| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 30, June 2025, pages 6-18
Pharmacokinetic profiles and improvement of resveratrol and derived stilbenes
Jie Penga, #, Wenyu Zhangb, #, Yixing Zhub, Haiqing Zhub, Chi-Tang Hoa, *
aDepartment of Food Science, Rutgers University, New Brunswick, New Jersey, USA
bCollege of Food Science and Pharmacy, Xinjiang Agricultural University, Urumqi 830052, China
#These authors contributed equally to this work.
*Corresponding author: Chi-Tang Ho, Department of Food Science, Rutgers University, 65 Dudley Road, New Brunswick, NJ 08901, USA. E-mail: ctho@sebs.rutgers.edu
DOI: 10.26599/JFB.2025.95030409
Received: May 29, 2025
Revised received & accepted: June 18, 2025
| Abstract | ▴Top |
Numerous studies have demonstrated the health-promoting benefits of resveratrol and its close derivatives in various aspects of disease prevention and management, yet due to their highly conjugated 1,2-diphenylethylene structural skeleton, the in vivo application of stilbenoids could be limited. Therefore, the metabolic profiles of these stilbene compounds warrant further attention and investigation. The bioavailability of a nutrient or a drug is significantly influenced by ADME (absorption, distribution, metabolism and excretion). In this review, we summarize the study results of drug metabolism and pharmacokinetics (DMPK) profiles of resveratrol and its close oligomeric derivatives, including oxyresveratrol, piceatannol, pterostilbene, rhaponticin, rhapontigenin and 2,3,5,4′-tetrahydroxystilbene-2-O-β-glucopyranoside (THSG). This review also addressees explored delivery strategies, such as stilbenoids-loaded nanoparticles or Pickering emulsions, to enhance their aqueous solubility, stability, and thus bioavailability.
Keywords: Resveratrol; Resveratrol related stilbenes; Bioavailability; DMPK profile; Pterostilbene
| 1. Introduction | ▴Top |
Resveratrol represents a family of monomeric stilbene phytochemicals heterogeneously distributed in plants, many of which are edible plants, usually fruits and vegetables such as grapes and berries. This family of 1,2-diphenylethylene derivatives is mainly found in dietary plants. In chemistry, resveratrol and its structural analogs are a group of stilbene compounds with benzene rings whose hydrogen atoms are replaced by varying numbers of hydroxyl and methoxy groups, among others (Peng et al., 2024; Wang, Zhao et al., 2020). It has been revealed that these stilbene compounds possess antioxidant and inhibitory effects on chronic inflammation (Soufi et al., 2015; Truong, Jun, and Jeong, 2018), which are closely associated with their potential protective effects in metabolic syndrome (Hou et al., 2019), particularly in diabetes and its complications (Huang et al., 2020; Peng et al., 2024). These stilbenoid compounds also exhibited beneficial effects in liver injury (Jia et al., 2019; Wu et al., 2019), brain diseases such as brain injury, dementia and Alzheimer’s disease (Hornedo-Ortega et al., 2018; Pasinetti et al., 2015), cardiovascular disease (Fan et al., 2022; Breuss and Atanasov, 2019), anti-aging effect (Li, Li and Lin, 2018) and the modulation of gut microbiota is involved in the mitigation of some diseases (Koh et al., 2020).
However, despite potential health effects exhibited by phytochemicals from the stilbene family, their utilization as therapeutic candidates either as supplements or potential medications is hindered by a few factors. In the last few decades, researchers have studied the pharmacokinetic profile of resveratrol and its structural analogs, although much less investigation has been conducted for the latter. These works provide us with important insight into how they are absorbed and metabolized and identify potential factors contributing to their low bioavailability, such as poor solubility and absorption and fast blood clearance. To solve these limitations associated with not only stilbenes but also many other natural phytochemicals, various formulation systems were created to enhance their stability against environmental stress and bioavailability.
To date, several delivery systems have been investigated for stilbenes compounds. A key strategy employed in these delivery methods is to protect these bioactive substances by encapsulating them within shell-like structures. This could be achieved by structuring lipid- or polymer-based nanoparticles, nanostructured lipid carriers (NLSc), or liposomes, among others approaches. Alternatively, strategies often focus on reducing the droplet size or improving the product’s permeability through cell membrane to boost bioaccessibility. Nanoemulsions and Pickering emulsions are two representative choices.
In this review, focusing on several key stilbenes, namely resveratrol, pterostilbene, piceatannol, oxyresveratrol, polydatin, rhaponticin, rhapontigenin and THSG, we primarily summarize their pharmacokinetic profiles and current formulation strategies employed to enhance their bioavailability. Formulation such as nanoemulsions, nanoparticles and liposomes are highlighted. Additionally, their structural characteristics, dietary sources, bioactivities and associated mechanisms are briefly discussed.
| 2. Structure characteristics, dietary sources and brief bioactivities of resveratrol and its close derivatives | ▴Top |
The study of structure-activity relationship starts with structural analysis. Stilbenes possess a basic C6-C2-C6 skeleton, comprising two phenyl rings linked by an ethylene group. The diversity among stilbenes arises from distinct substituents on these phenyl rings. As one or multiple hydrogen atoms on the basic skeleton are substituted by hydroxylation, glycosylation, and polymerization among others, more than 400 stilbene phytochemicals have been identified ((Pecyna et al., 2020; Shen, Wang, and Lou, 2009). The chemical structures of resveratrol and its close analogs are shown in Figure 1.
