then, the cells were washed twice with PBS again, and each sample (0.5% DMSO, 10 or 30 µM AITC, or 200 µM H2O2) was added and incubated at 37°C for 30 min. After washing twice with PBS, the chamber was removed, and a coverslip was mounted with 50% glycerol/PBS and observed using a confocal laser microscope (FV3000, Olympus, Tokyo, Japan). For semi-quantification, RGM1 cells (5 x 105/mL) were preincubated in 200 μL of 50% DMEM/Ham’s F12 medium containing 10% FBS on a 96-well black plate (clear bottom, Thermo Fisher Scientific) overnight. After washing the cells with PBS, FBS-free DMEM containing DCFH-DA (final concentration: 10 μM) was added and incubated at 37°C for 30 min. Then, the cells were washed twice with PBS again, and each sample was added and incubated at 37°C for 30 min. After washing with PBS, the fluorescence of media was measured at Ex505 nm and Em525 nm by a microplate reader (Synergy H1, BioTek, Winooski, VT).
2.4. Protein solubility assay
Proteostress was evaluated by the method as previously reported (Suihara et al., 2021). Briefly, RGM1 cells (1 x 106/mL) were precultured in 50% DMEM/Ham’s F12 media containing 10% FBS on a 24-well plate overnight. Each well was washed twice with PBS, replaced with FBS-free DMEM, and each sample (0.5% DMSO or 30 µM AITC) was added. After incubation at 37°C for 0, 2 or 4 hr, the cells were washed twice with PBS and extracted with regular lysis buffer (BD Pharm LyseTM, BD Biosciences, Franklin Lakes, NJ) or high detergency lysis buffer (regular lysis buffer containing 2% sodium dodecyl sulfate, SDS) for determination of the amounts of the soluble and the whole protein, respectively, which was quantified by the BCA method. The amounts of insoluble proteins were estimated by subtracting those of soluble proteins from whole ones.
2.5. Real-time reverse transcription polymerase reaction (RT2-PCR)
RGM1 cells (1 x 106/mL) were preincubated in 1 mL of 50% DMEM/Ham’s F12 medium containing 10% FBS on a 12-well plate overnight. After washing the cells twice with PBS, the media were replaced by FBS-free DMEM containing AITC (0 or 15 μM) and incubated for 24 hr. After another washing, the cells were exposed to posttreatment with AITC (0 or 20 µM), and the total RNA was extracted using the RNeasy Mini KitTM (Qiagen, Venlo, The Netherlands). Total RNA concentrations were calculated by measuring the absorbance at 260 nm. cDNA was synthesized using total RNA (1 µg) with 25 mM MgCl2 (4 µL), PCR buffer (Takara Bio, Kusatsu, Japan) (2 µL), 2 mM dNTP (Takara Bio) (2 µL), 40 U/µL RNase inhibitor (Takara Bio) (0.5 µL), 5 U/µL AMV RTase (Takara Bio) (1 µL) and 1 g/L oligo-dT adaptor primer (Sigma-Aldrich, 1 µL) by RT reaction, which was performed by using a Programable Thermal Controller PTC-100 (MJ Research, St. Bruno, Canada). The conditions of cDNA synthesis were as follows: 30°C for 10 min, 55°C for 30 min, 95°C for 5 min, and 4°C for 15 min. For PCR, 5 µL cDNA was mixed with a sense- and antisense-primer (10 pmol, 4.5 µL), synthesized by Thermo Fisher Scientific, Power SYBR Green PCR Master Mix (25 µL) and ultrapure water (11 µL). PCR was performed using a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA) and the thermal conditions were as follows: 50°C 2 min and 95°C 2 min for 1 cycle, and 95°C for 15 sec, 60°C for 30 sec, 72°C for 30 sec for 99 cycles. The relative mRNA levels were estimated by the ΔΔCt method. The sequences of the primers are shown in Table 1.
 Click to view | Table 1. Primers used for RT2-PCR |
2.6. pH stress assay
RGM1 cells (1 x 106/mL) were preincubated in 200 μL of 50% DMEM/Ham’s F12 medium containing 10% FBS on a 96-well plate overnight. After washing the cells twice with PBS, they were pretreated with AITC (0, 2.5, 5, 10 and 15 μM) for 1 hr. After washing, the media were replaced by FBS-free DMEM, the pH of which was adjusted to 4.6, 7.4, or 9.8, and the cells were incubated for 1 hr. After washing the cells, the media were replaced to FBS-free DMEM (pH 7.4) followed by a 24 hr-incubation. Cell viability was determined as described above.
2.7. pH determination
For extracellular pH measurement, RGM1 cells (1 x 106/mL) were preincubated in 200 μL of 50% DMEM/Ham’s F12 medium containing 10% FBS on a 24-well plate overnight. After washing the cells twice with PBS, they were pretreated with AITC (0 or 15 μM) for 1 hr. After washing, the media were replaced by FBS-free DMEM, in which the pH was adjusted to 4.6. After incubation for 1 hr, the media pH was returned to 7.4, followed by a 24 hr-incubation. The pH of medium was measured using a handy pH meter (LAQUAtwin pH-11B, HORIBA, Kyoto, Japan). For intracellular pH determination, RGM1 cells (1 x 106/mL) were preincubated in 200 μL of 50% DMEM/Ham’s F12 media containing 10% FBS on a 96-well black plate (Thermo Fisher Scientific) overnight. After washing the cells with HEPES, FBS-free DMEM containing BCECF-AM (final concentration: 5 μM) was added and incubated for 30 min. After washing with HEPES, the fluorescence of medium was measured at Ex490 nm and Em535 nm by a microplate reader (Synergy H1). To make a calibration curve, RGM1 cells (1 x 106/mL) on a 96-well black plate were incubated in FBS-free DMEM, and then nigericin (DOJINDO Laboratories, final concentration: 10 μg/mL) Nigericin, an electroneutral K+/H+ ionophore derived from Streptomyces hygroscopicus, was added to equilibrate intra- and extracellular pH (Kaneko et al., 1991) to make a calibration curve. After 10 min, BCECF-AM (5 μM) was added, and the fluorescence of medium was measured using the same method as the above. A linear calibration curve regarding the intracellular pH was obtained in a range of pH 5.5 and 7.9 in the media (data not shown).
