| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 29, March 2025, pages 77-84
Propolis targets macrophage activity to ameliorate inflammatory bowel disease
Xinru Yanga, #, Jingwen Hea, #, Hui Zhaoa, Jie Pana, Yuting Gaob, *, Qi Tanga, *
aTianjin Key Laboratory of Food and Biotechnology, Tianjin International Joint Center of Food Science and Engineering, State Experimental and Training Centre of Food and Drug, School of Biotechnology and Food Science, Tianjin University of Commerce, No. 409 Guangrong Road, Beichen, Tianjin 300134, China
bCollege of Life Sciences, Shanxi Agricultural University, Jinzhong 030801, Shanxi, China
#These authors contributed equally to this article.
*Corresponding author: Yuting Gao, College of Life Sciences, Shanxi Agricultural University, Jinzhong 030801, Shanxi, China. E-mail: 1120190183@mail.nankai.edu.cn; Qi Tang, Tianjin Key Laboratory of Food and Biotechnology, Tianjin International Joint Center of Food Science and Engineering, State Experimental and Training Centre of Food and Drug, School of Biotechnology and Food Science, Tianjin University of Commerce, No. 409 Guangrong Road, Beichen, Tianjin 300134, China. E-mail: tangqi@tjcu.edu.cn
DOI: 10.26599/JFB.2025.95029407
Received: March 10, 2025
Revised received & accepted: March 29, 2025
| Abstract | ▴Top |
Propolis is a gelatinous mixture produced by honeybees for maintaining the structural integrity and aseptic environment of the hive. The bioactive compounds are crucial for the health effects of propolis. Given that propolis is generated by honeybees, its exact bioactives and health benefits are varied due to the sources of materials. Here we investigated whether propolis collected from Yaunqu County, Shanxi ,China, potentially protected against mice intestinal damage-induced by dextran sulfate sodium (DSS). Our results indicated that propolis effectively alleviated DSS-induced intestinal injury and the mechanisms behind it involving in targeting macrophage inflammation including pro-inflammatory cytokines, pro-inflammatory signal pathways Nuclear Factor kappa B (NF-κB) and the mitogen-activated protein kinase (MAPK), and tissue factors. This research enriches the evidence of propolis as a candidate for protection against intestinal injury.
Keywords: Propolis; Inflammation; Macrophage; Intestinal damage; Nuclear Factor kappa B (NF-κB)
| 1. Introduction | ▴Top |
Propolis is a gelatinous mixture produced by honeybees rich in plant resins, salivary enzymes (such as β-glucosidase), and beeswax, which is crucial for maintaining the structural integrity and aseptic environment of the hive (de Groot, 2013; Pasupuleti et al., 2017). Its chemical composition is complex, comprising 45% plant resins, 30% beeswax and fatty acids, 10% essential oils, 5% pollen, and 5% organic compounds. More specifically, propolis contains active components such as flavonoids, phenolic acids, and terpenoids, and is characterized by significant geographic and botanical diversity (Patel, 2016; Saavedra et al., 2016). For instance, temperate region propolis, primarily derived from poplar species (rich in chrysin, quercetin, and galangin), contrasts with Brazilian green propolis from the Baccharis genus, which contains prenylated phenylpropanoids (e.g., Artepillin C), while Russian sources are rich in birch propolis (Isidorov et al., 2016; Sforcin, 2007; Zabaiou et al., 2017). Recent studies have demonstrated the broad biological activities of propolis, including antioxidant, antibacterial, anti-inflammatory, anticancer, hepatoprotective, and neuromodulatory properties (Altuntaş et al., 2023; Asgharpour et al., 2019; Forma and Bryś, 2021; Guzmán-Gutiérrez et al., 2018; Kuo et al., 2019; Marunaka et al., 2019; Oryan et al., 2018; Rajendran et al., 2014; Tao et al., 2018; Vică et al., 2022; Wang et al., 2014; Ye et al., 2019).
Among its diverse biological functions, the anti-inflammatory potential of propolis has attracted significant attention. While inflammation is a critical mechanism of host defense, its dysregulation can lead to chronic diseases such as inflammatory bowel disease (IBD) and arthritis (Karin and Clevers, 2016; Shimizu and Suzuki, 2019). Propolis inhibits the inflammatory cascade through multiple pathways: flavonoid compounds (like chrysin and kaempferol) inhibit reactive oxygen species (ROS) production and block pro-inflammatory enzymes (COX-2, iNOS) activity (Guzmán-Gutiérrez et al., 2018; Marunaka et al., 2019); phenolic esters (like caffeic acid phenethyl ester, CAPE) attenuate NF-κB and MAPK signaling pathways, reducing the production of inflammatory cytokines (TNF-α, IL-6) in macrophages (Bueno-Silva et al., 2017; Zullkiflee et al., 2022); Brazilian red propolis (from Dalbergia species), rich in neovestitol, regulates TLR-mediated inflammatory pathways, showing significant therapeutic effects in both acute and chronic inflammation models (Bueno-Silva et al., 2017; Franchin et al., 2016). Notably, propolis also maintains intestinal epithelial barrier integrity by upregulating tight junction proteins (such as Claudin-1, Occludin) and inhibiting apoptosis, thereby alleviating intestinal inflammation (Khayyal et al., 2019; Khayyal et al., 2015; Shimizu and Suzuki, 2019).