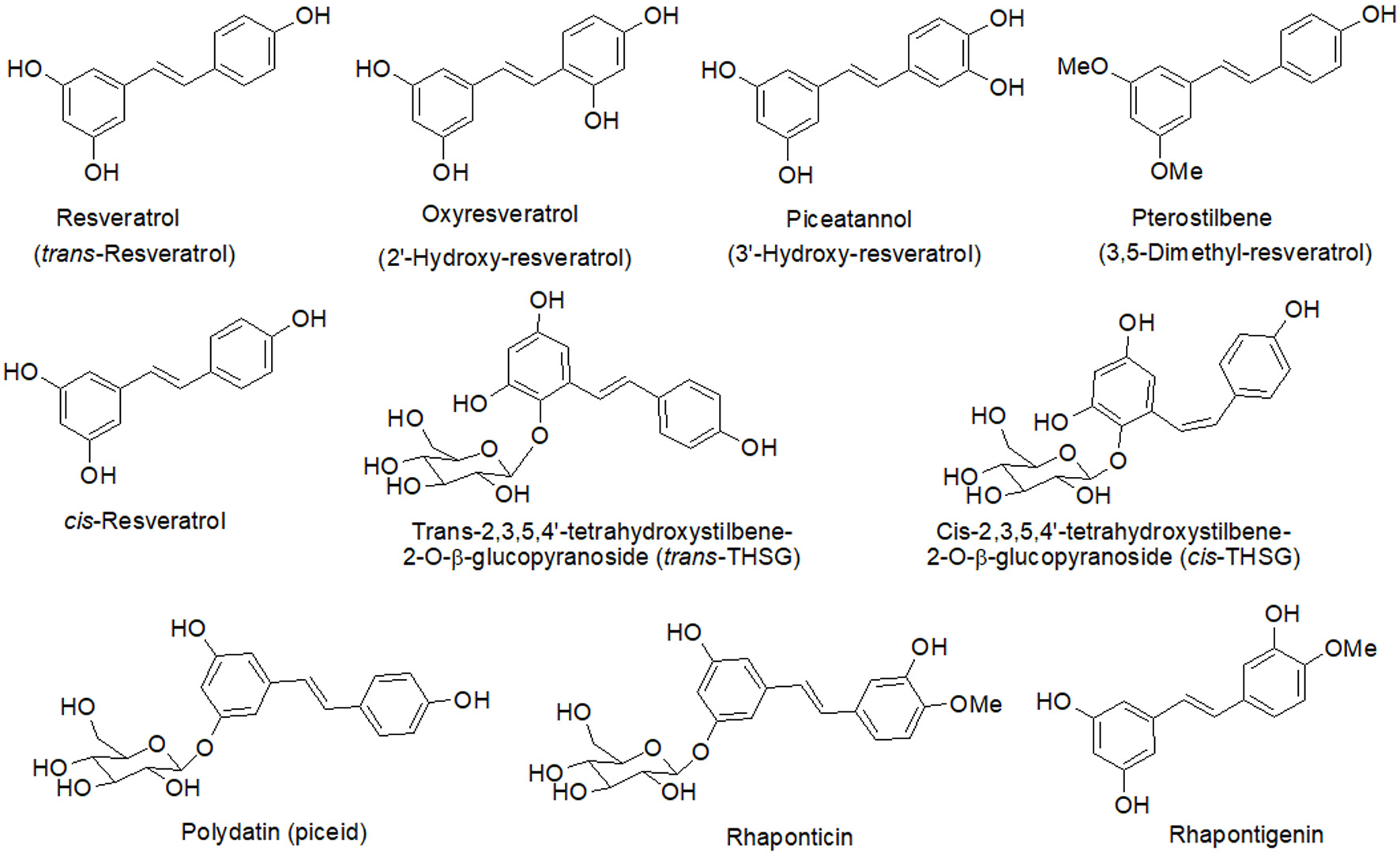 Click for large image | Figure 1. Chemical structures of resveratrol and its close derivatives. |
Resveratrol (trans-3,5,4′-trihydroxystilbene), present in plants like berries (e.g. grapes) and is rich in Polygonum cuspidatum (Tian and Liu, 2020). According to current reports, resveratrol possesses a few biological functions, ranging from antioxidant, cardioprotective and neuroprotective effects to anti-aging effect (Bastianetto, Ménard, and Quirion, 2015; Bonnefont-Rousselot, 2016; Li, Li, and Lin, 2018; Rauf et al., 2018).
Polydatin, a 3-glycoside derivative of resveratrol (Figure 1), is predominately found in the Vitaceae, Liliaceae, and Leguminosae families and its main plant source is Polygonum cuspidatum, which has a long history of use in traditional Asian herbal medicine (Imtiyaz et al., 2024). Similar to resveratrol, polydatin possesses multiple biological activities, such as cardio-protection (Huang et al., 2015), amelioration of diabetes nephropathy (Xie et al., 2012), neuroprotective effects (Chen et al., 2020; Li et al., 2012), and anti-cancer effects (Chen et al., 2020; Imtiyaz et al., 2024). Notably, comparing to resveratrol, polydatin has shown superior effects against oxidative stress in vivo, potentially by promoting the activity of serum superoxide dismutase, catalase and glutathione peroxidase (Wang et al., 2015).
