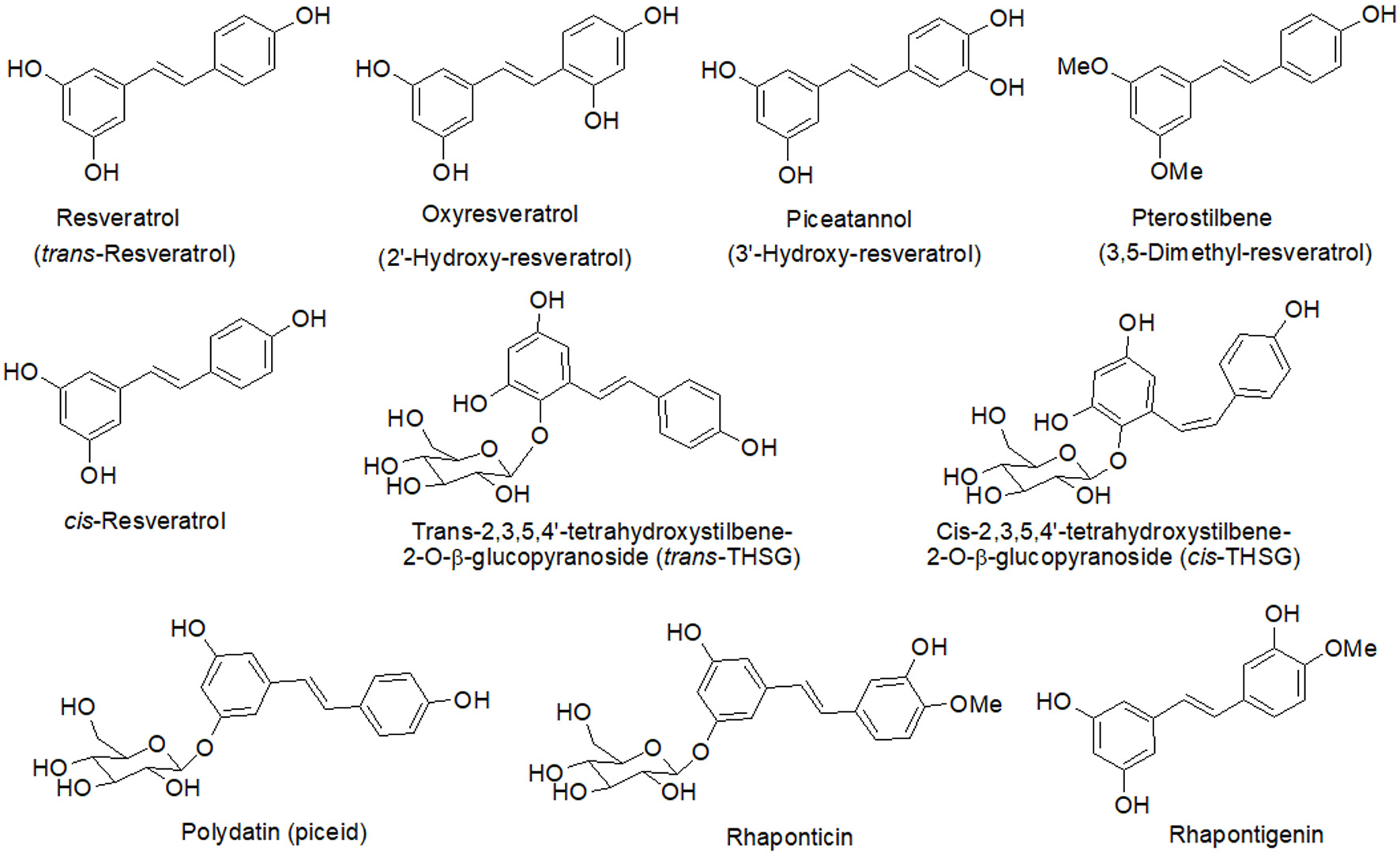
Figure 1. Chemical structures of resveratrol and its close derivatives.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 30, June 2025, pages 6-18
Pharmacokinetic profiles and improvement of resveratrol and derived stilbenes
Figure

Tables
| Stilbenoids | Solubility | Bioavailability | Cmax ( µg/mL) | Time to Cmax | Vd (L/kg) | T1/2 (h) | AUC (μg h/ mL) | Major metabolites | References |
|---|---|---|---|---|---|---|---|---|---|
| Resveratrol | <0.05 mg/mL | 2.6–46% (human); 20–29% (rat, dose 50 mg/kg) | 0.073–0.539 | 30 min low dosage; 1.5–2 h high dose | 2.24–2.49 | 1 to 3 | 5.05 (mice); 0.179 ± 0.079 (human) | Glucuronides Sulfates | Das et al., 2008; Ha et al., 2021; Kapetanovic et al., 2011; Sergides et al., 2015; Johnson et al., 2011; Wang and Sang, 2018; Wenzel and Somoza, 2005; Yang, Wang et al., 2024 |
| Polydatin | 0.16 mg/mL | 2.9% | 12.63 | 0.33 h | 63.83 | 2.48 ± 0.99 | 14.31 ± 0.35 | Glucuronides | Yang, Wang et al., 2024 |
| Pterostilbene | 0.018 mg/mL | 80% (rat, dose 20 mg/kg) | 0.415–1.038 | 90–300 min | 2.5–3.0 | 1.57 ± 0.37 | 4.43 – 11.9 (mice) | Sulfates; Glucuronides | Sun et al., 2018; Dellinger, Garcia, and Meyskens, 2014; Kapetanovic et al., 2011; Remsberg et al., 2008 |
| Trans-THSG | 10 mg/mL | na | 31.91 | 10–30 40min | 3.94 | 0.83 - 2 | 639.9 ± 347.2 (rat) | Monoglucuronides | Zhao, Cheng et al., 2013; Zhao, Zhang et al., 2013 |
| Piceatannol | <0.5 mg/mL | 6.99 ± 2.97% (human); 50.7% (rat, dose 10 mg/kg) | 2.0 | 15 min | 10.76 | 4.23 | 508.8 | Monoglucuronide; Sulfates; methylation; rhapontigenin | Roupe et al., 2006; Setoguchi et al., 2014 |
| Oxyresveratrol | 0.6 mg/mL | 10–15% | 1.5 | 1–2 h | 105 | 1.70 ± 0.77 | 5.43 ± 1.02 (rat) | monoglucuronide and monosulfate | Huang et al., 2010; Hu et al., 2014; Junsaeng et al., 2019 |
| Rhaponticin | 0.109 mg/mL | 0.03% | 1.71 | 10.32 min | 62.02 | 3.0 ± 1.35 | 52.8 | Rhapontigenin monoglucuronide and monosulfate | Roupe et al. (2006); Zhao et al., 2012 |
| Rhapontigenin | 0.11 mg/mL | na | 0.18 | 52.5 min | 0.011 | 3 | 42 | monoglucuronide and monosulfate | Roupe et al., (2006); Navarro-Orcajada et al., 2023 |
| Stilbene compounds | Delivery methods | Core components | Evaluation model | Results | References |
|---|---|---|---|---|---|