Macrophages, central regulators of innate immunity, exhibit dual functional states (polarized into pro-inflammatory M1 and anti-inflammatory M2 phenotypes) in inflammatory responses. M1 macrophages contribute to pathogen clearance and inflammatory responses by secreting pro-inflammatory cytokines such as TNF-α and IL-1β. In contrast, M2 macrophages promote tissue repair and resolution of inflammation through the secretion of anti-inflammatory cytokines like IL-10 and TGF-β1 (Foss et al., 2018; Li et al., 2018). In IBD, functional dysregulation of macrophages is considered a key factor in the onset and progression of the disease. Abnormally activated macrophages can cause persistent intestinal inflammation, disrupt intestinal barrier function, and affect motility (Hegarty et al., 2023). Therefore, regulating the functional states of macrophages has emerged as a potential therapeutic strategy for inflammation-related diseases.
Despite extensive research on propolis, its mechanisms in macrophage-mediated inflammation remain to be elucidated. Given propolis's rich flavonoid content and its regulatory effects on the NF-κB and MAPK pathways, we hypothesize that propolis can attenuate inflammatory responses by inhibiting macrophage activation. Utilizing an in vitro THP-1 macrophage model and an in vivo DSS-induced IBD model, this study aims to explore the mechanisms underlying propolis's effects on inflammatory bowel disease, thereby potentially providing new evidence for propolis as a multi-targeted anti-inflammatory therapeutic.
| 2. Materials and methods | ▴Top |
2.1. Determination and analysis of propolis components propolis
Propolis (donated by BoKang TianMi Technology Development Co., Ltd., Shanxi Yuanqu) was ground using a powder mill and sieved through a 40-mesh screen. Propolis powder was mixed with 70% ethanol at a solid-to-liquid ratio of 1:30 (w/v) and subjected to sonication for 30 minutes at 25°C. The mixture was centrifuged at 5,000 rpm for 10 minutes at 5°C, and the residue was re-extracted under identical conditions. The residue was filtered under vacuum after each extraction, and the combined filtrates were collected. Ethanol was removed by rotary evaporation, and the remaining material was recovered and lyophilized. The flavonoid composition of propolis was analyzed by UPLC-PDA. Chromatographic conditions: Waters CORTECS C18 (2.1 × 100 mm, 1.6 µm) with methanol (A) and 0.4% formic acid in water (B) as the mobile phases. Gradient elution: 0 min (18% A), 5 min (18% A), 15 min (35% A), 95 min (48% A), 180 min (73% A). Column temperature was 30°C; Flow rate was 0.2 mL/min; injection volume was 2 µL; Detection wavelength was 270 nm. Ion source: electrospray ionization (ESI); Ion spray voltage: +3,500 V/−3,500 V; Nebulizer pressure: 15 psi; Airflow rate 11 L/min; Evaporator temperature 300°C. A full-scan mode was used with a mass range set from m/z 100 to 1,500.
2.2. Cultivation of monocytic macrophages (THP-1) and establishment of inflammatory model
Cell culture: THP-1 cells (human monocytes) were purchased from Wuhan Shang En Biological Ltd. Cells were cultured in RPMI 1640 medium (HyClone, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin mixture. After revival, cells were maintained at 37°C in a 5% CO2 incubator and subcultured every 2–3 days, with densities kept between 0.5–1 × 106 cells/mL.
Construction of the inflammatory model: Propolis extract powder was dissolved in DMSO to prepare stock solutions. Cells were seeded at a density of 5 × 105 cells per well in 6-well plates. Once the cells reached 80–90% confluence, propolis solutions were added to final concentrations of 0.05 µg/mL and 0.1 µg/mL (with DMSO at 0.1%). Following a 2-hour pretreatment with propolis, TNF-α and IFN-γ were added to final concentrations of 100 ng/mL. After 8 hours, cells were harvested and processed for RNA and protein extraction for subsequent experiments.
2.3. IBD mouse experiment design
Forty-eight C57BL/6 mice (21–23 g), aged 8–10 weeks, were purchased from Beijing HFK Bioscience Co. Ltd. The mice were housed at the Animal Experimentation Center of the Chinese Academy of Medical Sciences, Institute of Radiation Medicine, maintained at 22±2°C under a 12-h light/dark cycle. Mice were acclimatized for one week before the experiments began, and only those without any abnormalities were used. All animal experiments were approved by the Ethics Committee of Tianjin University of Commerce.
The mice were randomly divided into three groups: a blank control group, a DSS-induced colitis group, and a 0.01% propolis intervention group, with 16 mice in each group. The blank control group received a normal diet and regular drinking water. The DSS-induced colitis group received 3% DSS (molecular weight: 36,000–50,000 Da) in drinking water and a normal diet. The propolis intervention group received 3% DSS in drinking water and a diet containing 0.01% propolis extract. All mice were fed for 7 days. At the end of the feeding period, mice were euthanized by orbital exsanguination, and fresh blood and colon tissues were collected for subsequent analyses.
2.4. RNA extraction and real-time qPCR analysis
Total RNA was extracted from cells and colon tissues using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol. RNA purity and concentration were measured using a spectrophotometer. cDNA was synthesized using PrimeScript™ RT Master Mix (Takara, Japan) with 1 μg of total RNA, Oligo(dT)18, and random hexamer primers according to the manufacturer’s instructions. The reaction was incubated at 37°C for 15 min, followed by 85°C for 5 s to terminate reverse transcription.
