| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 30, June 2025, pages 25-32
Components and bioactivities of sea cucumber: an update
Xinru Yanga, Hui Zhaoa, Yanfei Liua, Jie Pana, Guliang Yangb, Qi Tanga, *
aTianjin Key Laboratory of Food and Biotechnology, Tianjin International Joint Center of Food Science and Engineering, State Experimental and Training Centre of Food and Drug, School of Biotechnology and Food Science, Tianjin University of Commerce, No. 409 Guangrong Road, Beichen, Tianjin 300134, China
bNational Engineering Laboratory for Rice and By-Products Processing, Food Science and Engineering College, Central South University of Forestry and Technology, Changsha 410004, China
*Corresponding author: Qi Tang, Tianjin Key Laboratory of Food and Biotechnology, Tianjin International Joint Center of Food Science and Engineering, State Experimental and Training Centre of Food and Drug, School of Biotechnology and Food Science, Tianjin University of Commerce, No. 409 Guangrong Road, Beichen, Tianjin 300134, China., E-mail: tangqi@tjcu.edu.cn
DOI: 10.26599/JFB.2025.95030411
Received: March 20, 2025
Revised received & accepted: June 4, 2025
| Abstract | ▴Top |
Sea cucumbers (holothurians), a classic marine invertebrate echinoderms, were found worldwide mainly as benthic organisms attached to sediments on the ocean floor. In particular, Sea cucumbers have long been esteemed as a valuable food due to their unique high nutritional value. Bioactives from Sea cucumbers play a crucial role in favor of human wellness. In this review, we collected recent research advances regarding this topic. First, we summarized the bioactive components from sea cucumbers including saponins, polysaccharides, polypeptides, proteins, fatty acids, cerebrosides, and gangliosides. Furthermore, we also outlined the role of sea cucumbers in anti-tumor, metabolic disease prevention , immune regulation and anti-aging. In a word, this review intends to cause further attention on the researches and development of sea cucumbers on the basis of the health protective mechanisms associated with the bioactives.
Keywords: Sea cucumbers; Bioactive compounds; Health benefits; Diseases
| 1. Introduction | ▴Top |
Sea cucumbers have long been esteemed as a valuable food due to their unique high nutritional value. Recent studies indicate that they play an important role in maintaining health and preventing diseases, making them a quintessential functional food. Sea cucumbers primarily inhabit tropical or subtropical marine rocky reef areas from 0 to 28°C. As benthic organisms, they clung to sediments, rocks, or algae, and feed on tiny organisms or other organic matter in coral sand(He and Li, 2015; Aminin et al., 2015). Belonging to the phylum Echinodermata and the class Holothurioidea, sea cucumbers are invertebrates further classified into three subclasses, six orders, and 24 families (He and Li, 2015). Currently, more than 900 species of sea cucumbers have been identified worldwide, yet only around 40 species are considered edible. To date, over 140 species have been discovered in China, of which 21 are edible. These 21 edible species include: from the order Dendrochirotida, the family Cucumariidae such as Pentacta quadrangularis, P. inornata, and P. anceps; from the order Aspidochirotida, the family Holothuriidae including Bohadschia marmorata, B. argus, B. graeffei, Actinopyga lecanora, A. mauritiana, A. miliaris, Holothuria nobilis, H. leucospilota, and H. scabra; from the order Aspidochirotida, the family Stichopodidae comprising Apostichopus japonicus, Thelenota ananas, T. anax, Stichopus chloronotus, S. variegatus, S. horrens, and S. flaccus; and from the order Apodida, the family Synaptidae including Acaudina molpadioides and Paracaudina chinensis var. ransonnetii. The geographical distribution of sea cucumbers is characterized by a gradual decrease in both diversity and abundance from the equator toward the poles. The Indian Ocean and the western Pacific host the greatest variety and number of sea cucumbers. Notably, Apostichopus japonicus is found in northern China, while the remaining edible species predominantly inhabit the southern marine areas near Hainan Island and the Xisha Islands (He and Li, 2015; Zhang et al, 2012).