Oxyresveratrol (trans-2,4,3′,5′-tetrahydroxystilbene, Figure 1) is a natural stilbenoid primarily found in the Moraceae family (mulberry family), particularly in Artocarpus (jackfruit etc.) and Moru genera (white mulberry etc.) (Likhitwitayawuid, 2021). Structurally, it differs from oxyresveratrol by having an extra hydroxyl group at the 2-position of resveratrol’s mono-hydroxyl phenyl ring. While sharing similar biological activities with resveratrol (Likhitwitayawuid, 2021), this extra hydroxyl group brings oxyresvertrol stronger antioxidant and anticancer activities(Yang et al., 2019). Its diverse biological and pharmacological activities include anti-melanogenesis effects by inhibiting tyrosinase, anti-inflammatory activity, gut microbiota modulation, and neuroprotective effects, among others (Likhitwitayawuid, 2021).
Pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene, Figure 1), a natural 3,5-dimethylated analog of resveratrol with similar biological functions, is notably abundant in blueberries (Lin et al., 2020; Liu et al., 2020). The substitution of the two hydroxyl groups on the A-benzene ring of resveratrol with two methoxyl groups improves its lipophilicity and reduces its overall reaction with phase II metabolic enzymes like glucuronidase in the human body (Dellinger, Garcia, and Meyskens, 2014; Wang and Sang, 2018). Therefore, in comparison with resveratrol, pterostilbene may exhibit higher membrane permeability and metabolic stability, potentially resulting in better bioavailability (Dellinger, Garcia, and Meyskens, 2014; Kapetanovic et al., 2011; Liu et al., 2020).
Rhaponticin (trans-3,5,3′-trihydroxy-4′-methoxystilbene 3-O-β-D-glucoside), and its aglycone, rhapontigenin, are primarily found in various rhubarb species from the Rheum family and could accumulate up to 40.8 mg/g in the dry root of Rheum rhaponticum (Kolodziejczyk-Czepas and Czepas, 2019; Yang, Dai et al., 2024). Like other stilbenoids, these compounds occur naturally in both cis and trans form in plants, though chiefly in trans isoform. Rhapoticin shares a similar molecular structure with resveratrol, distinguished by a substituted glucose group at 3-position and a methyl group at 4′-position. Notably, rhaponticin exhibits strong anti-inflammatory effects. Both rhaponticin and rhapontigenin have been shown to effectively inhibit pro-inflammatory enzymes and transcription factors (Kageura et al., 2001; Kutil et al., 2015). Moreover, rhaponigenin demonstrates stronger inhibitory activity on NO production than resveratrol in macrophages (Yamamoto et al., 2017).
Structural analysis of resveratrol and its analogs enables us to recognize that structural variations, particularly the numbers and position of hydroxyl or methoxyl groups on the rings, are closely related to anti-inflammation, anti-oxidative and free-radical scavenging capacity, and other redox-related properties, which are believed to be pivotal in their overall bioactivities (Murias et al., 2005).
| 3. Major mechanisms involved in stilbenoids bioactivities | ▴Top |
The antioxidant effects of resveratrol and its stilbene analogs have protected ROS-induced tissue damage in organs like the retina, kidney, heart, and nerves in diabetic complications in in vivo studies. Such protection is mediated by various mechanisms, including reduced production of reactive oxygen species (ROS) and nitric oxide (NO) level (Ates et al., 2007; Kutil et al., 2015), attenuated lipid peroxidation ( Torres-Cuevas et al., 2021), downregulation of NADPH oxidase (Wu et al., 2016), elevated levels of glutathione (GSH), and detoxifying enzymes such as superoxide dismutase (SOD) (Fang et al., 2018; Sadi and Konat, 2016; Shen and Rong, 2015), glutamine synthetase (GS) (Zeng et al., 2016), catalase (CAT) (Kumar et al., 2007) and glutathione peroxidase (GPx) (Zhang et al., 2019). Key signaling pathways involved in these effects include Nrf2/HO-1 up-regulation (Kosuru et al., 2018; Li et al., 2019; Lv, Du, Zhang and Zhang, 2019; Zhang et al., 2021), PKC inhibition (Giordo et al., 2021), PI3K/Akt/GSK-3β/Nrf2 activation (Malik et al., 2019), Sirt1/FOXO3 modulation (Wang et al., 2017) and AMPK pathway (Wang, Li et al., 2020). Furthermore, rhapontigenin and polydatin have also shown anti-oxidative effect by reducing lipoxygenase (LOX) level (Ngoc et al., 2008) and activating SIRT1 (Liu, Liu, Xu and Ding, 2024; Kawakami et al., 2014).
Resveratrol and its related derivatives also exhibit effective inhibition on inflammation. Studies reveal that these oligomeric stilbenoids can suppress inflammation under in vitro and in vivo conditions and decrease the levels of inflammatory cytokines such as interleukin (I-1β), tumor necrosis factor (TNF)-α and IL-6 (Soufi et al., 2015; Cai et al., 2020). The inhibition of NF-κB with broad upstream and downstream targets is one of the primary anti-inflammation mechanisms (Huang, Xu et al., 2017; Soufi et al., 2015; Xie et al., 2012). Moreover, polydatin demonstrates anti-inflammation effects by regulating ICAM, ICAM-1, NLRP3 and the MAPK pathway (Lv, Du, Liu et al., 2019; Peritore et al., 2021). These stilbenoids also contribute to the inhibition of advanced glycation end products (AGEs) and their receptors (RAGE) (Xian et al., 2020). Furthermore, resveratrol stilbenoids have shown regulatory effects on inflammation-induced stress signals in microglia (Carey et al., 2013) and have attenuated cognitive behavioral dysfunction (Joseph et al., 2008). It was reported that compared to resveratrol, pterostilbene owns a better neuromodulatory activity in aging and Alzheimer’s disease (Chang et al., 2011).