2.8. Statistical analysis
Each experiment was performed at least 3 times and the values are shown as the mean ± standard deviations (SD). Statistically significant differences between the groups for each assay were determined using Student’s t-test. Differences were considered significant at p < 0.05.
| 3. Results | ▴Top |
3.1. Cytoprotective effects of AITC
ITCs are xenobiotics to animals and thus increase the expression of xenobiotic-metabolizing enzymes (Keum et al., 2005). Therefore, pretreatments with ITCs may potentiate cytoprotective activity toward xenobiotics, including ITCs themselves at cytotoxic concentrations. In addition, we were interested in the issue of how long the cytoprotective effects of ITCs can be prolonged. To test these questions, we pretreated RGM1 gastric normal mucosa cells from rats with AITC (0–30 μM) 24 hr or 48 hr prior to posttreatment with AITC (20 μM), or both. We selected pH 4.6 as an acidic stress condition because AITC could not protect RGM1 cells cultured at the pH of gastric juice (pH 1–2, data not shown). As shown in Figure 1, posttreatment with AITC (20 μM) with double vehicle pretreatments resulted in drastic cell death (viability <10%). On the other hand, pretreatments with AITC under several conditions dramatically protected cells from cytotoxicity. For instance, viability of the cells pretreated with AITC 24 hr and 48 hr (both 20 μM) before posttreatment were 85% and 72%, respectively. Double pretreatments at 20 and 30 μM were found to be most protective as compared with each single pretreatment, whereas the concentrations of AITC pretreatments 24 hr prior to posttreatment and those of double pretreatments to exhibit significant cytoprotection were notably lower than those of 48 hr. Meanwhile, it is notable that the cytoprotective effects of AITC pretreatments 24 hr before posttreatment abolished at the highest concentration of 30 μM.
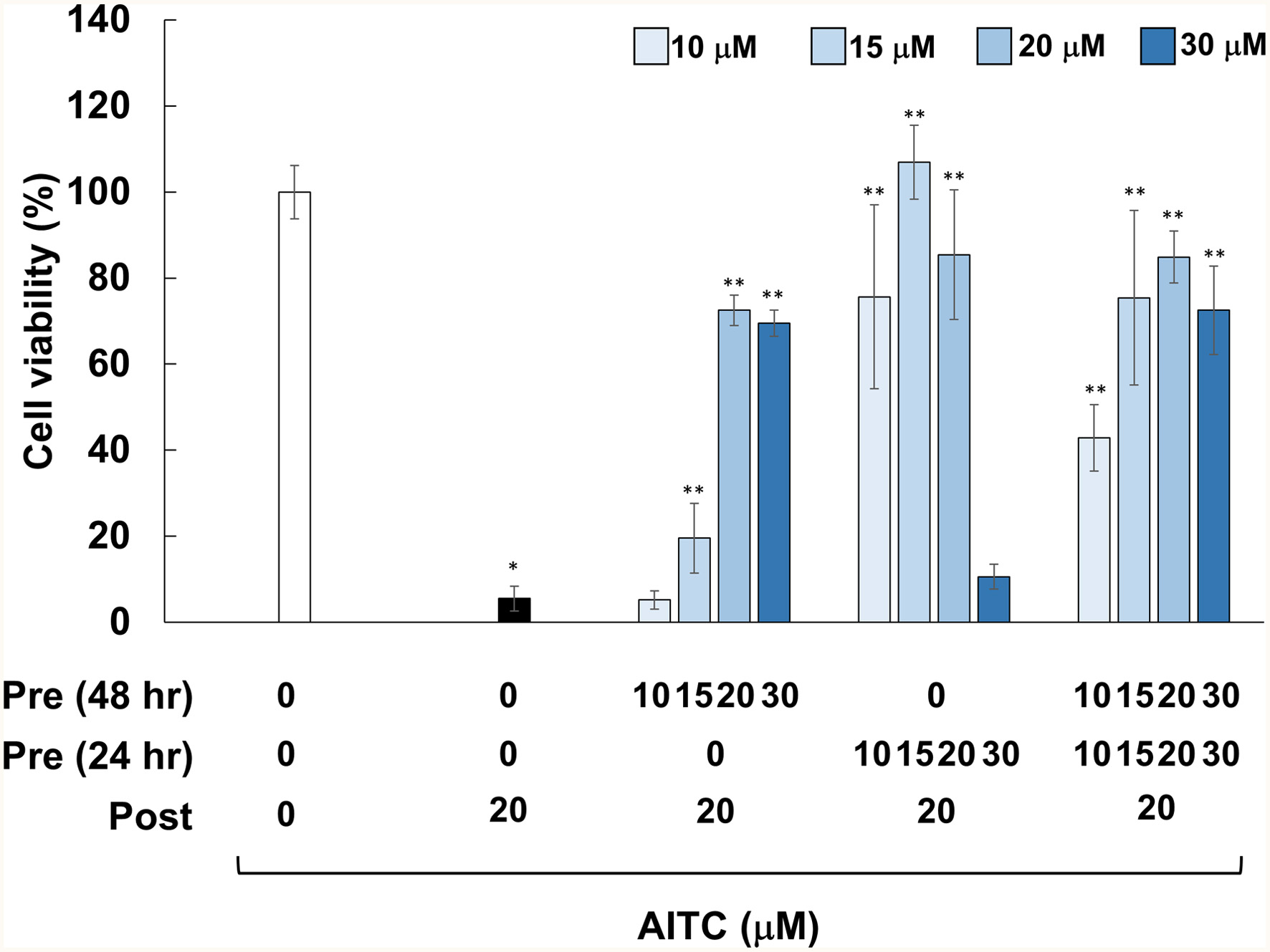 Click for large image | Figure 1. Effects of pretreatments with AITC on cytotoxicity induced by AITC posttreatment. RGM1 cells (2 x 105/200 μL) were exposed to the vehicle (DMSO) or AITC (10–30 μM) for 24 hr. After washing, the cells were treated with the vehicle or AITC (10–30 μM) for another 24 hr, and then incubated with the vehicle or AITC (20 μM) for 24 hr, followed by measurement of cell viability. The data are shown by means ± SD (n = 4), *p < 0.05 versus Control (vehicle treatments at 3 times), **p < 0.05 vs. AITC (20 μM) after vehicle treatment twice (black bar) in Student’s t-test. |