Quantitative real-time PCR (qPCR) was performed on a Roche LightCycler® 96 system (Roche, Switzerland) using SYBR® Green Premix Ex Taq™ II (Takara, Japan). The amplification protocol was as follows: 95°C for 30 s (initial denaturation), followed by 40 cycles of 95°C for 5 s (denaturation) and 60°C for 30 s (annealing/extension). All reactions were performed in triplicate technical replicates, with GAPDH as the internal reference gene. Relative gene expression levels were calculated using the 2−ΔΔCt method. Statistical significance was evaluated using Student’s t-test, with P < 0.05 considered statistically significant. Primer sequences are listed in Table 1.
 Click to view | Table 1. Primers for RT-PCR |
2.5. Total protein extraction and western blot analysis
Tissue or cell samples were lysed in pre-cooled RIPA lysis buffer (supplemented with 1 mM PMSF, 1× protease inhibitor cocktail, and 1× phosphatase inhibitor cocktail). Tissue samples were homogenized in liquid nitrogen prior to lysis, while cell samples were disrupted by sonication (30% amplitude, 10 s on/10 s off, 3 cycles). The lysate was incubated on ice for 30 minutes, then centrifuged at 12,000 g for 15 minutes at 4°C. The supernatant was collected, and protein concentration was determined using the BCA assay.
For SDS-PAGE, 20 μg of protein lysate supernatant was loaded and separated by electrophoresis through a stacking gel (80 V, 30 min) and a resolving gel (100 V, 90 min). Proteins were transferred to a PVDF membrane using a constant current of 200 mA for 2 hours. The membrane was blocked with 5% non-fat milk in TBST for 1 hour at room temperature, followed by incubation with primary antibody at 4°C overnight. After three washes with TBST, the membrane was incubated with HRP-conjugated secondary antibody for 1 hour at room temperature. Protein bands were visualized using ECL chemiluminescence and quantified with ImageJ software.
2.6. MTT assay
Cells were seeded in a 96-well plate at a density of 5 × 104 cells/well and pre-cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS, Gibco) at 37°C in a 5% CO2 incubator for 24 hours. The experimental groups were treated with propolis solutions at final concentrations of 0, 0.1, 0.2, 0.5, and 1 µg/mL, while the control group received an equivalent volume of culture medium. Each group was set up in six replicate wells.
After 24 hours of treatment, 20 μL of MTT solution (5 mg/mL, Sigma-Aldrich) was added to each well, followed by an additional 4-hour incubation. The culture medium was then removed, and 150 μL of DMSO was added to dissolve formazan crystals by shaking at 37°C for 10 minutes. Absorbance was measured at 570 nm using a microplate reader, with a reference wavelength of 630 nm. The cell viability calculation was performed according to the formula:
2.7. Hematoxylin and eosin (H&E) staining and immunohistochemical analysis
Tissue Processing: fresh tissue samples were fixed in 4% paraformaldehyde solution, followed by graded ethanol dehydration (70–100%), xylene clearing, and paraffin embedding. Serial sections (4 µm) were cut and processed for H&E staining. Deparaffinized and rehydrated sections were stained with hematoxylin, counterstained with eosin, dehydrated, cleared in xylene, and mounted for histological evaluation under an optical microscope.
Immunohistochemistry analysis: sections were deparaffinized in xylene, rehydrated through graded ethanol, and subjected to antigen retrieval by autoclaving in 10 mM sodium citrate buffer (pH 6.0) at 121°C for 15 minutes. After cooling, sections were washed three times with PBS (pH 7.4). Endogenous peroxidase activity was quenched with 3% H2O2 for 10 minutes at room temperature, followed by blocking non-specific binding with 5% BSA in PBS for 30 minutes. Sections were incubated with primary antibody overnight at 4°C, washed with PBS, and incubated with HRP-conjugated secondary antibody for 1 h at room temperature. Antigen-antibody complexes were visualized using DAB chromogen for 1–3 minutes. Sections were counterstained with hematoxylin, dehydrated, cleared in xylene, and mounted. Images were analyzed under an optical microscope, with positive signals defined as brownish-yellow cytoplasmic and/or nuclear granules.
2.8. Data analysis
Statistical analysis was performed using SPSS 18.0 software. Data are presented as Means ± SEM. One-way ANOVA was used for comparisons, with Duncan’s multiple range test for homogeneous variances and Dunnett’s T3 test for heterogeneous variances. A P-value of <0.05 was considered statistically significant, <0.01 as significant, and <0.001 as highly significant. GraphPad Prism 5.0 was used for graphical analysis.
| 3. Results analysis | ▴Top |
3.1. Determination and analysis of propolis components
Propolis exhibits significant biological activities, including antimicrobial, anti-inflammatory, antioxidant, and antitumor effects, which are closely associated with its chemical composition. In this study, UPLC-PDA was used to analyze the main components of propolis. The results showed that propolis contains seven compounds in relatively high concentrations, listed in descending order: chrysin (26.64 mg/g), rutin (10.70 mg/g), caffeic acid phenethyl ester (CAPE) (10.67 mg/g), quercetin (5.79 mg/g), apigenin (1.04 mg/g), kaempferol (0.86 mg/g), and luteolin (0.36 mg/g) (Figure 1 and Table 2). Except for CAPE, the remaining six compounds belong to the flavonoid class. These findings indicate that flavonoids are the major components of propolis, accounting for the highest proportion, followed by other phenolic compounds.
