| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 30, June 2025, pages 33-39
Elicitor-induced enhancement of bioactive content and α-amylase inhibition in cluster bean sprouts
Komal Solanki, Rushna Mansuri, Krutika Saurabh Abhyankar*
Navrachana University, Division of Biomedical and Life Sciences, School of Science, Vasna-Bhayli Road, 391410 and Vadodara, Gujarat- India
*Corresponding author: Krutika Saurabh Abhyankar, Navrachana University, Division of Biomedical and Life Sciences, School of Science, Vasna-Bhayli Road, 391410 and Vadodara, Gujarat- India. E-mail: krutikaa@nuv.ac.in
DOI: 10.26599/JFB.2025.95030412
Received: May 2, 2025
Revised received & accepted: June 10, 2025
| Abstract | ▴Top |
This study investigates the effects of elicitor treatments on bioactive compounds and α-amylase inhibitory activity in Cyamopsis tetragonoloba sprouts. Elicitors like glutamic acid, ascorbic acid, chitosan, and their combination were used on seeds to check their effects on germination, radicle length, phytochemical content, and antioxidant activity. Glutamic acid and a combination of 50 mg/kg low-molecular-weight chitosan with 5 mM glutamic acid significantly increased protein, phenol, and ascorbic acid contents. The highest DPPH radical scavenging activity (15.57%) was observed in sprouts treated with 50 mg/kg chitosan and 5 mM glutamic acid, while no notable ferric reducing antioxidant power (FRAP) activity was recorded. Elicited sprouts showed significantly higher α-amylase inhibitory activity than non-germinated seeds, with the chitosan-glutamic acid combination achieving the highest inhibition (53.92%). These findings underscore the potential of elicitors to enhance the nutritional and functional properties of cluster bean sprouts, warranting further research into specific phenolic compounds and other bioactive.
Keywords: α-Amylase inhibitory activity; Bioactive compounds; Cluster bean; Elicitors; Germination; Glutamic acid
| 1. Introduction | ▴Top |
Legumes such as beans, lentils, and peas are prized worldwide for their nutrient-dense composition, offering starch, dietary fiber, protein, healthy fats, and essential minerals (Lin and Lai 2006). However, antinutritional factors like phytates, tannins, and lectins can hinder nutrient absorption. Germination, a straightforward bioprocessing method, reduces these compounds by enzymatically breaking them down, enhancing nutrient bioavailability, digestibility, and taste (Urbano et al., 2005). It also decreases antinutritional factors while boosting bioactive compounds, which contribute to antioxidants, anti-inflammatory, and other health-promoting properties in legume sprouts (Gan et al., 2017).
To further increase bioactive compound levels, elicitors—compounds that activate plant defense mechanisms—are applied during germination. Elicitors are either biotic (e.g., chitosan, glutamic acid, salicylic acid) or abiotic (e.g., NaCl, light, temperature stress) and stimulate phytochemical production by mimicking stress responses (Baenas et al., 2014). According to Tang et al. (2021), chitosan elicitor was found to have a large accumulation of vitamin C, total phenolic, and total flavonoid content in the soybean sprouts (Tang et al., 2021). Treatment with glutamic acid has been shown to speed up sprouting because it offers an extra source of nitrogen for effective embryonic growth (Ampofo and Ngadi 2021). Using ascorbic acid as an elicitor, sprouted kidney beans, fava beans, and peas suggested de NOVO synthesis of phenolic chemicals. It is generally known that ascorbic acid promotes the phenyl propanol and pentose phosphate pathways, which in turn causes the production of phenolic compounds in legumes (Uchegbu and Amulu 2015). Biotic elicitors include phytohormones, amino acids, and polysaccharides, while abiotic ones involve salts, ultrasound, or high pressure (Liu et al., 2019). These enhance seedling growth and the synthesis of compounds like flavonoids, phenolic acids, and amino acids, which show α-amylase inhibitory activity (Limón et al., 2014; Teoh and Das 2018). Therefore, considering the response exerted by the elicitors on the seeds during germination, ascorbic acid, glutamic acid and combination of chitosan with glutamic and lactic acid were selected for the present study.