The anti-apoptotic effect represents another important bioactivity of these stilbenoids. Resveratrol and its closely derivatives significantly downregulate apoptotic markers sucha s caspase-3 and caspase-9 while upregulate Bcl-2, hence reducing cell apoptosis and associated tissue damages (Shah et al., 2019; Soufi et al., 2015; Li et al., 2019; Wang, Li et al., 2020). These actions involves the modulation on several signaling pathways, namely the PI3K/Akt/FOXO3a, (Wu et al., 2017), NLRP3 (Li et al., 2018), microRNA-29b/specificity protein 1 (Zeng et al., 2017) and AMPK/Sirt1/PGC-1α (Li et al., 2017) pathways. In addition, the stimulation of autophagy (Huang, Ding et al., 2017), attenuation of related endoplasmic reticulum stress (Guo et al., 2015), and activation of inositol requiring 1alpha (IRE-1α) /JNK pathways (Wang et al., 2011; Malaguarnera, 2019) could also contribute to the actions of these resveratrol-associated stilbenoids.
Beyond the bioactivities described above, resveratrol and associated stilbenes have also demonstrated effects in alleviating pathological changes, such as the inhibition of angiogenesis and fibrosis. For example, in diabetic retinopathy, stilbenoids compound can suppress the proliferation and migration of endothelial cells (Rokicki et al., 2014; Shen and Rong, 2015). The AMPK and PI3K pathways are suggested to contribute to the inhibition of retinal epithelial cell migration (Chan et al., 2013). Extensive studies on resveratrol have been conducted, revealing its effective inhibition of growth factors including vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β1 in retinal and renal cells as well as in diabetic retinopathy rats , thereby mitigating pathological angiogenesis (Chen et al., 2019; Kim et al., 2012; Wen et al., 2013). Studies have also revealed that resveratrol can attenuate renal fibrosis by regulating AMPK/NADPH oxidase 4/ROS pathway (He et al., 2016), decreasing fibronectin production (Gong et al., 2020), inhibiting p38 MAPK and TGF-β1 (Qiao et al., 2017), suppressing endothelial to mesenchymal-transition (EndTM) by activating SIRT1 (Du et al., 2021), and inhibiting PKC/NADPH oxidase/ROS pathway (Giordo et al., 2021). Similarly, pterostilbene and trans-2,3,5,4′-tetrahydroxystilbene (trans-THSG) effectively reduced fibronectin secretion by down-regulating TGF-β/drosophila mothers against decapentaplegic protein 1 (Smad1) pathway (Zhang, Ren et al., 2019) and inhibiting renin-angiotensin system (Chen, Yang et al., 2016). Moreover, resveratrol inhibited myocardial fibrosis through its antioxidant function (Wang et al., 2018). It exhibited strong anti-proliferative effects on mouse cardiac fibroblast cells through ROS/ERK signaling pathway and ameliorated myofibroblast cell differentiation through the ROS/ERK/TGF-β/periostin pathway (Wu, Li et al., 2016). Similar effects were observed in oxyresveratrol, pterostilbene and trans-2,3,5,4′-tetrahydroxystilbene, as they exhibited hepatic protective activity via inhibiting liver and renal fibrosis (Long et al., 2019; Yang et al., 2021) by elevating antioxidant activity, inhibiting expression of TGF-β and its downstream activation of ERK1/2 and Smad1/2 (Yang et al., 2021; Zhan et al., 2021).
| 4. Bioavailability and pharmacokinetics | ▴Top |
4.1. Resveratrol and polydatin
Following oral administration, resveratrol undergoes rapid absorption in the gastrointestinal (GI) tract, with peak plasma concentrations (Cmax) occurring within the first 30 min and 1.5–2 h after low and higher doses in fasting status, respectively (Table 1). Notably, fed states can greatly affect influences its pharmacokinetics. For instance, Cmax can be delayed from 3 h to 5 h post-administration in mice or rats when switching from a standard to a high-fat diet (Huang et al., 2019). Urine and feces analyses estimate the absorption of resveratrol in both human and rats at around 75%, mainly attributed to transepithelial diffusion (Li et al., 2003; Soleas et al., 2001; Walle et al., 2004). However, its oral bioavailability is limited due to extensive first-pass metabolism in the intestine and liver, and neither dose escalation nor repeated dosages significantly improved it (Soleas et al., 2001; Walle, 2011). Depending on the solubilizing formulation systems, the absolute oral bioavailability of resveratrol exhibits considerable variability ranging from 2.6% to 46.4% (Ha et al., 2021). While it is challenging to detect the free form of resveratrol in circulating plasma due to extremely low concentration, tissue accumulation has been observed in the kidney, liver, brain, and intestine (Huang et al., 2019; Walle, 2011). This finding is consistent with its relatively high volume of distribution (Vd), indicating substantial extravascular distribution of resveratrol (Huang et al., 2019; Walle et al., 2004). Metabolic studies reveal that resveratrol’s major metabolites post-absorption are glucuronides and sulfates, suggesting the involvement of enterohepatic circulation (Wenzel and Somoza, 2005). Unlike resveratrol which is absorbed in the intestine primarily through transepithelial diffusion, polydatin has an additional glucose group at the 3-position. While this enhance its solubility, it may concurrently impede its absorption into the bloodstream as active glucose transporters are required (He et al., 2007). Nevertheless, polydatin concentration in rat serum was observed to be 3–4 times higher than that of resveratrol following a 200 mg/kg oral does (Wang et al., 2015). Upon oral co-administration of polydatin and resveratrol in the form of Ramulus Cinnamomi extract, both compounds were quickly absorbed, reaching Tmax within 0.5 h. However, polydatin showed a 4.7-fold greater AUC0-t and 2.5-fold higher Cmax with a shorten t1/2, suggesting an enhanced absorption but more rapid elimination in the blood (Yang, Wang et al., 2024). Notably, it is crucial to recognize that other co-existing components within this herbal extract may have profound impacts on the pharmacokinetic profiles of these two components (Yang, Wang et al., 2024).