The edible portion of sea cucumbers consists primarily of their body wall, which is nutritionally rich in proteins while being low levels of carbohydrates and fats. Moreover, this marine delicacy provides an excellent source of vitamins, including vitamin A, B1, B2, and B3—as well as minerals such as calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), and zinc (Zn). Additionally, it contains beneficial trace elements like manganese (Mn), selenium (Se), molybdenum (Mo), cadmium (Cd), and strontium (Sr) (Shi et al., 2016). These nutrients play a vital role in human growth and overall health. The biological functions of sea cucumbers are largely attributed to their unique bioactive compounds. For example, Hu et al. analyzed the protein content and amino acid composition across 8 sea cucumber species, revealing that a distinct amino acid profile characterized by a low lysine/arginine ratio, a feature associated with cholesterol-lowering effects (Wen et al., 2010). Sea cucumbers are rich in bioactive substances that exhibit diverse physiological activities, including anti-angiogenic, antitumor, anticoagulant, antihypertensive, anti-inflammatory, antimicrobial, antioxidative, and antithromboticeffects (He and Li, 2015; Shi et al., 2016; Bordbar et al., 2011)[3,4,6]. This review focus on summarizing the structural characteristics and physiological activities of these bioactive compounds, thereby providing a theoretical foundation and practical insights to guide future research on sea cucumber derived bioactive substances and their health physiological activities. Additionally, this work supports the development of functional foods or dietary supplements leveraging these marine resources.
| 2. Bioactive components | ▴Top |
Sea cucumbers contain various bioactive components, including saponins, polysaccharides, polypeptides, proteins, fatty acids, cerebrosides, and gangliosides. Many of their physiological activities (e.g., immune regulation, antitumor effects, antidiabetic effects, and lipid-lowering properties, etc.) are closely linked to these active ingredients.
2.1. Saponins
Sea cucumber saponins (Figure 1) are a unique triterpenoid compounds characterized by their structural complexity. This complexity arises from variations in the substituent types and positions on the aglycone ring, as well as differences in the types, numbers, and linkage order of the attached sugars moieties (Zhao et al., 2018). Typically, these saponins have a relative molecular weight of approximately 1,000 Daltons, classifying them among the larger bioactive substances (Han et al., 2008; Kalinin, 2000). Structurally, each sea cucumber saponin features i an oligosaccharide chain attached to the C-3 position of the aglycone. In terms of solubility, these componds are readily soluble in polar solvents such as water, methanol, and aqueous ethanol, but exhibit low solubility in non-polar organic solvents such as benzene and diethyl ether.
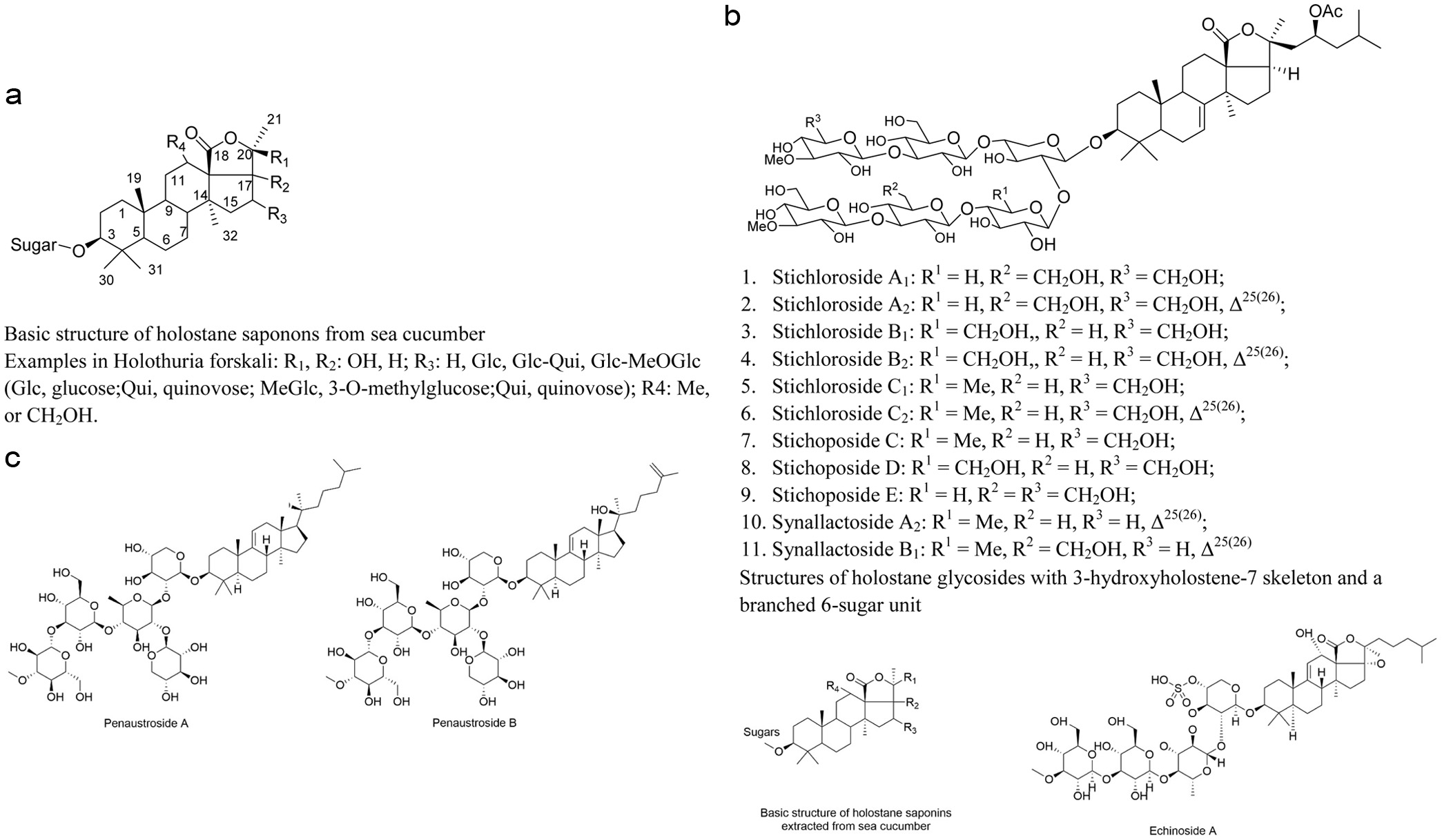 Click for large image | Figure 1. Structures of saponins extracted from sea cucumber. |