With the rising global prevalence of diabetes, there is an urgent need for effective therapeutic strategies. Guidelines from the American Diabetes Association and the European Association for the Study of Diabetes recommend α-amylase and α-glucosidase inhibitors to manage blood glucose levels by reducing carbohydrate absorption (Yan et al., 2019). This study focuses on Cyamopsis tetragonoloba (cluster bean), a nutrient-rich legume from the Fabaceae family, containing vitamins, amino acids, flavonoids, and phenolic compounds (Khare 2004). While cluster bean extracts have shown various health benefits in prior research, the effects of germination and elicitation on its sprouts are underexplored. This study examines the phytochemical composition, antioxidant activity, and in vitro α-amylase inhibitory activity of germinated cluster bean seeds treated with various elicitors, aiming to highlight their potential as functional foods with enhanced nutritional and bioactive properties.
| 2. Materials and methods | ▴Top |
2.1. Seed procurement and cleaning
Seeds of Cyamopsis tetragonoloba (L.) Taub (Variety - Gujarat Vegetable Guar 11-GVG 11_Anand Bahar) were obtained from the Main Vegetable Research Station, Anand Agricultural University, Anand, Gujarat, India (Latitude 22.5523° N, Longitude 72.9240° E) in September 2023. Upon collection, seeds were meticulously cleaned to remove debris and stored in an airtight container.
2.2. Chemicals
Chemicals and reagents used in this study were sourced as follows: α-amylase porcine pancreas (5 U/mg), ascorbic acid, chitosan (Low MW), 3,5-dinitrosalicylic acid (DNSA), gallic acid, glutamic acid, and 2,4,6-tripyridyl-S-triazine (TPTZ)were purchased from SRL Pvt Ltd India. α, α-diphenyl-β-picrylhydrazyl (DPPH)and α-amylase from malt (RM 638) were procured from Himedia Pvt Ltd India. All other chemicals were of analytical grade.
2.3. Elicitor treatments
Elicitor concentrations were selected based on the study by Burguieres et al., (2007). The elicitors employed included 500 µmol/L ascorbic acid, 5 mM glutamic acid, a combination of 50 mg/kg LMW chitosan with 5 mM glutamic acid, and a combination of 50 mg/kg LMW chitosan with 5 mM lactic acid. Distilled water (DW) was used as the control. Elicitor solutions were freshly prepared in DW and applied daily by spraying onto seeds during germination.
2.4. Germination of cluster bean seeds
The germination with elicitors was carried out as described by Limón et al., (2014). The cluster bean seeds were initially washed with DW to remove surface impurities and subsequently immersed in a 0.05% sodium hypochlorite solution for 30 minutes for disinfection. After thoroughly rinsing with tap water to neutralize pH and eliminate residual sodium hypochlorite, the seeds were soaked in DW for 6 hours with periodic stirring. Hydrated seeds were then distributed into five trays lined with filter paper for elicitor treatment. Elicitor solutions were applied daily, and germination was conducted at 37 °C in a hot air oven for 72 hours. Radicle length and germination rates were recorded, followed by drying of sprouts at 40 ± 2 °C for 48 hours. Dried sprouts were ground into fine powder and stored at −20 °C until further use.
2.4.1. Germination percentage
The germination percentage (%) was determined by dividing the number of germinated seeds by the total number of seeds started, multiplied by 100, using the formula:
This calculation was performed for each respective tray containing the seeds treated with different elicitors and the control group.
2.4.2. Aqueous sample preparation
One gram of powdered sprouts from each group (elicited and control) was suspended in 10 mL of DW, vortexed for 10 minutes, and centrifuged at 1,391 g for 10 minutes. The resulting supernatant was stored at −20 °C for subsequent analyses.