 Click to view | Table 1. Pharmacokinetic values of resveratrol and its derived stilbenes |
4.2. Pterostilbene
The pharmacokinetics of resveratrol and pterostilbene has been comprehensively compared by Wang and Sang (2018). As illustrated in Table 1, pterostilbene exhibits a superior pharmacokinetic profile over resveratrol due to dimethyl ether structure, manifesting as higher metabolic stability and bioavailability (Azzolini et al., 2014; Liu et al., 2020; Wang and Sang, 2018). Moreover, dose-escalation lead to an increase in Tmax, suggesting that the pterostilbene absorption is a capacity-limited process (Yeo, Ho, and Lin, 2013). The distribution of pterostilbene in various tissues has been reported, with Deng et al. (2015) specifically highlighting its ability to pass through the blood-brain barrier. In parallel, another research reveals that pterostilbene concentrations in certain tissues can be much higher than that in blood, potentially explaining its bioactivity despite low circulating plasma level (Azzolini et al., 2014). In mice, rats, and human, pterostilbene undergoes phase II metabolism, with sulfation and glucuronidation being the dominant metabolic pathways. In particular, a comparative study in human liver microsomes demonstrated pterostilbene’s better metabolic stability over resveratrol, as more than 75% of pterostilbene remained unchanged while 68% of resveratrol went through glucuronidation (Dellinger, Garcia, and Meyskens, 2014). Elimination of pterostilbene occurs mainly through renal and hepatic excretion. Notably, increasing the dosage resulted in a reduced plasma elimination rate, leading to non-linear pharmacokinetics, which indicates the saturation of related enzymes (Yeo, Ho, and Lin, 2013).
4.3. THSG
The pharmacokinetic profile of THSG remains comparatively underexplored. Limited studies in rats show that THSG can be rapidly absorbed after oral administration, with a Tmax of 10–30 min and a half-elimination time (T1/2e) of 50–120 min (Table 1) depending on the dose (Dong et al., 2014; Sun et al., 2018; Zhao, Cheng, et al., 2013). THSG is widely distributed following absorption, mainly to the heart, kidney, liver, and lung (Zhao, Zhang et al., 2013). This widespread tissue distribution is also supported by a Vd of approximately 3.94 L/kg, thereby suggesting its potential biological function at extrahepatic sites (Zhao, Cheng, et al., 2013). While one study reported that around 81% THSG was present as phase II metabolites (Dong et al., 2014), other two studies revealed a low recovery of unchanged THSG, implying that it was primarily excreted as metabolites through feces, most likely as monoglucuronides (Zhao, Cheng, et al., 2013; Zhao, Zhang, et al., 2013).
4.4. Piceatannol
In rat, the total area under the curve (AUC) of piceatannol (include both intact and conjugate form) is lower than that of resveratrol. However, the ratio of intact piceatannol AUC to total AUC was 3.7- to 4.3-fold higher than that of resveratrol (Table 1), suggesting greater metabolic stability of piceatannol (Setoguchi et al., 2014). While phase I metabolism appears to be insignificant in the metabolism of piceatannol, it can be metabolized to a few compounds following intravenous administration, through glucuronidation, sulfation, and methylation, with piceatannol-monoglucuronide being the most abundant (Dai et al., 2020; Setoguchi et al., 2014). Also, piceatannol can be effectively converted to active monomethylated derivatives, rhapontigenin and isorhapontigenin, after oral administration, compensating for oxyresveratrol’s lower plasma exposure as parent compound (Dai et al., 2020). Although piceatannol undergoes extensive hepatic glucuronidation and exhibits a relatively low absolute bioavailability of 6.99 ± 2.97% (Dai et al., 2020), it has a relatively high Vd of 10.76 L, indicating its wide distribution in tissues (Roupe et al., 2006). It also possesses a long half-life of 2–3 h and is predominantly eliminated via the hepatic pathway with a limited clearance rate (Dai et al., 2020; Roupe et al., 2006). Moreover, the pharmacokinetic profile of piceatannol can be improved when co-administrated with α-cyclodextrin (Inagaki et al., 2016).