The aglycone moiety of sea cucumber saponins contains of 30 carbon atoms and is predominantly of the holostane type, characterizing by a pentacyclic nucleus with adjacent rings connected in a trans configuration. Based on the position of the lactone ring, sea cucumber saponins are classified into two types:Saponins with an aglycone comprise of lanostane-3β-ol with a γ(18,20)-lactone in the E-ring of the pentacyclic triterpene are referred to as holostane saponins (Figure 1b). For instance, Stichlorosides (Figure 1b), isolated and identified from Eupentacta fraudatrix, Holothuria lessoni, Bohadschia marmorata, Stichopus chloronotus and Staurocucumis liouvillei belong to this category. Penaustroside A and Penaustroside B from Pentacta australis (Figure 1c) belong to non-holostane-type triterpenoid oligoglycosides. Notably, holostane saponins represent the majority of identified sea cucumber saponins accounting for over 70 out of more than 100 known structures. (Miyamoto et al., 1992). Conversely, if the lactone ring is located at the C-18(16) position or if the aglycone lacks a lactone ring entirely, the saponin is termed a non-holostane saponin. Cumarioside G2, isolated and identified from Eupentacta fraudatrix, is an example of a non-holostane saponin, a group that is less commonly found (Avilov et al., 1994).
At the C-3 carbon atom, an oligosaccharide side chain composed of 2–6 monosaccharide units is generally attached. This side chain exhibits structural diversity, existing either linear or branched configurations, and is composed of various monosaccharides such as glucose, 3-O-methylglucose, xylose, 3-O-methylxylose, and quinovose. There are significant differences among various sea cucumber saponins regarding both the number and the types of monosaccharides constituting the oligosaccharide chain. The hydroxyl groups on certain monosaccharides are often undergo sulfation, with one or several hydroxyl groups being esterified by sulfate moieties. Additionally, a hydroxyl group at the C-3 position of the aglycone forms a glycoside bond with the sugar via a β-O-glycosidic linkage. Hydroxyl or acetyl groups are usually attached to the C-12, C-16, and C-17 positions of the aglycone. Furthermore, one or more double bonds are typically present at positions such as Δ7(8), Δ8(9), Δ9(11), Δ24(25), and Δ25(26) of the aglycone, and conjugated double bonds occasionally appear on the side chain (Miyamoto et al., 1992).
2.2. Polysaccharides
Sea cucumber polysaccharides consitute another nutritionally and physiologically significant component of the body wall. Their content varies differently among species, generally accounting for about 6% of the total organic matter in dried sea cucumbers, while some species may contain as much as 31% polysaccharides. The composition andabundance of polysaccharides are key indicators for assessing the nutritional quality of sea cucumbers. Based on the structural characteristics of the sugar chains, sea cucumber polysaccharides can be classified into two categories. One category comprises glycosaminoglycans (GAGs), also termed acidic mucopolysaccharides, are heteropolysaccharides with branched structures. Their sugar residues primarily consist of D-N-acetylgalactosamine, D-glucuronic acid, and L-fucose, with a relative molecular weight ranging between 40,000 and 50,000. The other category consists of holothurian fucans (HF), which are linear homopolysaccharides predominantly of L-fucose residues, with a higher molecular weight of 80,000 and 100,000. Although these two types differ in monosaccharide composition and chain structure, both types undergo partial sulfation of hydroxyl groups on their sugar chains. Variations in sulfation sites and degrees contribute to structural diversity and variations in molecular weight, complicating the purification and structural characterization (Shi et al., 2016; Yin et al, 2009).