2.5. Phytochemical analysis
The protein content was determined using BSA as a standard and expressed as mg/g of BSA equivalents following Lowry et al., (1951). A known amount of sample was added to the alkaline copper solution, incubated, and then folin reagent was added to the test tube in the dark and incubated for half an hour. The blue color absorbance was measured at 660 nm. Phenol content was measured with gallic acid as a standard and reported as mg/g of gallic acid equivalents as described by Thimmaiah (1999). A known amount of sample was combined with FCR reagent and incubated for three minutes at room temperature. Twenty percent sodium carbonate was added in tubes and kept in a boiling water bath for one minute. The absorbance of the reaction mixture was measured at 650 nm after the tubes had cooled. Total ascorbic acid content was quantified following the method of Schaffert and Kingsley (1955). The seed samples were treated with thiourea and DNPH reagent, followed by incubation in a boiling water bath at 100 °C for 15 minutes and cooled under tap water. 85% chilled sulphuric acid was added in the treated sample and further incubated for 15 minutes in an ice bath. The absorbance of the reaction mixture was measured at 515 nm.
2.6. Antioxidant activity
Antioxidant activity was assessed using DPPH radical scavenging and FRAP assays. The DPPH test was carried out in accordance with Brand-Williams et al. (1995). The seeds aqueous extracts were incubated with DPPH solution (0.1 mM in methanol) in dark for 30 minutes at 37 °C and absorbance was measured at 517 nm against blank. Using the following formula, the scavenging activity was determined:
The FRAP assay used ascorbic acid as the reference standard and was conducted according to Benzie and Strain (1996) procedure. Aqueous samples were mixed with FRAP solution (containing TPTZ, FeCl3 and acetate buffer) incubated for 30 min at 37 °C in the dark. The absorbance readings were taken at 593 nm wavelength.
2.7. α-Amylase inhibitory activity
The α-amylase inhibition assay was conducted following a modified method based on Nair et al., (2013). Triplicate samples were incubated with 100 µl of α-amylase from porcine pancreas (5 U/mg) or α-amylase from malt source and incubated at 37 °C for 10 minutes. Subsequently, 50 µl of 1% soluble starch solution was added to each sample and further incubated for 10 minutes at 37 °C. The reaction was terminated by adding 100 µl of 96 mM DNSA reagent and boiling the mixture at 100 °C for 5 minutes, followed by cooling to room temperature. Diluted samples were then analyzed for absorbance at 540 nm using a spectrophotometer.
The α-amylase inhibitory activity (%) of each sample was calculated using the formula:
2.8. Statistical analysis
Statistical evaluations were conducted using one-way ANOVA followed by Tukey’s test using SPSS (version 16.0). Results are presented as Mean ± SEM (n = 3), and statistical significance was set at p < 0.05.
| 3. Results and discussion | ▴Top |
3.1. Germination percentage and radicle length
All elicited groups of Cyamopsis tetragonoloba seeds exhibited germination percentages ranging from 47% to 100%, with the highest rate (100%) observed in seeds treated with a combination of chitosan and lactic acid or glutamic acid combined with chitosan (Figure 1). Radicle lengths in elicited samples ranged from 3.9 cm to 5.8 cm, with the longest (5.8 cm) recorded in seeds treated with 50 ppm chitosan and 5 mM lactic acid, and the shortest (3.2 cm) in distilled water (DW)-treated seeds (Figure 2). Germination, a critical developmental stage, involves biochemical changes driven by endogenous enzyme activation, enhancing nutritional quality compared to non-germinated seeds (Nkhata et al., 2018). The 100% germination rate in chitosan-lactic acid and chitosan-glutamic acid treatments suggest these elicitors significantly boost germination efficiency, likely by stimulating metabolic activity. The increased radicle length, particularly with chitosan and lactic acid, indicates improved early seedling growth, consistent with Tang et al., (2021), who reported enhanced nutritional and physiological functions in soybean sprouts treated with 0.01% chitooligosaccharide, and Zayed et al., (2017), who demonstrated nano-chitosan’s role in promoting germination under salt stress in bean plants.