| Cl: clearance; MCT: medium chain triglyceride; MRT: mean residence time; SD: Spraw-Darley. | |||||
| Resveratrol | Nanosuspension | Polyvinylpyrrolidone, mannitol | Wistar rats | Bioavailibilty (AUC & Cmax)↑ ,Vd↓ | Hao et al., 2015 |
| Nanoemulsion | long-chain triglycerides | Wistar rats | Bioavailibilty (AUC & Cmax)↑ , absorption↑, permeability↑ | Singh and Pai, 2015; 2016 | |
| Polymer nano-particles | carboxymethyl chitosan | Wistar rats | Bioavailibilty (AUC & Cmax)↑, tmax↑ | Zu et al., 2014 | |
| Protein nano-particles | Zein | Wistar rats | Bioavailibilty (AUC & Cmax)↑, tmax↑ | Penalva et al., 2015 | |
| Solid lipid nanoparticles | Stearic acid | Wistar rats | Bioavailibilty (AUC & Cmax)↑, tmax↑, Cl↓ | Pandita et al., 2014 | |
| Cyclodextrin | β –cyclodextrin | SD rats | Cmax↑ | Das et al., 2008 | |
| Polydatin | Liposome | Lethicin, cholesterol | SD rats | AUC↑, Tmax↑, T1/2↑, MRT↑ | Zhang et al., 2024 |
| Oxyresveratrol | Microemulsion | Tween80®, Labrasol® | Wistar rats | Bioavailibilty (AUC & Cmax)↑ | Sangsen et al., 2016 |
| Bioenhancement | Piperine | Wistar rats | Bioavailibilty↑(2–6 fold), Cmax↑ | Junsaeng et al., 2019 | |
| Piceatannol | Emulsion | α-Cyclodextrin | SD rats | Bioavailibilty (AUC0-3h & Cmax)↑ | Inagaki et al., 2016 |
| Pterostilbene | Nanoemulsion | Lipids: MCT, sunflower oil or olive oil | Caco-2 cell monolayer | Bioaccesibility↑; Apparent permeability coefficient↑ | Liu et al., 2019 |
| Nanoparticle | Soybean lecithin, D-alpha-tocopheryl polyethylene glycol succinate | N/A | Solubility↑ anti-tumor acitivity↑ | Zou et al., 2021 | |
| Trans-THSG | Nanoparticle | Carboxymethyl chitosan and chitosan hydrochloride | Simulated environmental stress | Heat and solar radiation stability↑; Improved pH release profile | Liu et al., 2022 |
| Vesicles-containing gel system | Oleic acid, calcium chloride, sodium alginate | Franz diffusion cell | Trasdermal flux↑ | Lai et al., 2020 | |
| Rhaponticin | Vesicles-containing gel system | Polyethylene glycol; Cholesterol, egg phosphatidylcholine, hydrogenated soybean phosphatidylcholine | Female nude mice | Cmax ↓, Tmax ↑, T1/2 ↑ AUC0-tn↑ | Sun and Zhao, 2012 |
| Emulsion | Folic acid | Balb/c mice | Water solubility↑ receptor affinity↑; Therapeutic effect↑, toxicity↓ | Liang et al., 2013 | |