2.3. Proteins and peptides
Proteins are the most abundant organic component in the sea cucumber body wall, accounting for approximately 90% of its weight. These proteins are composed of 18 amino acids, linked by peptide bonds, with glycine, glutamic acid, and arginine being relatively abundance. Among these, seven essential amino acids required for human nutrition are present. Sea cucumber peptides refer to low-molecular-weight polypeptides, typically composed of 3-10 amino acids, obtained through protease hydrolysis of fresh sea cucumbers (He and Li, 2015). These peptides are predominantly collagen-derived peptides, along with glycopeptides, neuropeptides, antimicrobial peptides, and so on (Liu et al., 2010). Due to their lower molecular weight, these peptides exhibit a broader range of physiological activities compared to intact sea cucumber proteins, including lowering blood pressure and lipid levels, preventing atherosclerosis, enhancing fatigue resistance, exhibiting antimicrobial and antiviral properties, boosting immune function, as well as delaying aging and providing antioxidant activity. Moreover, in terms of solubility, stability, and bioavailability through digestion and absorption, these peptides outperform sea cucumber proteins, leading to significantly higher bioavailability compared to conventional sea cucumber products (Zhong et al., 2007).
2.4. Other components
Sea cucumbers contain 0.24–0.83% lipid substances, including fatty acids, gangliosides, cerebrosides, and others. The predominant fatty acids are of the structural types 16:0, 18:0, 20:1, 20:4 (n-6), and 20:5 (n-3). Variations in fatty acid composition exist among sea cucumbers from different marine habitats. For instance, those inhabiting temperate waters have approximately 15.5% branched-chain fatty acids, a higher content of 20:5 (n-3), and a lower content of 20:4 (n-6), whereas sea cucumbers from tropical waters contain only about 1% branched-chain fatty acids. Approximately 12.5–29.0% of the fatty acids are phospholipids, such as gangliosides and cerebrosides (Yuan et al., 2008). Cerebrosides have been shown to possess various biological activities, including inhibition of tumor cell growth, anti-HIV effects, and hepatoprotective properties. To date, 29 distinct cerebrosides have been isolated and identified from species such as Stichopus, Apostichopus, and Holothuria.
Research by Gowda et al. (Gowda et al., 2008) has demonstrated that lectins isolated from different sea cucumber species exhibit remarkable diversity in their biological activities. For example, lectins isolated from Stichopus display potent hemolytic activity against both human and murine erythricytes, while those obtained from Holothuria scabra show additional antibacterial properties. Liu et al.(Liu, 2008) purified a Stichopus lectin with a molecular weight of 31,000 that exhibits unique biochemical properity. This lectin’s agglutination of rabbit erythrocytes is specifically inhibited by bovine thyroglobulin, but remains unaffected by D-fructose, D-mannose, or D-glucose. Moreover, its activity demonstrates exceptional stability, being independent of metal cations such as Ca2+, Mg2+, Mn2+, Zn2+, or by EDTA, mataining functionality across a broad pH values ranging from 4.0 to 10.14, and retaining hemagglutinating capacity even after exposure to 90°C for 30 minutes.
Additionally, sea cucumbers contain pigments—primarily naphthoquinones, carotenoids, melanins, porphyrins, echinochrome, and astaxanthin—as well as organic compounds such as methionine, taurine, and niacin, and trace elements including Fe, Cu, Zn, Mn, Se, Mo, Ge, and Sr. These substances are essential for human growth and development, fulfilling irreplaceable physiological functions (Zhang et al., 2012).