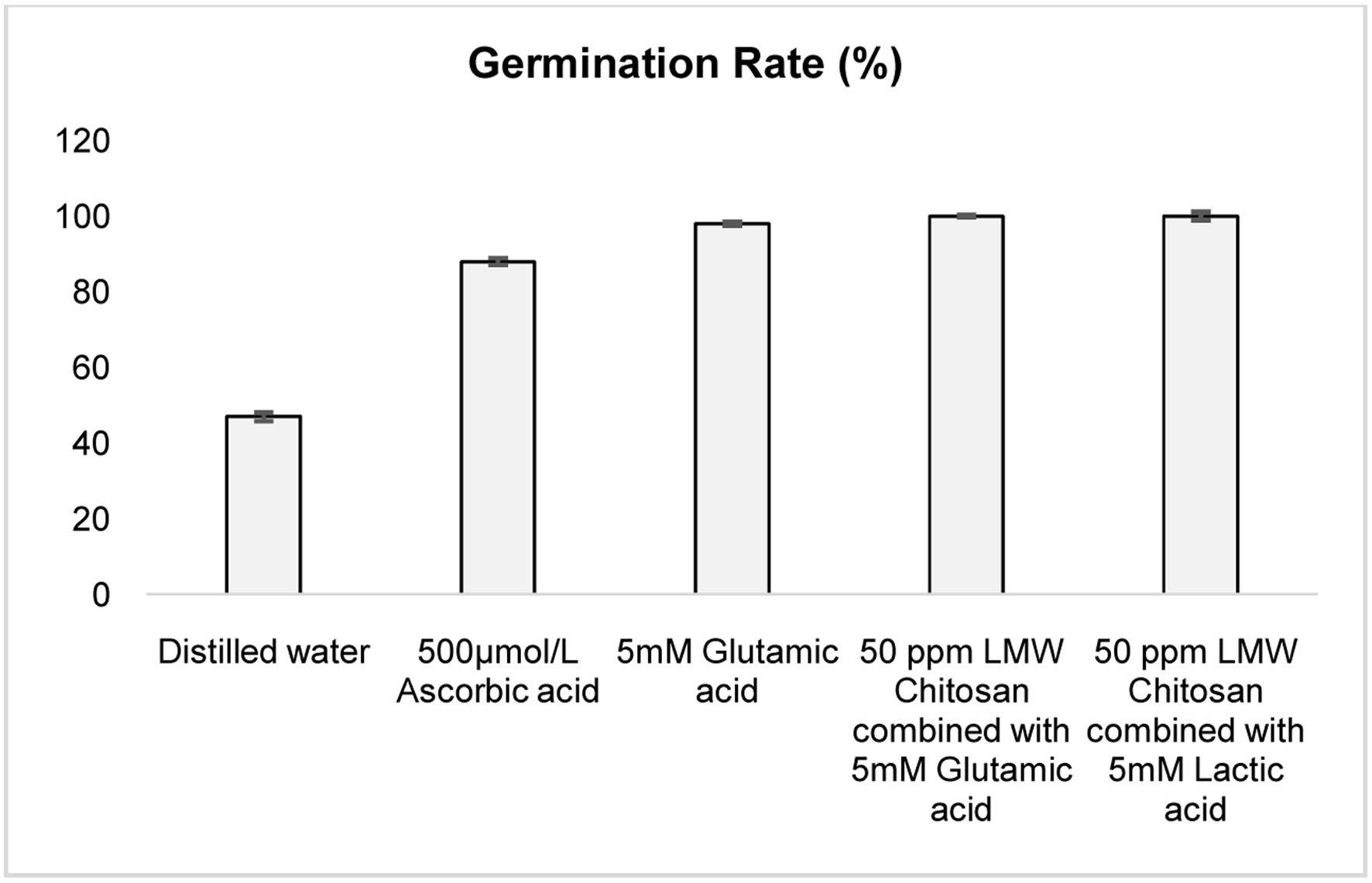 Click for large image | Figure 1. Germination percentage of cluster bean seeds following various chemical elicitor treatments. All the values are expressed as Mean ± SEM (n = 3). |
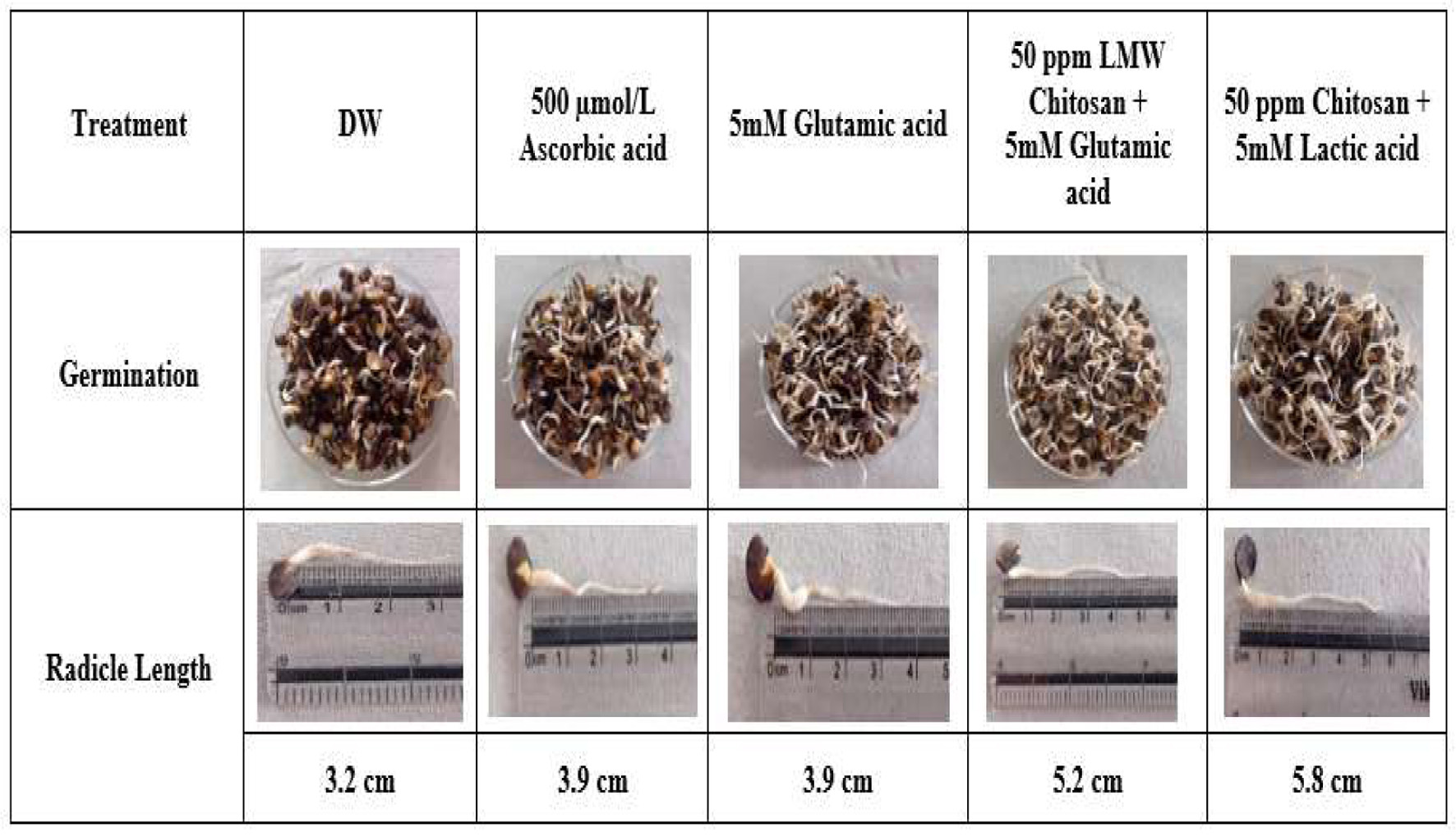 Click for large image | Figure 2. Germination and radicle length of cluster bean sprouts after treatment with various elicitors. |
3.2. Phytochemical analysis
Protein content significantly increased in all elicited groups compared to non-germinated seeds and the DW control (Table 1). Glutamic acid-treated seeds exhibited the highest protein content (285.94 ± 3.763 mg/g), followed by glutamic acid combined with chitosan (266.487 ± 1.675 mg/g), with statistically similar values (p < 0.05). DW and ascorbic acid-treated seeds showed comparable protein contents, while non-germinated seeds had the lowest (115.173 ± 0.7185 mg/g). Phenol content ranged from 12.12 to 15.95 mg/g gallic acid equivalent, with the highest in glutamic acid-treated seeds (15.95 ± 0.15 mg/g) and glutamic acid combined with chitosan (14.7 ± 0.34 mg/g) (Table 2). Statistically similar phenol contents