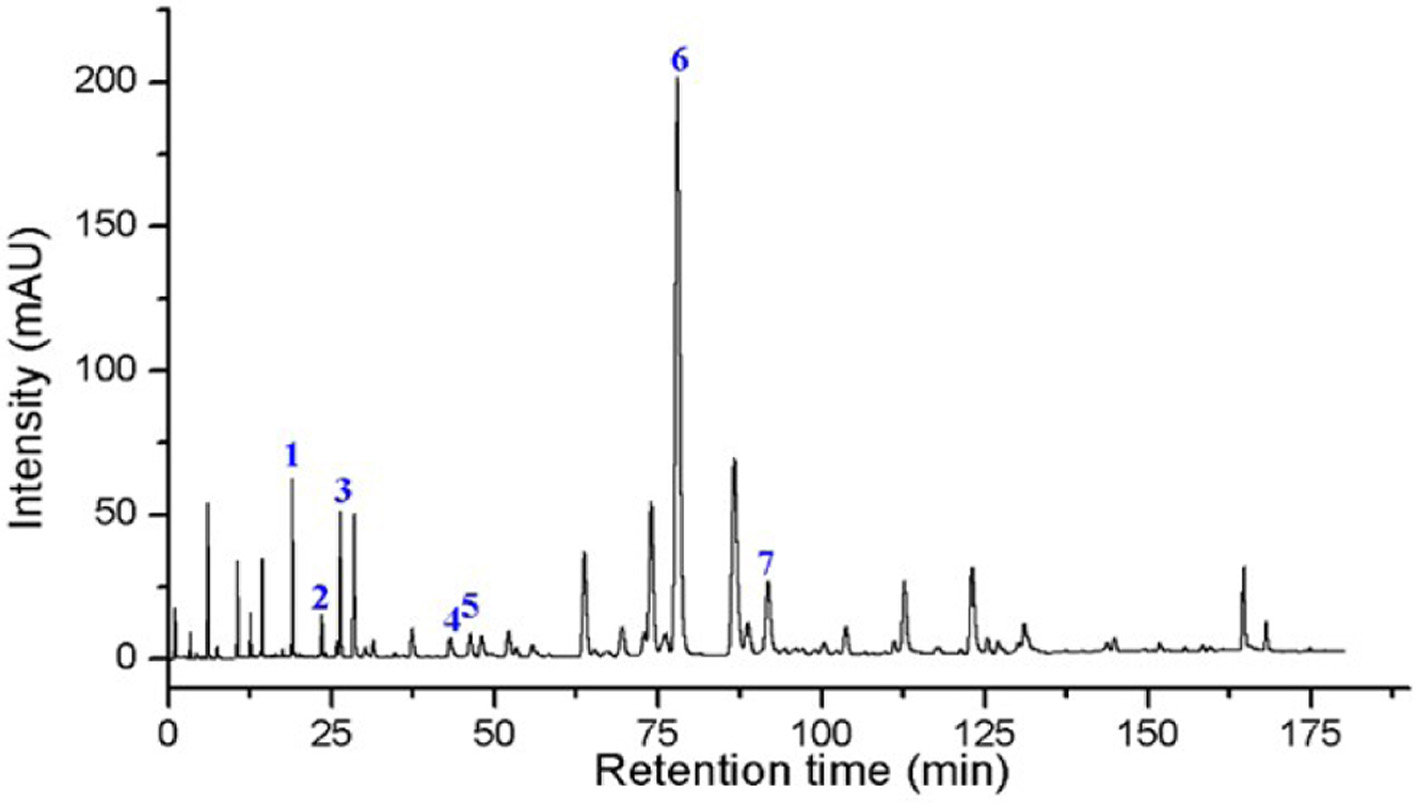 Click for large image | Figure 1. Chromatogram of Ethanol Extract of Propolis 1. Rutin; 2. Quercetin; 3. Luteolin; 4. Sinapyl alcohol; 5. Apigenin; 6. Populin; 7. Caffeic acid phenethyl ester (CAPE). |
 Click to view | Table 2. Compound Concentrations in Propolis |
3.2. Propolis reduces inflammatory response in THP-1 cells
The component analysis indicated that propolis is rich in flavonoids, suggesting its potential for significant anti-inflammatory activity. To validate this hypothesis, in vitro cell experiments were conducted. Initially, the cytotoxicity of propolis against THP-1 cells was evaluated using the MTT assay. Results showed no cytotoxicity within the concentration range of 0.1–1 μg/mL, with cell viability maintained above 95% (Figure 2a).