3.2. AITC induces oxidative stress and proteostress
ITCs, including AITC, have been reported to induce oxidative stress that is involved in its neuroprotective effects via Nrf2-dependent mechanisms (Calabrese and Kozumbo, 2021). As shown in Figure 2a, treatment with AITC (10 and 30 μM) for 30 min resulted in significant generation of DCFH-DA-positive cells, a hallmark of ROS generation, as compared with the vehicle control. We also explored their properties to induce proteostress (Suihara et al., 2021; Valentine et al., 2019) because ITCs are known to covalently bind both the cysteine and lysine residues of biological proteins to form protein adducts (Kumar and Sabbioni, 2010). Cellular proteins were extracted before and after AITC treatments using conventional lysis buffer or that containing 2% SDS, and resultant cell lysates were designated as the soluble and the whole proteins, respectively. The amounts of insoluble proteins were estimated by subtracting those of soluble proteins from whole ones. Treatment with AITC (30 μM) for 2 and 4 hr significantly decreased the amounts of soluble proteins and accordingly increased the insoluble proportions (Figure 2b).
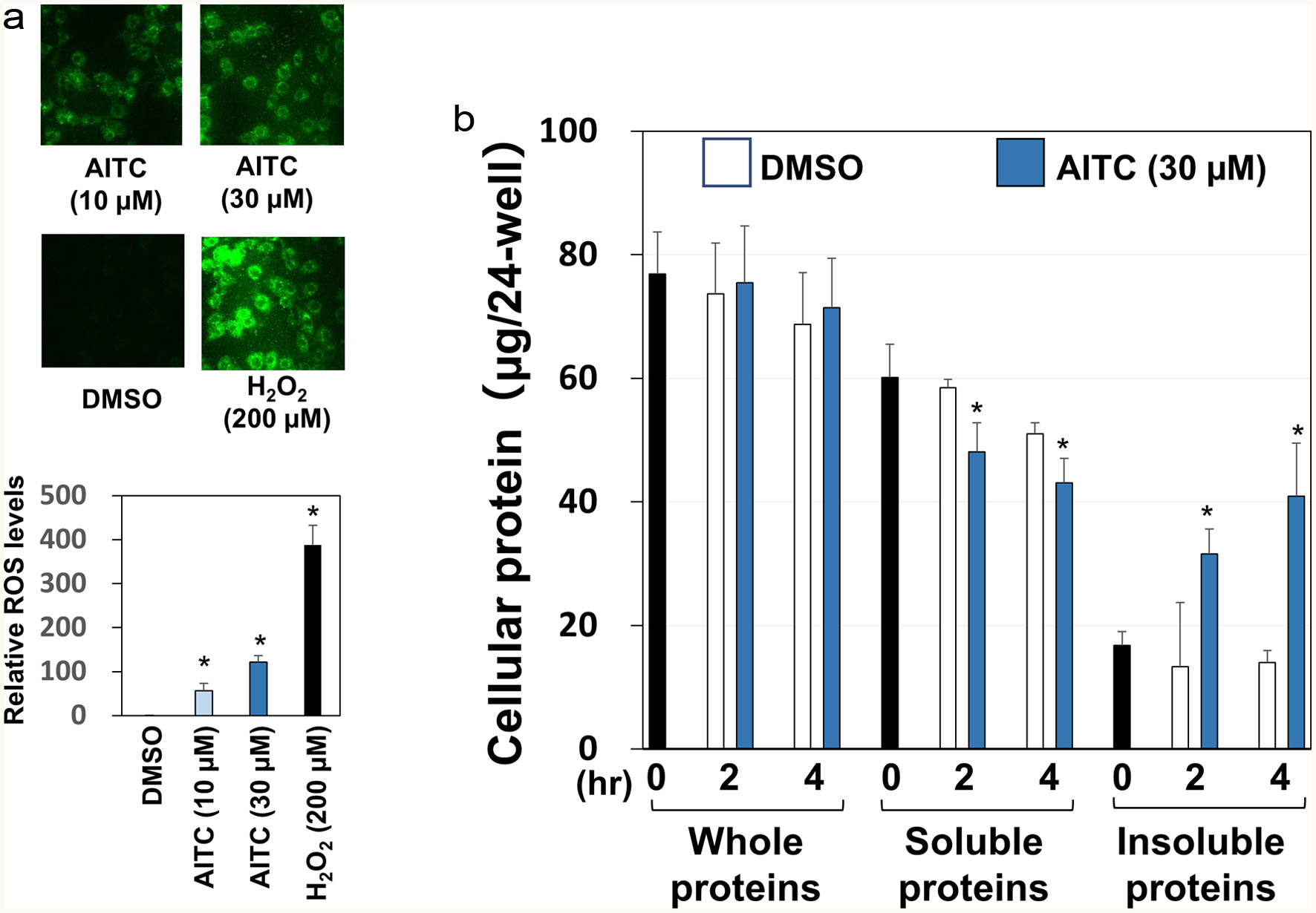 Click for large image | Figure 2. Oxidative stress and proteostress induced by AITC. Panel A, RGM1 cells (5 x 105/mL), seeded on eight consecutive chamber slides, were exposed to DCFH-DA (10 µM) for 30 min. Then, each sample (10 or 30 µM AITC, 200 µM H2O2, 0.5% DMSO) was added and incubated for 30 min. After the chamber was removed, a coverslip was mounted with 50% glycerol/PBS and observed using a confocal laser microscope for detecting intracellular ROS generation. For semi-quantification, RGM1 cells (2 x 105/200 µL) were preincubated on a 96-well black plate (clear bottom) overnight. After washing, DCFH-DA was added and incubated for 30 min. Then, the cells were washed, and each sample was added and incubated at 37°C for 30 min. After washing with PBS, the fluorescence of the media was measured at Ex505 nm and Em525 nm by a microplate reader. Panel B, after RGM1 