| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 29, March 2025, pages 60-76
Presence of γ-glutamyl and β-aspartyl isopeptides, diketopiperazines, pyroglutamyl peptides, in addition to normal peptides in fish and soy sauces: Structures, contents and their bioavailability
Yudi Rahmadiana, b, Satoshi Miyauchia, Sri Wijanartia, c, Tomoko Asaia, Kenji Satoa, *
aDivision of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwakecho, Kyoto, Japan
bDepartment of Animal Products Technology, Faculty of Animal Science, Universitas Andalas, Padang, Indonesia
cDepartment of Bioresources Technology and Veterinary, Vocational College, Universitas Gadjah Mada, Sleman, D.I. Yogyakarta, Indonesia
*Corresponding author: Kenji Sato, Division of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwakecho, Kyoto, Japan. E-mail: sato.kenji.7x@kyoto-u.ac.jp
DOI: 10.26599/JFB.2025.95029406
Received: March 21, 2025
Revised received & accepted: March 29, 2025
| Abstract | ▴Top |
This study identified peptides in fish and soy sauces and elucidated their bioavailability in rats. Over 96 peptides including γ-glutamyl, pyroglutamyl, β-aspartyl peptides, and diketopiperazines were detected. The content of these peptides varied greatly between the products tested. After the administration of Vietnamese fish sauce which had the highest peptide content among samples tested, most normal peptides did not significantly increase in the blood; whereas γ-glutamyl and pyroglutamyl peptides significantly increased in the small intestine and some hydrophobic γ-glutamyl isopeptides and pyroglutamyl-proline significantly increased in the blood. Diketopiperazines and β-aspartyl isopeptides significantly increased in the small intestine and the blood. These findings highlight the presence of modified peptides in fish and soy sauces, which are commonly consumed in daily dishes in East Asia. Only modified peptides such as diketopiperazines, β-aspartyl isopeptides and hydrophobic γ-glutamyl isopeptides survived gastrointestinal digestion, entering blood circulation, suggesting their potential biological activities.
Keywords: Fermented food; Fish sauce; Soy sauce; Peptide; Isopeptide; Diketopiperazine; Bioavailability
| 1. Introduction | ▴Top |
Salt has been used since ancient times to preserve food products. During salting, endogenous and microbial enzymes can cause changes in ingredients, which can alter the texture, taste, and flavor, and sometimes produce paste and liquid products. This process is known as fermentation. In East Asia, salted and fermented fish, meat, vegetables, beans, and cereals are used as seasonings owing to their strong umami taste. Fish, soy sauce, and soy paste are the prevalent seasonings in this area. Fermentation is also used to produce non-salted products, such as yogurt and fresh cheese. These products are usually prepared with a shorter fermentation period than East Asian fermented seasoning. The consumption of East Asian fermented seasonings in this area has decreased in recent decades (Okouchi et al., 2019), possibly because of high salt concentrations. On the other hand, it has been reported that fermented seasonings, especially Japanese-style soy paste miso may show beneficial effects on human health, such as lowering blood cholesterol, immune-enhancing, antidiabetic, anti-obesity, anticancer, antimicrobial, and risk-lowering activities against atherosclerosis, osteoporosis, stomach illnesses, and lactose intolerance symptoms (Chatterjee et al., 2018; Endres, 2001; Ito, 2020; Kokubo et al., 2007, 2013; Kondo et al., 2019; Mano et al., 2018; Marco et al., 2017; Nozue et al., 2017; Minamiyama and Okada, 2003).
These fermented seasoning products produce strong umami and kokumi sensations and enhance the flavor of daily dishes. Monosodium glutamate in these products is mainly responsible for the umami taste. In addition, some peptides such as pyroglutamyl-glutamine (pGlu-Gln) (Kaneko et al., 2011) and γ-glutamyl-valyl-glycine (γ-Glu-Val-Gly) (Kuroda et al., 2020) have been identified to enhance umami taste and kokumi sensation, respectively. However, the compounds in these products responsible for their health-promoting activities are poorly understood. Recently, some modified peptides such as pyroglutamyl peptides, diketopiperazines (cyclic dipeptides), and β-aspartyl isopeptides have been identified in miso, a Japanese-style salted and fermented soy paste (Nagao et al., 2024). These modified peptides show high bioavailability, especially diketopiperazines and β-aspartyl isopeptides, while normal peptides do not. Diketopiperazines are cyclic dipeptides formed from linear peptides (Otsuka et al., 2021) that have been reported to exhibit antioxidant (Zhong et al., 2018), anti-inflammatory (Zhang et al., 2019), antifungal, and antimicrobial activities (Kwak et al., 2018). Pyroglutamyl peptides are generated from a glutamine residue at the amino terminus of peptides by non-enzymatic intramolecular cyclization in a time- and temperature-dependent manner (Suzuki et al., 1999). Oral administration of pyroglutamyl-leucine (pGlu-Leu) in a wheat gluten enzymatic hydrolysate has been reported to have hepatoprotective activity by oral administration (Sato et al., 2013), to ameliorate colitis caused by dextran sulfate sodium in mice by oral administration at 0.1 mg/kg body weight (Wada et al., 2013). Recently, β-aspartyl peptides have shown anti-fatigue activity in a mouse model (Nakagawasai et al., 2021) and inhibitory activity against angiotensin converting enzyme (Nagao et al., 2024). These modified peptides may be responsible for the bioactivities of fermented foods. However, the presence of these modified peptides in other types of salted and fermented seasonings such as soy and fish sauces is poorly understood. Miso is fermented and aged under anaerobic conditions and not sterilized by heat treatment. However, fish and soy sauces are fermented and aged under more aerobic conditions with occasional stirring, and soy sauces are usually heated to terminate fermentation, which can generate new products.