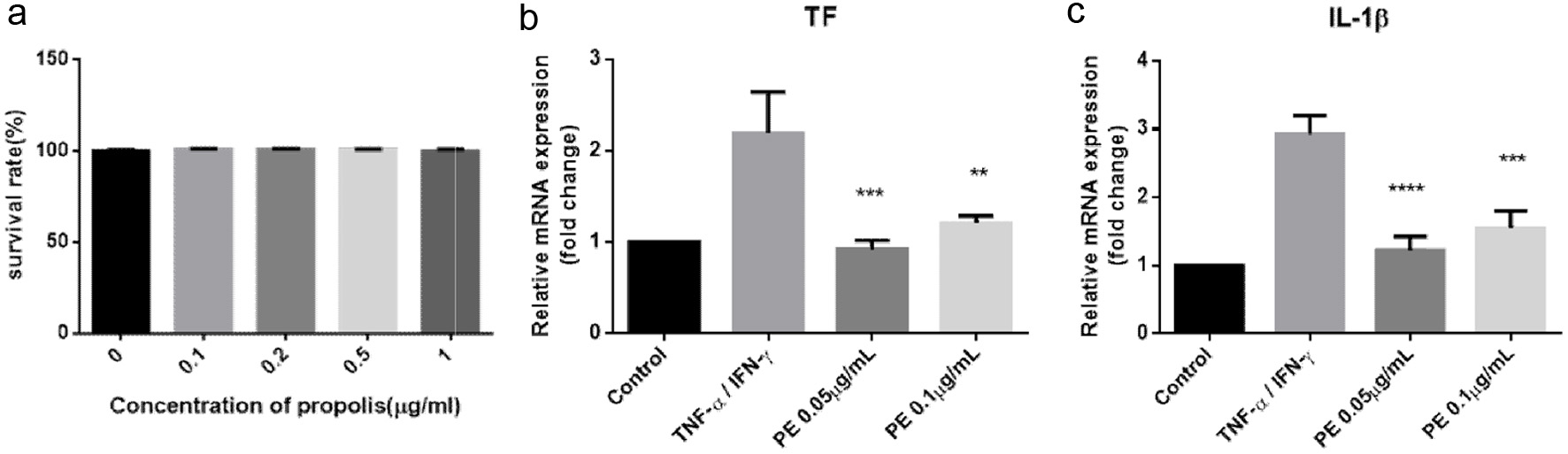 Click for large image | Figure 2. Propolis Exhibits Significant Anti-inflammatory Effects in the THP-1 Inflammatory Cell Model. (a) Cell viability post-treatment with various concentrations of propolis (n = 6); (b-c). Gene expression levels of TF and IL-1β in THP-1 cells (n = 3). |
Subsequently, an inflammatory cell model was established by stimulating THP-1 cells with TNF-α and IFN-γ. RT-PCR was used to measure the expression of inflammatory genes TF and IL-1β. As shown in Figure 2b, c, TNF-α/IFN-γ treatment significantly upregulated TF and IL-1β expression by 2–3 fold compared to the control. However, propolis treatment attenuated the expression of these inflammatory markers to levels comparable with the control. Notably, lower concentrations of propolis (0.05 μg/mL) demonstrated a more marked anti-inflammatory effect than higher concentrations (0.1 μg/mL), though statistical significance was not reached. Collectively, these results confirm that propolis exerts significant anti-inflammatory effects, particularly at lower concentrations.
3.3. Propolis modulates phosphorylation of MAPK and NF-κB pathways to reduce inflammatory response in THP-1 cells
To further investigate the anti-inflammatory mechanism of propolis, Western blot analysis was performed to assess the phosphorylation status of key kinases in inflammatory signaling pathways. As shown in Figure 3, stimulation with TNF-α and IFN-γ significantly increased phosphorylation of p65, p38, and ERK, particularly p38. However, propolis treatment markedly reduced phosphorylation of these kinases, indicating that propolis attenuates phosphorylation and activation of the p65/NF-κB, p38/MAPK, and ERK/MAPK pathways, thereby suppressing excessive macrophage activation.
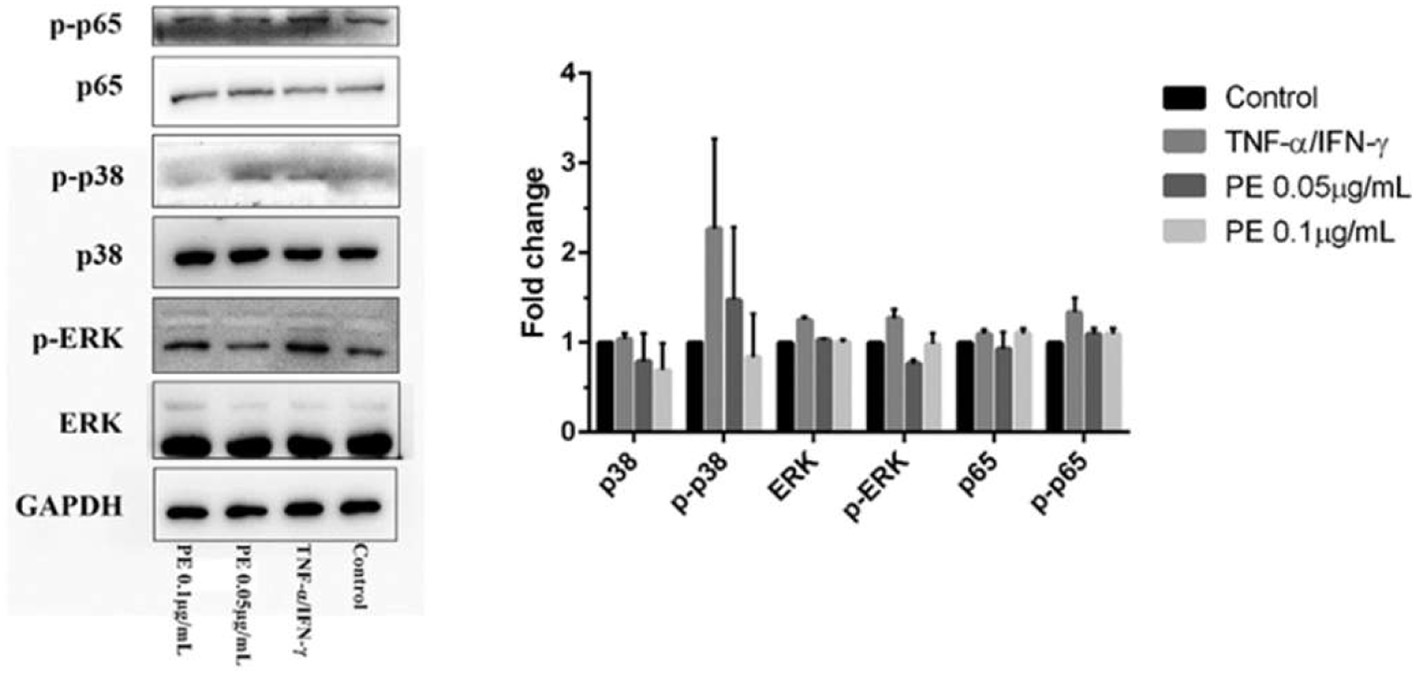 Click for large image | Figure 3. Western Blot Analysis of p65 NF-κB, p38/MAPK, and ERK/MAPK Pathways in THP-1 Cells. |
3.4. Propolis ameliorates intestinal damage in IBD mice
The in vitro results demonstrated that propolis has significant anti-inflammatory effects. To evaluate its therapeutic potential in vivo, we used a DSS-induced IBD mouse model and administered a diet supplemented with propolis extract to assess its ameliorative effects on intestinal inflammation.