cells (5 x 105/mL) were precultured on a 24-well plate overnight, each sample (30 µM AITC or 0.5% DMSO) was added and incubated for 0, 2 or 4 hr. Then, the cells were washed and extracted with regular lysis buffer or high detergency lysis buffer (regular lysis buffer with 2% SDS) for soluble and whole protein determination, respectively, which was quantified by the BCA method. The amounts of insoluble proteins were estimated by subtracting those of the soluble proteins from the whole ones. The data are shown by means ± SD (n = 3), *p < 0.05 versus DMSO by Student’s t-test. |
3.3. AITC increased expression of self-defensive genes
We then examined whether AITC increases the mRNA expression levels of the anti-oxidation enzymes (catalase and HO-1), the molecular chaperone (heat shock protein 27, HSP27), and the phase II detoxification enzyme (GSH-S-transferase-α, GST-α). In addition, we compared the effects of single and double treatments on these expressions since double treatments were distinctly cytoprotective (Figure 1). RGM1 cells were pretreated with AITC (0 or 15 μM) for 24 hr, and then exposed to another AITC treatment (0 or 20 μM) for 6 hr, which was followed by RT2-PCR. Interestingly, the combination of pre- and posttreatment with AITC significantly upregulated the expressions of catalase and GST-α, whereas each treatment was ineffective (Figure 3a and d). In addition, while posttreatment alone significantly increased both HO-1 and HSP27 expressions, double treatments were significantly more inducible (Figure 3b and c).
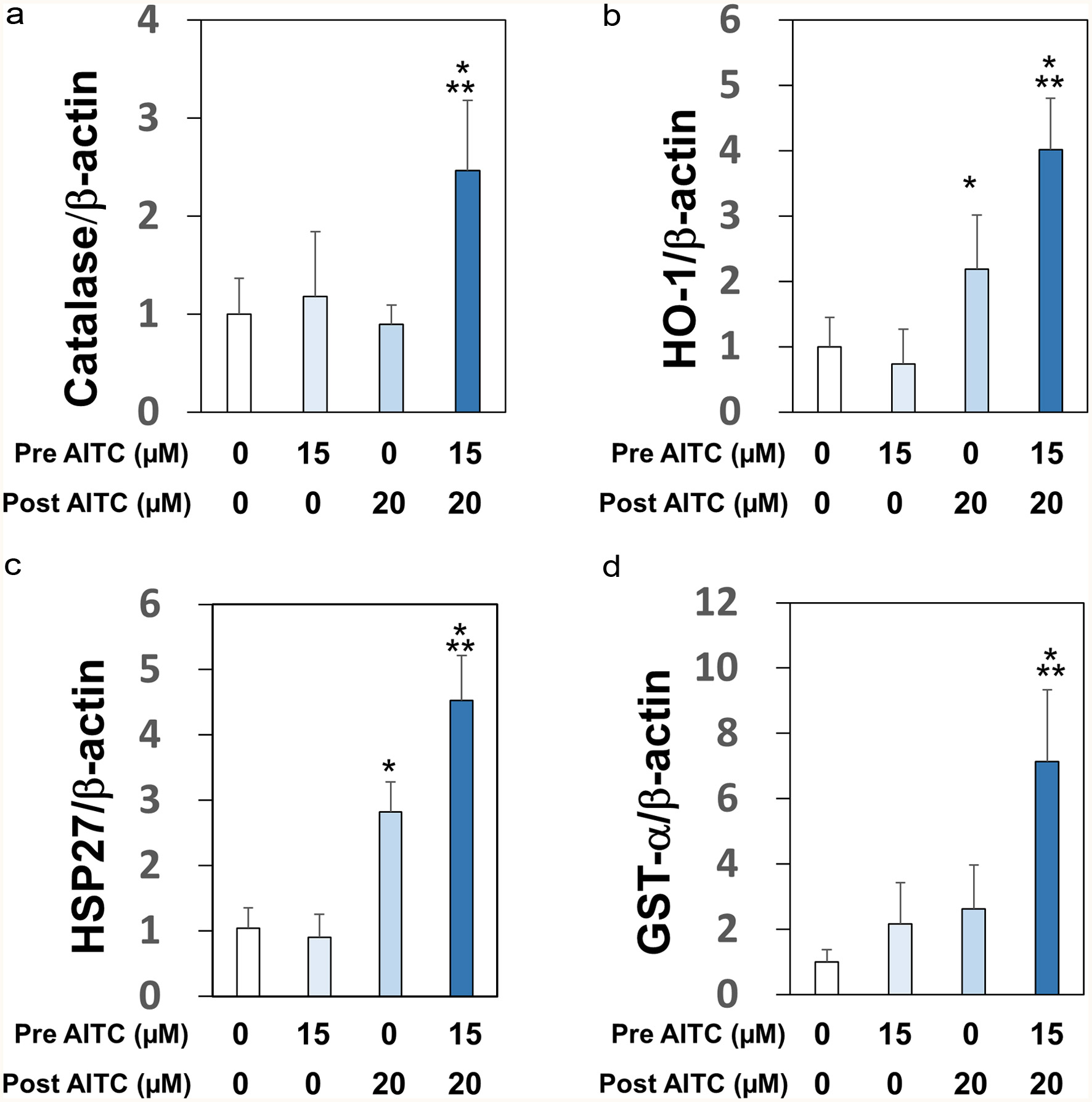 Click for large image | Figure 3. Upregulations of mRNA expression levels of self-defensive enzymes. RGM1 cells (1 x 106/mL) preincubated on a 12-well plate overnight were exposed to AITC (0 or 15 μM), and incubated for 24 hr. After another washing, the cells were exposed to AITC (0 or 20 µM). cDNA was synthesized using total RNA (1 µg). PCR was performed with a sense- and antisense-primer for catalase (Panel A), HO-1 (Panel B), HSP27 (Panel C), GST-α (Panel D), and β-actin (internal standard). The data are shown by means ± SD (n = 4), *p < 0.05 versus vehicle, **p < 0.05 versus post AITC (20 μM) by Student’s t-test. |
3.4. AITC confers resistance to low-pH stress