4.5. Oxyresveratrol
Based on limited data, oxyresveratrol was rapidly absorbed from the GI tract after oral administration, with a short Tmax of 15 min. It is further excreted in both bile and urine, predominantly as monoglucuronide and monosulfate (Huang et al., 2010; Huang et al., 2008). Specifically, oxyresveratrol-2-O-β-D-glucuronide has been identified as the primary glucuronide metabolite in human liver and intestine (Hu et al., 2014). Despite a relatively low absolute bioavailability of around 10% after a single oral dose, oxyresveratrol demonstrates a long residence time in circulation. While dose escalation was correlated with a significantly improved absolute bioavailability, consecutive doses for 1 week did not yield further enhancement (Chen, Yeo et al., 2016). The pharmacokinetic profile of oxyresveratrol was improved when used in combination with piperine (Junsaeng et al., 2019).
4.6. Rhaponticin and rhapontigenin
The glucose group on the rhaponticin enhance its solubility compared to oxyresveratrol. However, rhaponticin’s oral bioavailability was remarkably low, at only 0.03%, primarily due to its rapid metabolism (Zhao et al., 2012). Roupe et al. (2006) reported that rhapontigenin, administrated at a dosage of 10 mg/kg, has a half-life around 3 h in rat plasma, implying a fast elimination from blood, although it is longer than that of resveratrol. The Cmax of rhapontigenin in murine plasma was only 8.91 µg/mL 5 min after a 100 mg/kg oral dosage (Zhao et al., 2012) while a lower plasma concentration of rhaponticin at 1.71 µg/mL was observed. Overall, both rhaponticin and rhapontigenin exhibit characteristics of poor solubility and rapid metabolism (Zhao et al., 2012).
In brief, the therapeutic promise of resveratrol and its close derivatives is often hindered by their pharmacokinetic profiles, characterized by poor oral bioavailability and rapid metabolism and clearance. As the dominance of Phase II metabolism appears as a recurring theme for stilbene metabolism, rapid first-pass metabolism is identified as a major contributor to their low bioavailability. However, studies reported above also demonstrate that structural variation often result in different metabolic patterns and that in some cases, stilbene absorption is a capacity-limited process by which higher dose can mitigate the first-pass barrier. Neverthelss, rhaponticin, despite possessing a higher solubility, still suffers from exceptionally low bioavailability, hence suggesting that improved solubility alone is insufficient to guarantee a favorable pharmacokinetic profile if rapid metabolism and clearance predominates. Therefore, close examination and in-depth understanding of stilbene’s metabolic fates are crucial for effective formulation strategies.
| 5. Improvement of metabolic profiles | ▴Top |
In addition to the pharmacokinetic limitations, the poor aqueous solubility and instability against environmental conditions such as moisture, light, heat or high temperature, oxygen and the combination of two or more above factors are also involved (He et al., 2018; Zhang et al., 2014; Zupančič et al., 2015). Therefore, improvement of their water solubility and bioavailability using different strategies is vital for unlocking their preventive or therapeutic potentials. Since structural modification aimed at enhancing bioavailability may interfere with essential physiological functions and introduce uncertain toxicity, research have focused on developing diverse oral delivery systems, such as the recent trends on nano-based systems (Peng et al., 2018).
5.1. Summary of phytochemical delivery systems
Among nano-based systems, fabrication of stilbenoids-loading nanoparticles is a theme of current relevance as it allows the use of broad biocompatible materials, particularly food-grade materials which are considered safe to use. This nanotechnology enables researchers to design a smart stilbene delivery system in which the timing and spot for the deconstruction of the coating layer can be controlled by choosing materials with different chemical properties. In general, owing to the protection from the coating layer, fabricated nanoparticles of stilbenoids have a higher resistance to the change in pH and metabolic enzymes during digestion and metabolism, thus contributing to better stability, prolonged residence time, and achieving an improved controlled-release profile. Meanwhile, reduced particles size and modified surface facilitate an enhanced cellular uptake of encapsulated compounds. Coupled with increased solubility, these attributes collectively improve their overall bioavailability. Some original research and reviews that reported the improvement in the stability, solubility and/or bioavailability of stilbenoids’ nanoemulsions or nanoparticles are summarized in Table 2 and also in the following list: resveratrol (Mohseni et al., 2019; Santos et al., 2019; Yang, Wang, et al., 2019), pterostilbene (Liu et al. 2019; Peng et al., 2018; Tzeng et al., 2021; Zou et al., 2021), THSG (Liu et al., 2022), piceatannol (Aljabali et al., 2020), and oxyresveratrol (Sangsen et al., 2016). Moreover, in the last five years, research focusing on the comparison of bioactivities between free form stilbenoids and their nanoparticles in various disease models (Chung et al., 2020; Yang, Wang, et al., 2019) have gradually emerged, among which a more potent effect of nanoparticles in diabetes and diabetic complications was also indicated (Dong et al., 2019; Mohseni et al., 2019).