| 3. Physiological activities | ▴Top |
3.1. Antitumor activity
Numerous bioactive components in sea cucumbers exhibit antitumor properties, Among which saponins have been the most extensively studied due to their potent antitumor activity (Aminin et al., 2015; Kalinin et al., 2008; Zou et al., 2005) (Table 1). As early as 1976, Pettit et al. (Pettit et al., 1976) isolated and purified three active compounds, namely Stichostatin 1, Thelenostatin 1, and Actinostatin 1, from the Cuvierian tubes of sea cucumbers. These pioneering findings revealed the compounds’ remarkable ability to inhibit the proliferation of both mouse leukemia cells (P-388, L-1210) and human oral carcinoma cells (KB). Zhang et al. (S.-Y. Zhang et al., 2006) isolated and identified three novel triterpene glycosides—Fuscocinerosides A, B, and C—from the metabolites of H. fuscocinerea. These compounds not only exhibited cytotoxicity against tumor cells but also inhibited angiogenesis. Recent studies have further confirmed the broad-spectrum antitumor potential of sea cucumber saponins,with compelling evidence showing their significant growth-inhibitory effects on multiple cancer cell lines, including human gastric cancer cells, mouse leukemia cells, prostate cancer cells, ovarian cancer cells, liver cancer cells, Hela cells, and colorectal cancer cells (Wang et al., 2006; Wu et al., 2007). Dong et al (2012) isolated and identified eight sea cucumber saponins from Pearsonothuria graeffei, with structure-activity relationship studies revealing a critical finding:the length of the glycoside chain attached to the aglycone correlate significantly influences antitumor activity. lAdditionally, structural modifications on the side chain—such as hydroxylation, the presence of double bonds or epoxy structures—can reduce the antitumor efficacy of sea cucumber saponins. Avilov et al. (Avilov et al., 2000) isolated and identified four monosulfated triterpene saponins from Pentamera calcigera: Cucumarioside G2, Calcigerosides B, Calcigerosides C1, and Calcigerosides C2. Among these, Calcigerosides B, C1, and C2 are novel triterpene saponins. The desulfated derivatives of Calcigerosides B, C1, and C2 exhibited strong inhibitory activities with IC50 values under 50 µg/mL againstmultiple cancer cells: P-388 (mouse leukemia cells), A-549 (human lung cancer cells), HT-29 (human colon cancer cells), and Mel-28 (human malignant melanoma cells).
 Click to view | Table 1. The antitumor activities of various sea cucumber saponins summarized on the basis of a previous report |
Philinopside E (Tian et al., 2007) inhibits angiogenesis by interacting with the extracellular domain of the kinase insert domain-containing receptor (KDR) for vascular endothelial growth factor (VEGF). This interaction blocks the binding of KDR to VEGFand downstream signaling, thereby suppressing neovascularzation. Tong et al. (Tong et al., 2005) isolated a sea cucumber saponin, Philinopside A, from P. quadrangulari, which exhibits multiple receptor tyrosine kinases, including the VEGF receptor, fibroblast growth factor receptor-1 (FGFR-1), platelet-derived growth factor receptor-β (PDGFR-β), and epidermal growth factor receptor (EGFR). This multi-receptor inhibition induces apoptosis in both tumor cells and tumor-associated endothelial cells, leading to reduced tumor volume in mouse sarcoma (S-180) models. Intercedensides A, B, and C, isolated by Zou et al. (Z.-R. Zou et al., 2003) from Mensamaria intercedens, exhibit potent antitumor activity against 10tumor cell lines, including Lewis lung carcinoma and S-180, with ED50 values ranging from 0.6–4.0 µg/mL.
Another key bioactive component in the sea cucumber body wall is polysaccharides. Janakiram et al. (Janakiram et al., 2010) demonstrated that Frondanol A5, a glycolipid extract from Cucumaria frondosa, inhibits colon cancer with an IC50 value of 0.13 mg/mL, while showing no toxicity normal human cells. Gao et al. (Gao et al., 2008) investigated the effects of acidic polysaccharides from Stichopus japonicus on the HepG2 human liver cancer cell line. They found that these polysaccharides suppress HepG2 proliferation in a time- and dose-dependent manner by downregulating the expression of the Bcl-2 gene and upregulating nm23-H1 gene expression (Lu et al., 2010). Furthermore, Song et al. (Song et al., 2013) employed a diethylnitrosamine (DEN)-induced rat model of hepatocellular carcinoma to evaluate the therapeutic effects of Stichopus japonicus acidic polysaccharides administered via intraperitoneal injuction and oral gavage. Their findings revealed that these polysaccharides significantly suppressed serum alpha-fetoprotein (AFP) and proliferating cell nuclear antigen (PCNA), expression, promoted p21 expression, enhanced cellular immunity, and inhibited DEN-induced hepatocarcinoma growth.