Subsequently, we investigated whether AITC provides resistance capacity against low-pH stress conditions because RGM1 cells are derived from the normal gastric mucosa, where they are exposed to acidic pH in pathological conditions, such as gastritis. After being pretreated with AITC (0–15 μM) for 1 hr, RGM1 cells were incubated in the media of pH 4.6, pH 7.4 or pH 9.8 for 1 hr. Then, the cells were recovered in the media of pH 7.4 for 24 hr, followed by viability determination. As shown in Figure 4a, AITC (10 and 15 μM) significantly suppressed the decrease of cell viability in acidic media. Intriguingly, however, AITC did not protect cells from alkaline pH stress conditions, and rather, it concentration-dependently decreased cell viability (Figure 4b).
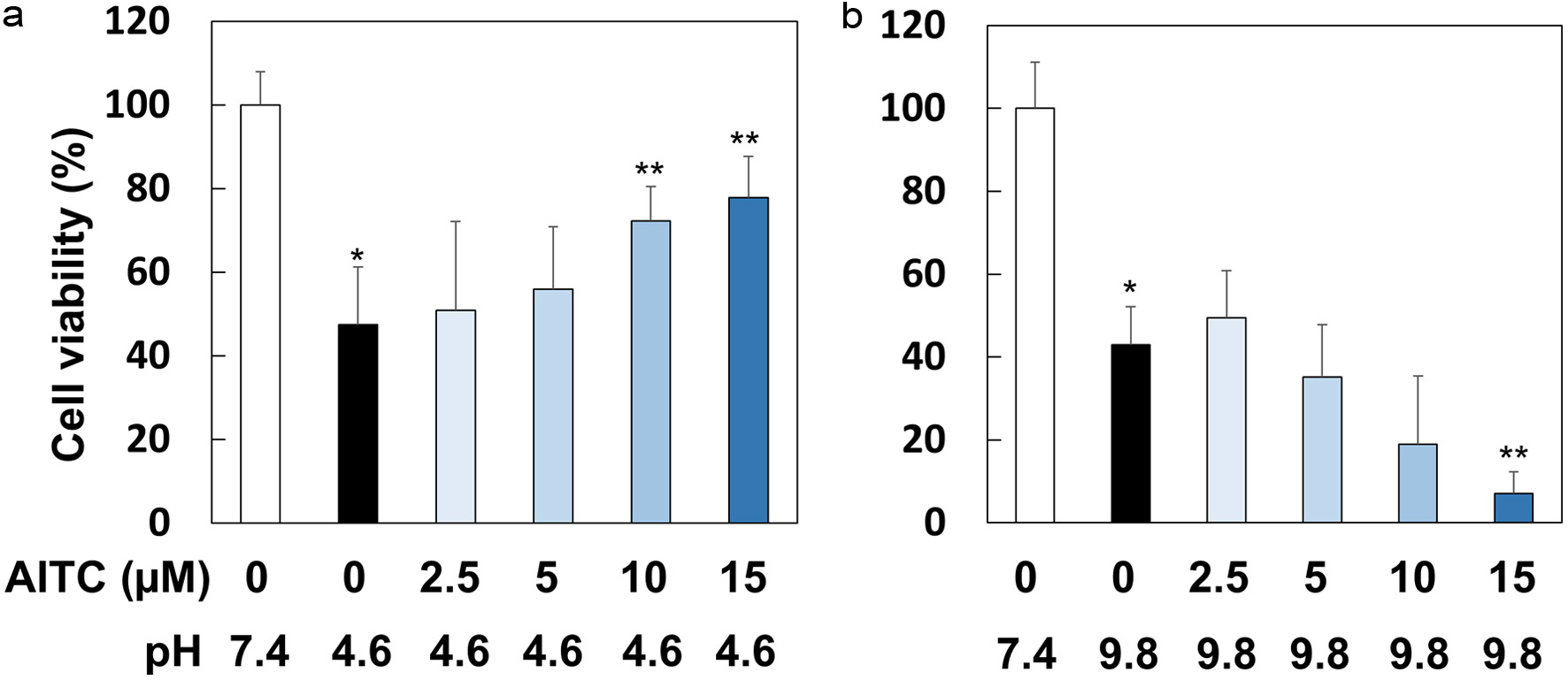 Click for large image | Figure 4. AITC protected RGM1 cells from acidic but not alkaline stress conditions. RGM1 cells (2 x 105/200 μL) were preincubated on 96-well plate overnight. After washing, they were pretreated with AITC (0, 2.5, 5, 10 and 15 μM) for 1 hr, and then the media were replaced by FBS-free DMEM, in which pH was adjusted to 7.4, 4.6, or 9.8, followed by incubation for 1 hr. Then, the media were replaced to FBS-free DMEM (pH 7.4) followed by a 24 hr-incubation. The data are shown by means ± SD (n = 6–8), *p < 0.01 versus pH 7.4, **p < 0.05 versus AITC (0 µM), pH 4.6 or pH 9.8 by Student’s t-test. |
3.5. Effects of AITC on the extracellular and intracellular pH
Given the aforementioned results, we measured both the extra- and intracellular pH before and after AITC exposure. After being treated with AITC (0 or 15 μM) in media of pH 7.4 for 1 hr, RGM1 cells were incubated in pH 4.6 for 1 hr. Then, the cells were recovered in pH 7.4 for 24 hr. Both extra- and intracellular pH were measured 1 hr after AITC exposure, 1 hr after replacing with pH 4.6 media, and 24 hr after recovery. As a result, AITC did not affect the extracellular pH in both acidic and neutral conditions (Figure 5a). For intracellular pH measurement, BCECF-AM, which is hydrolyzed to the membrane impermeable BCECF (Garrido et al., 1996), was used as a pH indicator. Interestingly, while not having affected intracellular pH in neutral media, pretreatment with AITC significantly suppressed pH reduction, and this tendency was maintained 24 hr after recovery. Meanwhile, Na+/H+ exchanger (NHE) has been reported to play an essential role in the adaptation against acidic stress conditions in RGM1 cells (Furukawa and Okabe, 1997). In addition, phosphatidylinositol-3 kinase (PI3K) may be responsible for the activation of NHE (Furukawa et al., 1999). Therefore, we attempted to examine their roles in the mechanisms underlying the protective activity of AITC against acidic stress conditions using pharmacological inhibitors. As shown in Figure 5b, both LY294002 (10 μM, PI3K inhibitor) and amiloride (100 μM, NHE inhibitor) significantly disrupted the protective effects of AITC.
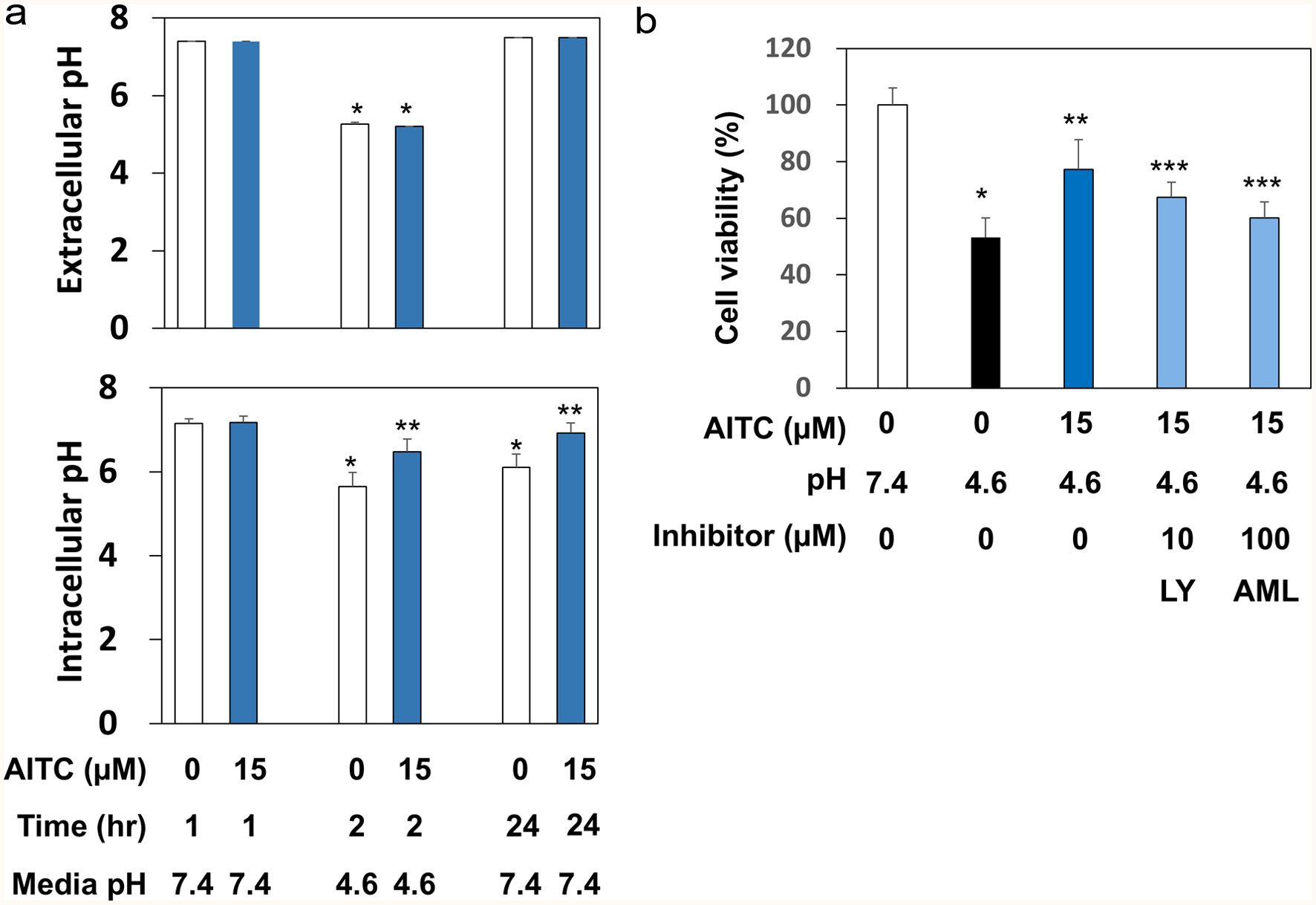 Click for large image | Figure 5. AITC regulated the intracellular pH possibly by targeting PI3K and NHE. Panel A, for extracellular pH measurement, RGM1 cells (1 x 106/mL) were preincubated on a 24-well plate overnight. After washing, the cells were pretreated with AITC (0 or 15 μM) for 1 hr. Then, the media were replaced by FBS-free DMEM, in which the pH was adjusted to 7.4, 4.6, or 9.8. After incubation for 1 hr, the pH of medium was measured. For intracellular pH determination, RGM1 cells (2 x 105/200 μL) were preincubated on a 96-well black plate (clear bottom) overnight. After washing, FBS-free DMEM containing BCECF-AM (final concentration: 5 μM) was added and the cell were incubated for 30 min. After washing, the fluorescence of the media was measured at Ex490 and Em535 nm. The data are shown by means ± SD (n = 4), *p < 0.05 versus pH 7.4, 1 hr, **p < 0.05 versus AITC (0 µM), 2 hr or 24 hr by Student’s t-test. Panel B, RGM1 cells (2 x 105/200 μL) were preincubated on a 96-well plate overnight. After washing, the cells were pretreated with the vehicle, LY294002 (10 μM, PI3K inhibitor), or amiloride (100 μM, NHE inhibitor) for 30 min and then exposed to AITC (15 μM) for 1 hr. After washing, the media were replaced by FBS-free DMEM, in which the pH was adjusted to 4.6, and the cells were incubated for 1 hr. After washing the cells, the media were replaced with FBS-free DMEM (pH 7.4) followed by a 24 hr-incubation. Cell viability was determined as described above. The data are shown by means ± SD (n = 4), *p < 0.05 versus pH 7.4, **p < 0.05 versus AITC (0 µM), pH 4.6, ***p < 0.05 versus AITC (15 µM), pH 4.6 by Student’s t-test. LY, LY294002 (PI3K inhibitor); AML, amiloride (NHE inhibitor). |