During DSS treatment, mice in the DSS group began to exhibit significant weight loss from day 3 onwards. Notably, propolis-treated mice showed more pronounced weight loss than DSS group mice on day 3 but recovered gradually from day 4, leading to a widening gap in body weight (Figure 4a). Weight loss percentage, diarrhea severity, and rectal bleeding were monitored, and Disease Activity Index (DAI) scores were calculated based on these parameters. Compared to the control group, DSS treatment significantly increased DAI scores, whereas the increase in the propolis group was significantly attenuated, although inter-individual variability was observed (Figure 4b). Additionally, colon length was significantly increased in the propolis group compared to the DSS group, further confirming the beneficial effects of propolis on the health of IBD mice (Figure 4c).
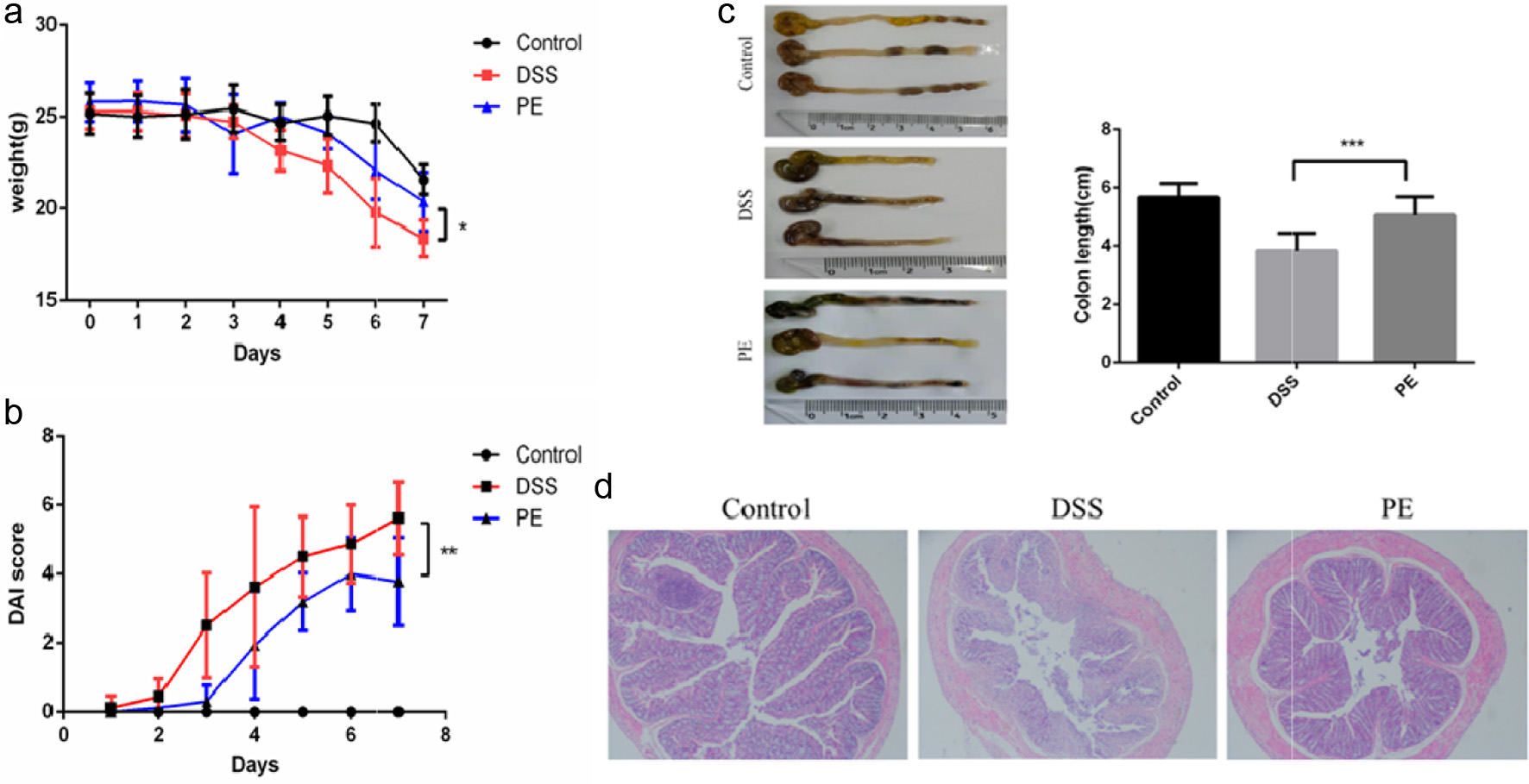 Click for large image | Figure 4. Effects of Propolis Treatment on the Colon of IBD Mice. (a) Weight changes in different treatment groups; (b) DAI scores in different treatment groups; (c) Display of colon length in different treatment groups; (d) H&E staining of the distal colon in different treatment groups. |
H&E staining analysis further validated these findings. Compared to the control group, intestinal architecture in DSS-treated mice was severely disrupted, characterized by shortened villi, crypt loss, extensive inflammatory cell infiltration, and villous atrophy/detachment. In contrast, intestinal architecture in the propolis-treated group remained largely preserved with intact villous and crypt structures, well-defined goblet cell architecture, and reduced inflammatory infiltration (Figure 4d). Collectively, these results demonstrate that propolis effectively ameliorates DSS-induced intestinal inflammation and exerts protective effects in the IBD model.
3.5. Propolis inhibits macrophage activation in IBD mice
Previous studies have shown that colonic inflammation in IBD mice is often accompanied by macrophage activation, which exacerbates intestinal damage. To investigate the in vivo anti-inflammatory mechanism of propolis, immunohistochemistry was performed to evaluate macrophage activation status in the distal colon. In DSS-treated mice, marked macrophage infiltration was observed, whereas F4/80-positive immunoreactive areas in the propolis-treated group were significantly reduced, approaching normal levels (Figure 5a). These results indicate that propolis effectively inhibits macrophage infiltration in the colon of IBD mice.