 Click to view | Table 2. Delivery examples of resveratrol and close stilbenoid compounds |
5.2. Examples of stilbene delivery systems
5.2.1. Resveratrol
A near-spherical shaped nanosuspension of resveratrol was prepared containing 0.38% of polyvinylpyrrolidone K17 and surfactant F188 (3.63%) and study of in vivo pharmacokinetics revealed that the bioavailability of resveratrol suspension was improved with the increase of Cmax and AUC values by 3.35- and 1.27-fold comparing to that of resveratrol alone, respectively (Hao et al., 2015). In a solid lipid nanoparticle system with stearic acid as the solid-lipid core, the encapsulation efficiency of resveratrol reached 79.9%. In the oral feeding to Wistar rats, the resulted nanoparticles of SLN-resveratrol exhibited an initial burst release followed by a sustained resveratrol release in natural conditions. Both AUC and Cmax of SNL-resveratrol were increased compared to those of resveratrol suspension, and t1/2 was 2.37 and 11.51 h for suspension and SNL of resveratrol respectively. In terms of efficacy, oral administration of SLN- resveratrol exhibited stronger effect in preventing weight loss and reducing blood glucose levels compared to RES alone (Pandita et al., 2014).
The application of emulsion formulation of resveratrol has been widely explored. Employing liquid and semi-solid self-emulsifying drug delivery systems, micro-emulsions of resveratrol was created with a droplet size of approximately 100 nm. An ex vivo study with the jejunum of rats demonstrated that the permeability of the micro-emulsion resveratrol was significantly enhanced, showing an 8.5-fold increase for the resveratrol-nanoemulsion compared to a resveratrol dispersion in an ethanolic medium with the preservation of the intestinal functional viability (Mamadou et al., 2017).
The encapsulation of resveratrol in liposomes has also demonstrated effectiveness. A study by Soo et al. (2016) developed a dual carrier system to co-encapsulate pure resveratrol with cyclodextrin-resveratrol inclusion complexes in both the lipophilic and hydrophilic compartments of liposomes. The final formulation exhibited a particle size of 131 ± 1.30 nm, a polydispersity index of 0.089 ± 0.005, and a zeta potential of −2.64 ± 0.51 mV. In contrast to free resveratrol and conventional liposomal formulations that showed a drug release profile of 40–60%, this nanoformulations exhibited complete (100%) drug release within 24 h (Soo et al., 2016). Recently, different particle sizes of resveratrol nanoliposomes were created using a thin-film hydration technique and results showed that small size liposomes (<100 nm) more effectively enhanced aqueous solubility, cellular permeability and cellular antioxidant activity (Baek, Jeong and Lee, 2023).
5.2.2. Polydatin
A liposome formulation of polydatin has been developed using a membrane dispersion method (Zhang et al., 2024). The liposome was prepared from lethicin, DSPE-PEG-2000 and cholesterol. The resulted polydatin liposome had a sustained release of polydatin, prolonged in vivo circulation time and dramatically improved bioavailability. Comparing to free polydatin, the yielded polydatin liposome had an increased Tmax (60 min vs 30 min), T1/2 (5.15 ± 0.47 vs 1.02 ± 0.07 h), AUC (13.03 ± 0.30 vs 3.58 ± 0.14 mg h/mL), and mean residence time (MRT, 12.02 ± 2.28 vs 3.32 ± 1.20 h) in SD rats bioavalability evaluation (Zhang et al., 2024). Moreover, the liposome of polydatin demonstrated more effective hypoglycemic effects than that of free form.
5.2.3. Oxyresveratrol
Formulation of oxyresveratrol has been challenging for its low aqueous solubility, poor bioavailability and instability. Cyclodextrins were often used for oxyresveratrol encapsulation to improve bioavailability and stability. Solid lipid nanoparticles were also used to entrap oxyresveratrol to improve stability and bioavailability (Likhitwitayawuid, 2021). Sangsen et al. (2016) examined the influence of different ratios of surfactants in oxyresveratrol microemulsifyings on the bioavailability enhancement and they found that Cmax, AUC and relative bioavailability were increased dramatically compared to free form of oxyresveratrol. For example, for oxyresveratrol delivered in the microemulsion using high percentage of Tween80®, Cmax increased to 2.36 µg/mL from 0.66 µg/mL of unformulated oxyresveratrol (Sangsen et al., 2016). Another bioavailability-enhancing method was the combination of oxyresveratrol with a bioenhancer piperine (Junsaeng et al., 2019). The combination increased oxyresveratrol bioavailability by 2–6 folds, and the Cmax reached 1.5 mg/mL within 1–2 h after oral dosing to rats.
5.2.4. Piceatannol
α-Cyclodextrin has been used in the emulsion formulation of piceatannol (Inagaki et al., 2016). When mixed with artificial gastric juice, the solubility of piceatannol was higher as a result of co-formulation with α-cyclodextrin and reached 35.0 ± 0.8 mM immediately, while that of piceatannol in its free form is about 10 folds lower (3.2 ± 0.1 mM). The bioavailability of piceatannol was significantly enhanced, indicated by the increase in AUC0-3h from 4.8 to 7.0 µmol h/L or more, depending on the amount of α-cyclodextrin used among different formulations. The Cmax value of piceatannol increased from 2.5 µmol/L in its free form to 5.3, 4.8 and 5.8 µmol/L when formulated with low, medium and high concentration of α-cyclodextrin in emulsions, respectively (Inagaki et al., 2016).