| 4. Discussion | ▴Top |
AITC has long been anticipated to mitigate chemical stress conditions by upregulating phase II detoxification enzymes (Munday et al., 2002). For instance, this phytochemical may contribute to the reduction of toxicity derived from mycotoxins in feed and food (Adegbeye et al., 2020). However, to the best of our knowledge, there has been no study that examined whether pretreatment with AITC can protect against toxicity induced by AITC itself. As shown in Figure 1, pretreatments with AITC in most of the experimental conditions dramatically protected RGM1 gastric normal epithelial cells from cytotoxicity induced by posttreatment with AITC. Pretreatment with AITC 24 hr before posttreatment was more protective than that with 48 hr, suggesting that cytoprotective effects may have decayed within 24 hr after pretreatment. In addition, biphasic responses, a hallmark of hormesis, are noticeable for each pretreatment because their cytoprotective effects reached peaks at a concentration of 15 or 20 μM, and then declined or abolished at 30 μM (Figure 1). Meanwhile, it may be confusing that, while posttreatment with AITC alone at a concentration of 20 μM was highly cytotoxic, pretreatments at the same concentration was not cytotoxic, and rather apparently protective. These data were well reproduced, and the reason for these puzzling results could be because serum starvation and the lack of chemical stimuli during 48 hr before AITC posttreatment reduced cellular defensive ability.
ITCs evoked pro-oxidative responses in biological systems (Valgimigli et al., 2009; Sestili et al., 2015), which corresponds with our present results (Figure 2a). In addition, electrophilic ITCs covalently bind cysteine and lysine residues to form cellular protein adducts (29,30), which is described as proteostress. In accordance with these findings, several electrophilic phytochemicals and their metabolites, including curcumin (Valentine et al., 2019), zerumbone (Ohnishi et al., 2013), and the o-quinone metabolite of (-)-epigallocatechin-3-O-gallate (Suihara et al., 2021), have been demonstrated to induce proteostress. Taken together, our results showing the increase of insoluble cellular proteins by AITC (Figure 2b) may be associated with the formation of protein adducts.