Sugawara et al. (Sugawara et al., 2006) treated human colorectal adenocarcinoma cells (DLD-1), human colon cancer cells (WiDr), and human colon adenocarcinoma cells (Caco-2) with sea cucumber cerebrosides, observing chromatin condensation, increased Caspase-3 activity and subsequent apoptosis. Wang (Wang, 2007)[36] isolated collagen-derived peptides from Stichopus japonicus (Japanese sea cucumber) and administered the homogenized peptide solution via oral gavage to mice with transplanted tumors models. The treatment significantly suppressed the growth of S-180 sarcoma, increased spleen and thymus indices, and elevated serum hemolysin levels in tumor-bearing mice, suggesting that these peptides enhanc immune function to control and eliminate tumor cells. Zhou et al. treated sea cucumber (S. japonicus) proteins with trypsin followed by ultrafiltration membrane filtration, to obtain the peptide LSCP-2, which significantly inhibited the growth of gastric cancer cells (SGC-7901) and human breast cancer cells (MCF-7) (Zhou et al., 2012). Du et al. (Du et al., 2012) investigated the antitumor activity of sea cucumber cerebrosides in S-180 sarcoma-bearing mice and found that a dose of 50 mg/kg reduced tumor weight by 45.24%, prolonged survival time by 55.28%, downregulated the expression of Bcl-2 and Bcl-xL, and upregulated Bax, Cytochrome C, Caspase-9, and Caspase-3 in tumor cells. These results indicate that sea cucumber cerebrosides induce apoptosis via the mitochondrial apoptosis pathway.
3.2. Improvement of metabolic diseases
Rodriguez et al. (Rodríguez et al., 2000) demonstrated that dietary supplementation with sea cucumber polypeptides in rats resulted in increased serum high-density lipoprotein (HDL) levels and decreased serum triglyceride concentrations compared to the control group, indicating the lipid-modulating bioactivity of these peptides. Taboada et al. (Taboada et al., 2003) observed that rats fed with sea cucumber- supplemented diets for 16 consecutive days exhibited significantly lower triglyceride levels than those receiving casein. In addition, the sea cucumber diet was associated with enhanced activities of intestinal maltase, lactase, alkaline phosphatase, and leucine aminopeptidase, while decreasing hepatic leucine aminopeptidase and glutamyl transpeptidase activities compared to the casein group. These results suggest that sea cucumber contains active ingredients capable of modulating lipid metabolism and influencing enzymatic activies in both intestinal and hepatic tissues.
Zhao et al. (Zhao et al., 2009) utilized enzymatic hydrolysis to break down sea cucumber body wall proteins and subsequently applied membrane separation techniques to obtain an angiotensin I-converting enzyme (ACE) inhibitory peptide (MEGAQEAQGD). At a dose of 3 μmoL/kg, this peptide significantly reduced blood pressure in spontaneously hypertensive rats. Wang et al. (Wang et al., 2009) administrated sea cucumber to rats via oral gavage, observing a marked reduction in lipid content in both the blood and liver, along with an increase in fecal fat content. These findings indicate that sea cucumber can inhibit lipid absorption and promote lipid degradation, thereby improving overall lipid metabolism.
Furthermore, Hu et al. (Hu et al., 2009) found that supplementation with sea cucumber saponins effectively attenuated obesity-related metabolic disturbances in rats. The treatment not only suppressed adipose tissue accumulation, particularly in perirenal fat mass,but also significantly lowered the concentrations of total cholesterol (TC) and triglycerides (TG) in both serum and liver. The study also demonstrated a direct inhibitory effect on fatty acid synthase (FAS). In addition, Xu et al. (Xu et al., 2011) investigated the impact of cerebrosides isolated from Acaudina molpadioides (AMC-2) on fatty liver in rats. Adding 0.03% and 0.006% AMC-2 to drinking water significantly decreased hepatic triglyceride (TG) and total cholesterol (TC) levels, seppressed stearoyl-coenzyme A desaturase (SCD) mRNA expression and activity, and reduced de novo lipogenesis, thereby ameliorating non-alcoholic fatty liver disease in rats.