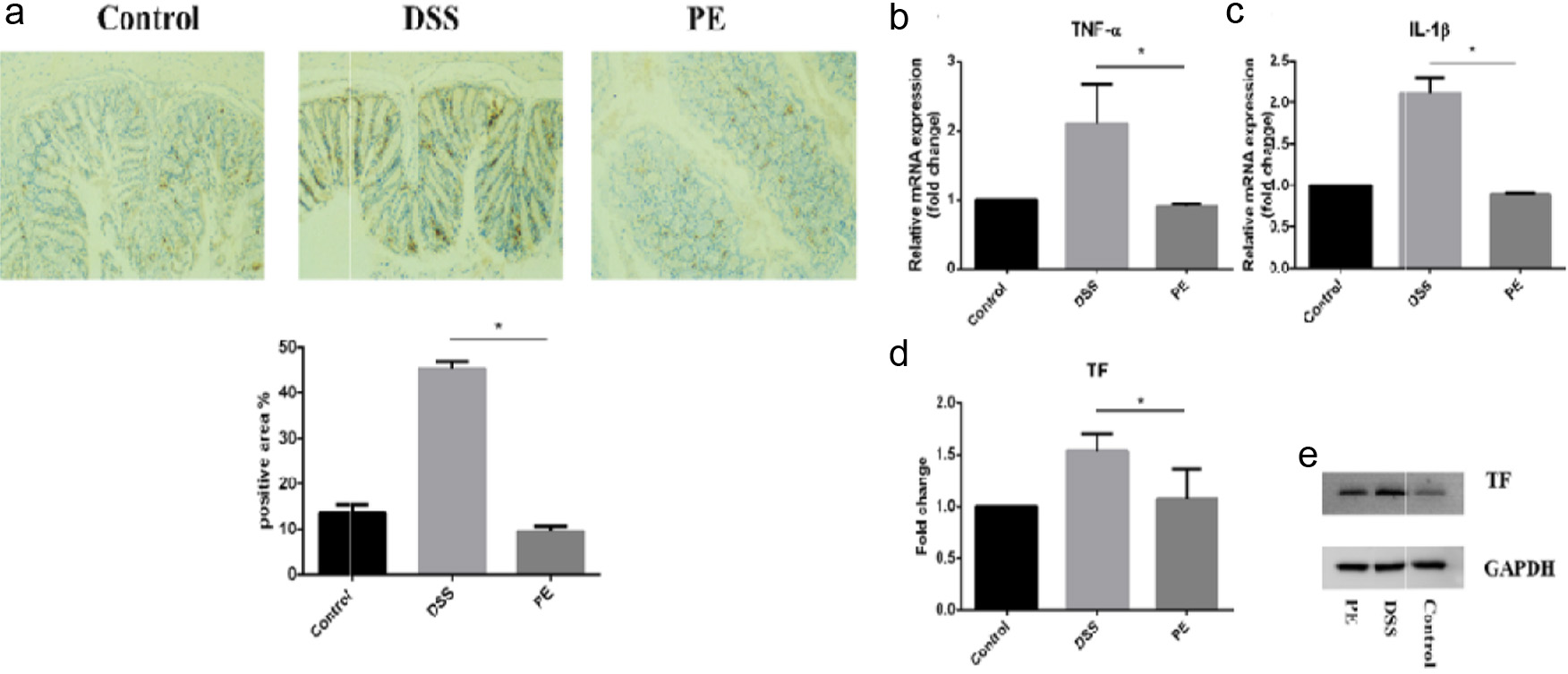 Click for large image | Figure 5. Effects of Propolis Treatment on Colonic Inflammation in IBD Mice. (a) Immunohistochemical analysis of macrophage infiltration in colon tissue (F4/80). (b-d) Real-Time PCR analysis of TNF-α, IL-1β, and TF RNA expression levels in colon tissue. (e) Western Blot analysis of TF protein expression levels in colon tissue. |
Excessive macrophage infiltration triggers the release of downstream inflammatory mediators, further amplifying the inflammatory response. Our findings corroborate this phenomenon: DSS stimulation significantly upregulated expression of multiple pro-inflammatory cytokines (TNF-α, IL-1β, and TF) in colonic tissues, whereas propolis treatment attenuated their overexpression (Figure 5b–e). Collectively, these results demonstrate that propolis protects against DSS-induced intestinal inflammation by inhibiting macrophage infiltration and suppressing pro-inflammatory cytokine production.
| 4. Discussion | ▴Top |
This study, using UPLC-PDA analysis, demonstrated that propolis is rich in flavonoids and phenolic esters, which effectively alleviate macrophage-mediated inflammation in both in vitro and in vivo IBD models. Our findings reveal that propolis attenuates macrophage activation via inhibition of NF-κB and MAPK signaling, thereby reducing pro-inflammatory cytokine expression (TNF-α, IL-1β, and TF). These results provide evidence for propolis as a multifaceted therapeutic for inflammatory bowel disease.
The anti-inflammatory properties of propolis are closely linked to its flavonoid components, particularly chrysin (26.64 mg/g) and caffeic acid phenethyl ester (CAPE, 10.67 mg/g). Chrysin, a dominant flavonoid in temperate propolis, inhibits NF-κB activation by blocking IκBα degradation, thereby reducing downstream cytokine production (Franchin et al., 2018). Similarly, CAPE, a major phenolic ester in Brazilian propolis, directly suppresses MAPK phosphorylation (p38, ERK) and NF-κB nuclear translocation in macrophages (Fitzpatrick et al., 2001; Zissel et al., 2015). Our data further confirm that propolis treatment downregulates phosphorylated p65, p38, and ERK in TNF-α/IFN-γ-stimulated THP-1 cells. Notably, lower propolis concentrations exerted stronger anti-inflammatory effects than higher concentrations—a paradox explained by the biphasic response common in phytochemicals, where supraphysiological doses may trigger cellular stress responses that counteract therapeutic benefits (Vauzour et al., 2010; Zhang et al., 2017).