In response to the oxidative stress and proteostress induced by AITC, RGM1 cells are forced to activate self-defensive machinery for homeostasis and survival. In fact, treatments with AITC upregulated mRNA expression levels of anti-oxidative enzymes (catalase and HO-1), detoxification enzyme (GST-α), and molecular chaperone (HSP27) (Figure 3). It is important to indicate that double treatments are more effective than each single one. Although the mechanistic reason(s) for these additive effects remains to be elucidated, it is worth noting that increased histone acetylation promotes the formation of euchromatin (uncompacted form of chromatin) and thereby rapidly facilitates protective gene expression under stressful conditions (Gu et al., 2013). Interestingly, pretreatment with trichostatin A, a euchromatin formation inducer (Williams et al., 2011), significantly protected RGM1 cells from AITC-induced cytotoxicity (data not shown). In addition, AITC has been reported to increase histone acetylation to promote euchromatin formation in several types of cultured cells (Lea et al., 2001; Mitsiogianni et al., 2020). Collectively, we hypothesized that AITC may have promoted euchromatin formation via epigenetic mechanisms and thereby increased the capacity for stress adaptation, which is currently under investigation.
We attempted to examine whether AITC exhibits cross-resistance, which has been reported to be associated with epigenetic mechanisms (Horowitz et al., 2017). Notably, AITC protected RGM1 cells from low-pH, but not high-pH, stress conditions by regulating the intracellular pH (Figures 4 and 5). Previous reports have shown that the epidermal growth factor protected cells from acidic stress conditions via PI3K for activation of NHE (Furukawa et al., 1997, 1999), a membrane-bound Na+/H+ exchanger. Our present results with pharmacological inhibitors (Figure 5) suggest the involvement of both PI3K and NHE in the regulation of acidic pH. Importantly, several ITCs, including AITC, have been shown to activate PI3K in many types of cells (Yang et al., 2020; Du et al., 2024; Jakubíková, et al., 2005; Liu et al., 2017). Meanwhile, our present results may contribute to the understanding of the mechanisms underlying the preventive effects of an ITC on gastritis (Yanaka, 2006) where oxidative and acidic stresses become dominant.
Upregulation of anti-oxidation and detoxification enzymes, together with molecular chaperones, by AITC (Figure 3) is quite reasonable because it is a xenobiotic and has pro-oxidative and proteostress properties (Figure 2). On the other hand, the acquisition mechanisms of low-pH stress resistance may not be directly associated with the stress-inducing properties of AITC. However, it can be described as cross-resistance since AITC did not affect the intracellular pH in a normal culture condition (Figure 5a). Delgado-Jarana et al. have previously reported cross-resistance, in which hyperosmotic stress conditions conferred tolerance against oxidative stress in yeasts (Delgado-Jarana et al., 2006). Also, Spitz and Li have shown that heat shock increased the adaptive capacity against oxidative stress in CHO cells (Spitz et al., 1987). However, as far as we know, this study is the first to demonstrate cross-resistance by a phytochemical. Further studies that aim to uncover other types of cross-resistance conferred by phytochemicals are warranted.
Adaptation is the fundamental mechanism provided with every organism or homeostasis and survival. In principle, continuous exposures to xenobiotics at a tolerable level frequently result in decay of responsiveness. For example, repetitive exposure to anti-cancer drugs often lead to reduced therapeutic effects, and similar phenomena are known for antibiotics, steroids, and insulin, etc. Likewise, unfavored taste and smell of vegetables, most of which are derived from phytochemicals, gradually become bearable with increasing age. For instance, tolerance to capsaicin, a pungent in chill pepper that infants and children tend to dislike, is associated with the release of β-endorphin to induce reward effects (Bach et al., 1995). Such adaptation generally occurs after repetitive and long-time ingestion of them. ITCs are the major sharp and pungent flavors of the cruciferous family of plants, although their effects on β-endorphin release remain to be clarified. In any case, the tolerance to AITC in cruciferous vegetables, including Wasabi, might also be related to the increased detoxification ability, which is presumably acquired through its continuous ingestion. Our present data (Figure 3d) showing marked expression levels of GST-α by double AITC treatments may support this hypothesis.
| 5. Conclusions | ▴Top |
We have demonstrated that pretreatments with AITC notably protected RGM1 cells from cytotoxicity induced by its posttreatment. The underlying protective mechanisms may involve the increased expression of self-defensive enzymes, which were derived from the oxidative stress and proteostress induced by AITC. Increased activities of defensive enzymes derived from continuous ingestion of phytochemicals may strengthen our ability to detoxify environmental toxins, and such an adaptive situation has been coined as ‘Chemical training’ (Murakami, 2024).
Acknowledgments
We thank Prof. Mitsugu Akagawa (University of Tokushima) for providing RGM1 rat gastric mucosa cells. This work was supported in part by Grants-in-Aid for Scientific Research (C) (No. 22K05495, to A.M.) from the Japan Society for the Promotion of Science.
Data availability
The data that support the findings of this study are available from the corresponding author (A.M.) upon reasonable request.
Conflict of interest
There are no conflicts to declare.
Author contributions
A.M. designed and directed this study. S.K. and A.M. performed the experiments and analyzed the data. S.K., A.I. and A.M. discussed the experimental results and contributed to the preparation of the final version of this manuscript.
| References | ▴Top |