5.2.5. Pterostilbene
By encapsulating pterostilbene in oil-in-water nanoemulsion through high pressure homogenization, Liu et al. (2019) reported delivery systems that enhanced its solubility and bioavailability and demonstrate the potential impact of lipid compositions on the stability and performance of nanoemulsion. To mimic the in vivo digestion process, pterostilbene loaded-nanoemulsions was exposed to simulated stomach and intestinal conditions and after digestion, the bioaccessibility of pterostilbene in MCT oil-nanoemulsion(98.7 ± 3.9%) was significantly higher than control (< 20%) and other two nanoemulsions that utilized oils with a higher content of long-chain fatty acids such as sunflower oil (41.3 ± 0.4%) and olive oil (32.9 ± 1.9%) . In addition, Caco-2 cells were used to simulate intestinal epithelial cell barrier in gut and further assess the effect of nanoemulsion formulation on the permeability and bioavailability of pterostilbene. The apparent permeability coefficients of pterostilbene in micelles obtained from digesta of MCT based-nanoemulsion was the higher than that of unencapsulated pterostilbene by 5.7-fold, reaching 8.21 ± 2.09 × 10−6 cm S−1, which was attributed to higher solubility of pterostilbene in MCT oil.
5.2.6. THSG
To address THSG’s sensitivity to environmental stress, a nanoparticle composed of carboxymethyl chitosan and chitosan hydrochloride was developed. This formulation not only improved its stability against heat and solar radiation but also exhibited a great controlled release profile under gastrointestinal pH condition post-administration, thus offering a potential solution for overcoming the current limitations of THSG (Liu et al., 2022). Regarding the enhancemnet of THSG’s bioavailability especially intestinal absorption, one investigated strategy is its co-administration with polysaccharides from Ophiopogon japonicus. This approach resulted in a improved solubility and stability of THSG in aqueous solution and further improvement in metabolic profile, increasing the Tmax, Cmax and AUC0-tn to 3.5-, 1.45-, and 2.32-fold, respectively, relative to pure THSG. However, these were accompanied with a slight decrease in permeability (Sun et al., 2018). Another explored strategy is to apply THSG in transdermal drug delivery system through skin. In a recent study, a gel system was developed wherein THSG was loaded in oleic acid-containing vesicles, which were further incorporaed into a complex gel matrix. This novel gel system successfully increased the transdermal flux of THSG by about 4-fold, potentially due to the disruption of stratum corneum integrity (Lai et al., 2020).
5.2.7. Rhaponticin and rhapontigenin
Offering multiple health benefits, rhaponticin and rhapontigenin are considered as great candidates for dietary supplement. However, rhaponticin’s poor aqueous solubility and fast metabolism remain significant challenges that must be solved for further development. Therefore, several formulation methods were tested to improve their characteristics. PEGylated liposome, commonly employed in pharmaceutical research and industry, has been utilized to construct carrier for rhaphoticin and improve its solubility. Compared to oral administration of free rhaponticin, rhaponticin encapsulation by PEGylated liposome resulted in a smaller Cmax (0.7-fold), longer Tmax (4.5-fold) and T1/2 (1.9-fold), and higher AUC0-tn (5.45-fold), indicating a slower release, yet higher concentration of rhaponticin, and better capacity to withstand plasma clearance. Further in vivo study also revealed a stronger anti-tumor effect compared to free rhaponticin, potentially attributed to the delivery of a larger amount of rhaponticin and higher efficiency in cellular uptake (Sun and Zhao, 2012). Another formulation strategy to enhance rhaponticin bioavailability invovles the synthesis of folate-targeted rhaphonticin conjugate. Due to its linkage with folic acid, this conjugate exhibited improved water solubility and a high affinity for folate receptor-positive cell. Spontaneous release of rhaponiticin inside endosomes upon disulfide bond reduction was confirmed, and results from an in vivo tumor model showed an enhanced therapeutic effect, along with reduced toxicity (Liang et al., 2013).
| 6. Conclusion | ▴Top |
Resveratrol and related stilbenoids, characterized by a highly conjugated 1,2-diphenylethylene structural skeleton, are widely distributed in food sources. These phytochemicals have been reported to provide health benefits across various conditions, including but not limited to diabetes and its complications, brain and cardiovascular diseases. However, their utilization as therapeutic candidates, either as dietary supplements or as potential pharmaceutical interventions, needs further investigation. Despite extensive research on the pharmacokinetic behaviors of resveratrol over recent decades, comparatively limited works were conducted for other stilbenoid compounds. Collective studies reveal that resveratrol and related compounds share common characteristics such as poor absorption, extensive metabolism and rapid elimination in blood. These characteristics remain primary challenges for the in-depth discovery of stilbenes’ true biological effects, as therapeutically relevant concentrations often cannot be reached when delivered in their free form. Therefore, rising trends of investigation on formulation strategies and delivery systems have been observed. Currently, common approaches including nanoparticles, liposome and nanoemulsions are applied, and they are proved effective in improving stability against environmental stress and enhancing bioavailability, either through increased solubility or enhanced pharmacokinetic profiles such as permeability and absorption. However, further validation using in vivo study is needed given the prevalence of in vitro study in some case studies. In addition, beyond the aforementioned formulation strategies, alternative formulation methods, or the combination of multiple forms of delivery systems are also promising approaches for future research. For instance, incorporating nanoparticles into hydrogel may further enhance their release profiles and allow desired characteristics such as targeted release in the intestinal tract, topical application, or in-situ administration, thereby providing sustained and localized drug delivery with greater efficacy.
| References | ▴Top |