were observed in non-germinated, DW, ascorbic acid, and lactic acid with chitosan-treated seeds. Ascorbic acid content was statistically similar across non-germinated seeds (10.58 ± 0.13 mg/g), glutamic acid with chitosan (11.16 ± 0.60 mg/g), and lactic acid with chitosan (10.63 ± 0.28 mg/g), with the lowest in control and glutamic acid-treated seeds (Table 1).
 Click to view | Table 1. Phytochemical Analysis of Non-Germinated Cluster Bean Seeds and Germinated Seeds Treated with DW and Various Elicitors for 72 Hours |
 Click to view | Table 2. Pearson’s Correlation Coefficients of Antioxidant and Phytochemical Content in Cluster Bean Sprouts |
Germination enhances legume nutritional quality by altering bioactive compound profiles (López-Martínez et al., 2017). The significant protein increase, particularly with glutamic acid, likely results from endogenous protease activation, hydrolyzing storage proteins into soluble forms, and new protein synthesis during germination (Chen et al., 2017). This aligns with Świeca et al., (2014), who reported enhanced protein in chitosan-treated lentil sprouts, and Oh (2003), who observed similar effects in brown rice. The elevated phenol content in glutamic acid-treated seeds correlates with findings by Dueñas et al., (2015) and Ampofo and Ngadi (2021), who noted increased phenolic composition in elicited kidney and common bean sprouts, respectively. The rise in total phenol content can be linked to the activation of endogenous hydrolases, including phenylalanine ammonia-lyase in phenylpropanoid pathway, which is a crucial enzyme in phenol synthesis (Mao et al., 2024). While a detailed profile of specific phenolic compounds was not analyzed in this study, the focus on total phenol content is justified, as it is a widely accepted indicator of phenolic accumulation during germination and elicitation (Świeca et al., 2014; Pérez-Ramírez et al., 2018). Future studies could employ HPLC to characterize specific phenolic classes, such as flavonoids or isoflavonoids, to further elucidate elicitation effects. The limited variation in ascorbic acid content suggests minimal elicitor impact, possibly due to insufficient germination duration, as longer sprouting periods elevate ascorbic acid in legumes (Uppal and Bains, 2012).