3.3. Hemolytic activity
Sea cucumber saponins also exhibit significant hemolytic activity, a property intrinsically related to their unique molecular structure. These saponins bind to unsaturated sterols at the 5(6) position of biological membranes, forming saponin–steroid complexes that disrupt plasma membrane integrity by inducing large pore formation. At low concentrations (e.g., in the blood), smaller pores formed on erythrocyte membranesthat selectively permit K+ efflux, ultimately leading to cellular dysfunction and membrane rupture (Hu et al., 2005; Kalinin et al., 1996). Kalinin (Kalinin, 2000) observed that higher saponin concentrations induce larger pores form capable of facilitating the leakage of macromolecules like amino acids, resulting in complete cellular disruption and hemolysis. In his study of the relationship between saponin structure and hemolytic activity, Kalinin et al. (Kalinin et al., 1996) investigated the structural basis of saponin-induced hemolysis via K+ efflux kinetics, demonstrating a positive correlation between K+ leakage rate and pore density. For instance, sulfation at C-4 of xylose (first sugar in the glycan chain) or C-6 of glucose (third sugar) enhances K+ efflux. Methylation at C-3 of the terminal sugar in the glycan chain also exacerbates K+ loss. A 7(8)-double bond in the aglycone combined with C-16 ketone substitution reduces the rate of K+ efflux. Substitutions at different positions may alter the structure of sea cucumber saponins, ultimately determining their membrane-disruptive capacity and hemolytic potential.
Xiong et al. (Xiong et al., 2008) extracted Nobiliside A from Holothuria nobilis and investigated its hemolytic properties. At a low concentration (0.41 μg/mL), Nobiliside A caused hemolysis in 5% of red blood cells, while complete hemolysis was observed at a higher concentration (16.0 μg/mL). Notably, pre-incubation with cholesterol abolished hemolysis, confirming sterol-dependent pore formation (Anisimov, 1987). Due to their strong hemolytic effects, sea cucumber saponins are unsuitable for intravenous administration use. Structural engineering to minimize sterol binding may reduce toxicity and enable therapeutic applications.
Fonseca et al. (Fonseca et al., 2009) isolated a sulfated fucan and a fucosylated chondroitin sulfate from Ludwigothurea grisea and studied their effects on coagulation, thrombosis, and hemorrhage. They found that both compounds possess anticoagulant activity, albeit via entirely different mechanisms. Fucosylated chondroitin sulfate exerts its anticoagulant effect by inhibiting heparin cofactor II (HC-II), whereas sulfated fucan targets both antithrombin and HC-II to suppress thrombin. The sulfated fucan effectively inhibited venous thrombosis at lower doses, while FCS was more potent against arterial thrombosis. Li Zhiguang et al. (Li et al., 2000) examined the effects of glycosaminoglycans (GAGs) on human venous endothelial cells and found that GAGs reduced the procoagulant activity and tissue factor expression in these cells while increasing the expression of thrombomodulin. Chen et al. (Chen et al., 2012) isolated a sulfated deoxy-galactan fucan (Fucan-Ib) and a fucosylated chondroitin sulfate (fCS-Ib) from the Mexican sea cucumber Isostichopus badionotus. Fucan-Ib demonstrated excellent anticoagulant and antithrombotic activities by acting on thrombin, while fCS-Ib exerted its anticoagulant effects via heparin cofactor II.
3.4. Immune regulatory activity
Aminin et al. (Aminin et al., 2001) reported that injection of saponins extracted from C. japonica into mice stimulated innate immune responses by enhanceing the phagocytic activity and increasing the release of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), while promoting antibody formation. Cumaside, a monosulfated triterpene glycoside isolated from C. japonica by Aminin et al. (Aminin et al., 2011), has been shown to possess radioprotective properties. Echinoside A can activate metallothionein activity and protect against CCl4-induced liver damage in mice (Itoh et al., 1997). Moreover, sea cucumber polysaccharides have been found to enhance cellular immunity and can improve immune deficiency conditions. Huang et al. (Huang et al., 2001) isolated a polysaccharide (designated Dichotomous Sea Cucumber Polysaccharide-1) from dried sea cucumbers and demonstrated that it accelerates the secretion of interleukin-2 (IL-2), thereby promoting the proliferation of mouse splenic lymphocytes in vitro. The polysaccharide also increases spleen and thymus indices and enhances the delayed-type hypersensitivity response, indicating a marked immunostimulatory effect.
3.5. Antifungal and antiviral activity
Kumar et al. (Kumar et al., 2007) systematically evaluated 4 antifungal activities-Marmoratoside A, 17-α-hydroxy impatienside A, Marmoratoside B, and 25-acetoxy bivittoside D against 20 fungal strains, revealing particularly potent antifungal activity for Marmoratoside A and 17-α-hydroxy impatienside A with IC50 values ranging form 0.7 and 2.81 µM. Sedov et al. (Sedov et al., 1990) examined the effects of saponins derived from Cucumaria on various Gram-negative bacteria. In vivo, mice infected with different Gram-negative pathogens were treated via intraperitoneal injection of the saponins, achieving a cure rate of up to 90% when administered via intraperitoneal injection. In 1976, Kitagawa et al. (Kitagawa et al., 1976) isolated the saponin Holotoxin A and B from Stichopus japonicus,which were subsequently developed into effective treatments for tinea peids. Contemporary research has expanded our understanding of these antimicrobial effects, with multiple studies confirming the inhibitory activity of sea cucumber saponins against diverse pathogens including yeast, Salmonella, and Candida albicans (Pacheco et al., 2000; Mourão et al., 1998; Cong et al., 2006; Xiao et al., 2005; Wang et al., 2004). With ongoing research into the antifungal properties of sea cucumber saponins, they are poised to become a new focus in the development of antifungal drugs.