This study demonstrated that propolis inhibits tissue factor (TF, also known as coagulation factor III) expression in macrophages, a critical mediator linking inflammation and coagulation in IBD. Overexpression of TF in IBD patients exacerbates mucosal injury via thrombin-dependent activation of protease-activated receptors (PARs), thereby amplifying pro-inflammatory cytokine release (Anthoni et al., 2007; Leon et al., 2025; Palkovits et al., 2013). Our results showed that propolis reduced both TF mRNA and protein levels in inflamed colon tissues, suggesting disruption of the thrombotic-inflammatory cascade. These findings align with previous research by Fuliang Hu et al., who reported that propolis inhibits platelet activation and reduces platelet adhesion to fibrinogen- and collagen-coated surfaces, exerting anti-thrombotic effects (HU et al., 2005). Notably, flavonoids in propolis play a pivotal role in regulating blood lipid profiles, promoting vascular elasticity, reducing vascular fragility, enhancing microcirculation, and preventing atherosclerosis, earning them the reputation as "vascular endothelium protectors" (Mani et al., 2006; Sforcin and Bankova, 2011).
Despite the emphasized potential of propolis as a therapeutic agent for IBD, several limitations should be noted. First, although the THP-1 macrophage model is widely used, it does not fully recapitulate the heterogeneity of intestinal macrophages; primary macrophage cultures or epithelial cell co-culture systems would provide more profound mechanistic insights. Second, the DSS model primarily recapitulates acute colitis, and propolis should be evaluated in chronic or adoptive transfer models to assess its translational relevance. Lastly, the specific contributions of individual propolis components, such as chrysin and CAPE, to its anti-inflammatory effects require further clarification using gene knockout or inhibitor approaches.
In conclusion, this study confirms that propolis acts as an effective modulator of macrophage-driven inflammation in IBD by inhibiting NF-κB and MAPK signaling pathways and TF expression. Our findings underscore the therapeutic potential of propolis in inflammatory diseases and provide a preclinical rationale for clinical trials evaluating its efficacy in IBD treatment.
Conflict of interest
All authors declare no conflict of interest.
| References | ▴Top |