3.3. Antioxidant activity
DPPH inhibitory activity of sprouted samples (20 mg/ml) ranged from 4.83% to 15.57%, with the highest in glutamic acid (15.17 ± 0.78%) and glutamic acid combined with chitosan (15.57 ± 1.56%) treatments, showing statistically similar values (p < 0.05) (Figure 3). No significant improvement in ferric reducing antioxidant power (FRAP) was observed across treatments, with the highest activity in non-germinated seeds (68.08 ± 0.1 mg/g ascorbic acid equivalent) and the lowest in chitosan and lactic acid-treated seeds (32.49 ± 0.86 mg/g) (Figure 3). Elicitor application enhances antioxidant activity and secondary metabolite production, improving sprout health benefits (Szulc et al., 2024). The high DPPH activity in glutamic acid and chitosan-treated seeds, correlating with elevated protein and phenol contents (p < 0.01; p < 0.05), suggests these compounds drive antioxidant properties, as supported by Solanki et al., (2024) in Lab Lab sprouts. The lack of FRAP improvement, with higher activity in non-germinated seeds, may reflect metabolism of specific phenolic compounds during sprouting (Dueñas et al., 2015). The absence of correlation between phenol or ascorbic acid content and FRAP activity likely stems from differences in phenolic composition and structural complexity, as antioxidant activity depends on hydroxyl group configuration (Jing et al., 2012).
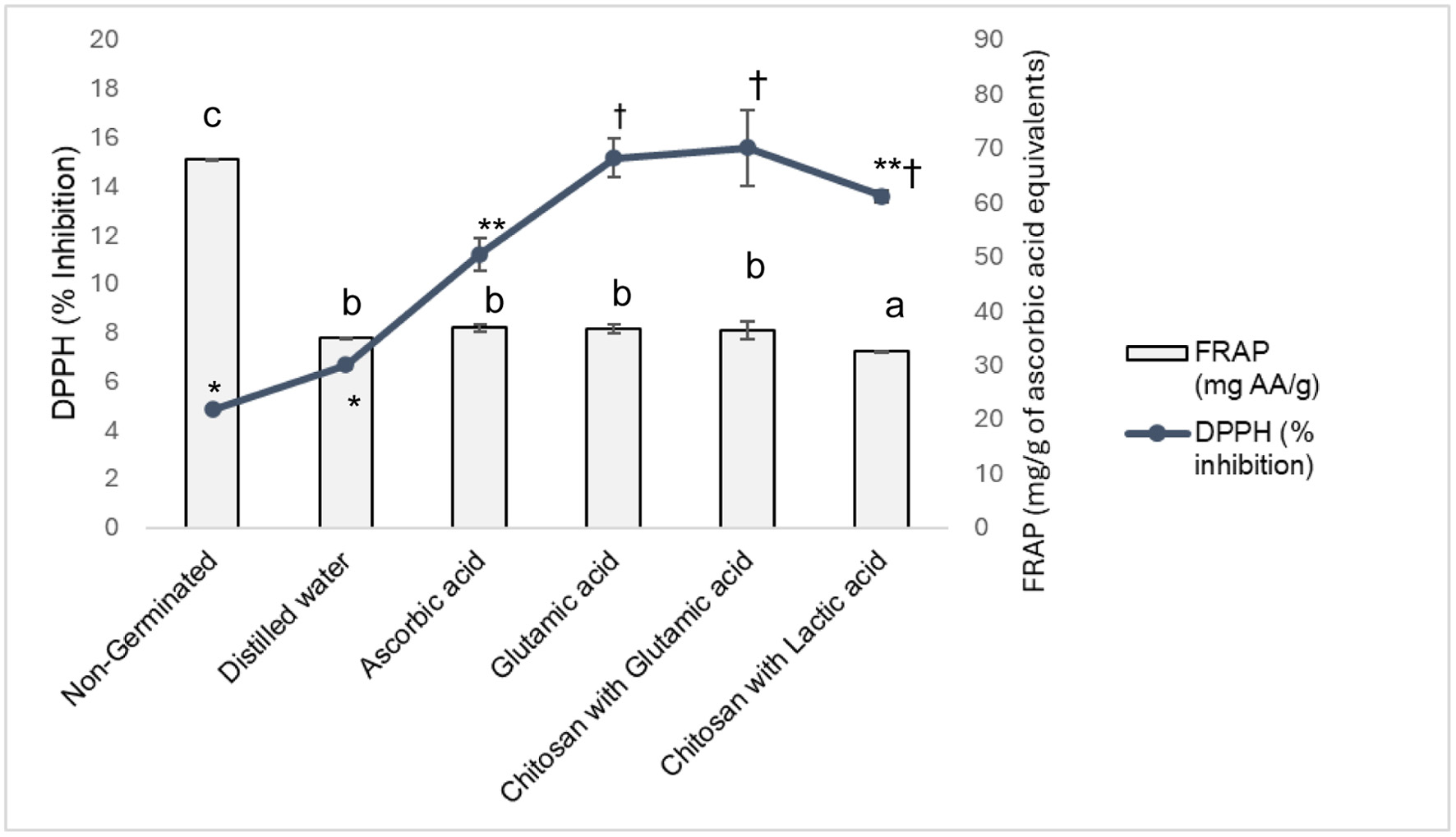 Click for large image | Figure 3. Antioxidant potential of cluster beans on treatment with varied elicitors. All values represent the Mean ± SEM (n = 3). Different lower-case letters within the FRAP clustered column on the graph indicate significant differences among elicitor treatments. For DPPH *, **, and † markers on the stacked line, significant differences among elicitor treatments are indicated. |
3.4. α-Amylase inhibitory activity
All elicited sprout extracts demonstrated superior α-amylase inhibitory activity compared to controls (Table 3). Aqueous extracts (10 mg/ml) exhibited inhibition ranging from 10.45 ± 0.87% to 49.55 ± 1.39% with malt as the enzyme source and 1.75 ± 0.55% to 53.92 ± 1.51% with porcine pancreas. Treatments with glutamic acid, glutamic acid combined with chitosan, and lactic acid with chitosan achieved up to 54% inhibition, outperforming the ∼31.76% reported for non-germinated cluster bean seeds (Riaz et al., 2022) and ∼20% for elicited Indian beans (Solanki et al., 2024). These differences may stem