Researches demonstrated the significant antiviral and antimicrobial potential of bioactive compounds derived from sea cucumbers. Minamiguchi K et al. (Minamiguchi et al., 2003) conducted a comparative study showing that sea cucumber glycosaminoglycan (sc-GAG) exhibited potent anti-herpetic activity against herpes simplex virus (HSV) with an half maximal effective concentration (EC50) of 10 μg/mL , representing a 2.5-fold greater potency than the conventional antiviral drug vidarabine (EC50 = 25 μg/mL).. Additionally, Schillaci et al. (Schillaci et al., 2013) isolated a peptide fragment with a molecular weight of 5 kDa from the coelomic fluid of H. tubulosa. At a concentration of 3.1 mg/mL, this peptide inhibited biofilmformation Staphylococcus aureus and Pseudomonas aeruginosa. These findings collectively underscored the diverse therapeutic applications of sea cucumber-derived compounds in combating viral infections and microbial biofilms.
3.6. Anti-aging effects
The accumulation of reactive oxygen species (ROS) in the body, promptly cleared, can cause oxidative tissues damage and accelerate aging if not efficiently eliminated. Fang Kun (Fang, 2013) demonstrated that papain-hydrolyzed fresh sea cucumbers peptides exhibited significant antioxidant potential, scavenging 25.10% of superoxide anions. When neutral protease A.S.1398 was employed for hydrolysis, the resulting peptides achieved a 77.00% hydroxyl radical scavenging activity. Wang et al. (Wang et al., 2010) isolated various peptide fractions of different molecular weights from autolyzed sea cucumbers via membrane separation and compared their antioxidant activities with that of vitamin C and all four peptide fractions demonstrated to display stronger antioxidant activity in a dose-dependent manner. Additionally, Wang et al (Wang, 2007) reported that Japanese Stichopus collagen peptides (AJCP) could enhance the activities of superoxide dismutase, glutathione peroxidase, and catalase in mice, while reducing malondialdehyde levels. Moreover, AJCP significantly increased the total hydroxyproline content in the skin, repaired damaged collagen fibers, and provided a protective effect against collagen degradation, suggesting its potential as a therapeutic agent for oxidative stress-related tissue damage and aging..
3.7. Other activities
During the peri-ovulatory period, the saponin content in sea cucumbers rises, which has been shown to inhibit the maturation of sea cucumber oocytes and prolongs their reproductive period. Mats et al. (Mats et al., 1990) isolated Holotoxins A1 and B1 from Stichopus japonicus, revealing that this mixture not only suppresses ovulation but also stimulates uterine contractions. In another study, sulfated polysaccharides extracted from Stichopus were combined with fibroblast growth factor (FGF) and applied to rat neural stem cells. This results demonstrated a notable enhancement in both proliferation and differentiation efficiency compared to FGF treatment alone. This effect possibly attributed to the ability of Stichopus sulfated polysaccharides to reduce apoptosis, prolong cell survival, and promote neurogenesis. Furthermore, Zhang et al. (Y. Zhang et al., 2010) demonstrated that sea cucumber sulfated polysaccharides at a concentration of 500 ng/mLstimulated the proliferation of neural stem/progenitor cells (NSPCs). Likewise, they also enhanced the proliferative effect of fibroblast growth factor-2 (FGF-2) on NSPCs, likely via reducing apoptosis in NSPCs.
| 4. Summary | ▴Top |
Sea cucumbers contain a variety of bioactive substances that are beneficial to human health. To the date, various bioactives have been isolated and identified. The separation and purification of sea cucumber compounds are particularly challenging due to the abundance of structural isomers. Current methods rely on multi-step chromatography, gel filtration, and prerarative HPLC. These methods are complex, time-consuming, and solvent-intensive, often resulting in low amounts of the target compounds. Therefore, establishing simplified, high-efficiency methods for isolation of sea cucumbers will greatly promote the identification of new bioactive substances and facilitate the development of functional foods.
| References | ▴Top |