from cultivar variations or improved extraction techniques. The strong performance of glutamic acid and lactic acid with chitosan treatments suggests enhanced phenolic content, which correlates with elevated α-amylase and DPPH inhibitory activities. Similar findings were noted in chitosan-treated barley sprouts, where prolonged germination reduced activity, highlighting the need to optimize elicitor dosage and timing (Ramakrishna et al., 2017). Elicitation with glutamic acid and chitosan markedly improves the bioactive profile and α-amylase inhibitory activity of Cyamopsis tetragonoloba sprouts, subtly supporting their potential as functional foods for applications like diabetes management, aligning with previous legume sprout studies.
 Click to view | Table 3. α-Amylase Inhibition Potential of Non-germinated and Elicited Cluster Bean Sprouts |
| 4. Conclusion | ▴Top |
The application of elicitors such as glutamic acid, chitosan, and their combinations significantly enhances the α-amylase inhibitory activity and bioactive compound content in Cyamopsis tetragonoloba sprouts, surpassing the performance of non-germinated seeds. These treatments elevate phenolic and protein levels, contributing to improved antioxidant and enzyme-inhibitory properties, which highlight the potential of elicited sprouts as nutrient-rich functional foods. The superior inhibitory activity observed, particularly with glutamic acid and chitosan combinations, underscores the efficacy of targeted elicitation in optimizing sprout functionality. Future research should identify specific phenolic compounds driving α-amylase inhibition and optimize elicitor dosages and germination times to maximize bioactivity. In vivo studies and assessments of processing stability and sensory properties are also needed to support the use of elicited cluster bean sprouts in functional foods.
Acknowledgments
Komal Solanki acknowledges support from the SHODH Government of Gujarat for providing a PhD Fellowship (Ref No: 202101544) that facilitated this work.
Funding
Not applicable
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
All authors contributed to the study conception and design. Ms. Komal Solanki and Ms. Rushna Mansuri were involved in carrying out experiments, data collection, data analysis and manuscript writing. Dr. Krutika contributed to conceptualization, data analysis, and project administration. All authors read and approved of the final manuscript.
| References | ▴Top |