This study aimed to comprehensively identify short-chain peptides in fish and soy sauces and elucidate their bioavailability in rats after oral administration, which can provide fundamental information for understanding the biological activities of East Asian fermented seasonings.
| 2. Materials and methods | ▴Top |
2.1. Reagents
Phosphate-buffered saline (pH 7.4, 10 × PBS), L-pyroglutamic acid, and acetonitrile (HPLC grade) were obtained from Nacalai Tesque (Kyoto, Japan). The amino acid mixture standard solution (Type H) was obtained from Fujifilm Wako Pure Chemical (Osaka, Japan). 9-Fluorenylmethoxycarbonyl (Fmoc) amino acid derivatives including Fmoc-L-Asp α-t-butyl-ester, Fmoc-D-Asp α-t-butyl-ester, Fmoc-L-Asp β-t-butyl-ester, Fmoc-D-Asp β-t-butyl-ester, Fmoc-L-Glu γ-t-butyl-ester and Fmoc amino acid-bound p-alkoxybenzyl alcohol (Alko) resin, and Fmoc proline-bound 2-chlorotrityl chloride (Barlos) resin were purchased from the Watanabe Chemical (Hiroshima, Japan). Cyclo-Ala-Pro was purchased from the Peptide Institute (Osaka, Japan). 6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate (AccQ) was purchased from Toronto Research Chemicals (Toronto, ON, Canada).
2.2. Fermented foods
Vietnamese fish sauce (VFS), Thai fish sauce (TFS), Japanese fish sauce (JFS), Japanese soy sauces (two Koikuchi-type; JSS-K1 and JSS-K2, and Tamari-type; JSS-T) were commercially obtained from local markets in Kyoto, Japan. These products are well-known brands, but they are not representative of the country’s products. As references of lightly fermented foods, Camembert-type cheese produced in Japan and German-style dry salami sausage produced in Japan were also obtained from online markets. All samples were kept at −30 °C until use.
2.3. Identification of peptides in samples without derivatization
Fish and soy sauces were diluted four times with distilled water. For extraction of peptides in the cheese and salami, samples (500 mg) were homogenized with 500 μL of distilled water in a BioMasher II (Nippi, Tokyo Japan). The homogenate was mixed with 500 μL of ethanol and then centrifuged at 12,000×g for 10 min at 4 °C. The supernatant was collected and kept at −30 °C. Aliquots of the extracts were further diluted 10 times with distilled water and then diluents were filtered through a Cosmonice filter (4 mm i.d., 0.45 μm, Nacalai Tesque). Aliquots of the filtrate (10 μL) were directly analyzed using a liquid chromatography-electrospray ionization tandem mass spectrometer (LC-MS/MS; LCMS 8040, Shimadzu, Kyoto, Japan) equipped with an Inertsil ODS-3 column (2.1 mm i.d. × 250 mm, GL Science, Tokyo, Japan). The column was kept at 40 °C. The mobile phases used were 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B). The peptides were resolved using a binary linear gradient at a flow rate of 0.2 mL/min. The gradient program was set up as follows: 0−2 min, 0% B; 2−30 min, 0−30% B; 30−40 min, 30−100% B; 40−45 min, 100% B; 45−45.1 min, 100−0% B; 45.1−55 min, 0% B. Detection was carried out by total ion scan and precursor ion scan targeting immonium ion of pyroglutamyl residue (mass to charge ratio, m/z 84.1) at collision energy at −35 V in positive mode. Scan ranges were set to m/z 100−200, 200−225, 225−250, 250−275, 275−300, 300−350, 350−400, 400−500, 500−600, and 600−1,000. The observed precursor ions of peptides were further analyzed by the product ion scan mode at collision energies of −15, −25, and −35 V to estimate the peptide sequence. The peptide sequences were estimated based on the m/z of the precursor and product ions, immonium ions, amino terminal series ions (a, b, and c product ions), and carboxy terminal series ions (x, y, and z product ions) defined by Medzihradszky and Chalkley (2015).
2.4. Identification of peptides in samples with AccQ derivatization
Aliquots (20 μL) of the diluents were added with 20 µL of 0.3% (w/v) AccQ-acetonitrile solution and 60 µL of 50 mM sodium borate buffer (pH 8.8). The reaction was carried out for 10 min at 50 °C. The reactant was mixed with 50 μL of 5 mM sodium phosphate buffer (pH 7.5) and clarified via passing through the Cosmonice filter. The filtrate (20 μL) was injected to the LC-MS/MS. Separation was carried out using same solvents by a binary gradient as 0–3 min, 0% B; 3–20 min, 0–30% B; 20–30 min, 30–100% B; 30–35 min, 100% B; 35–35.1 min, 100–0% B; 35.1–45 min, 0% B. Detection was carried out by total ion scan and precursor ion scan targeting the AccQ-derived product ion (b1 ion, m/z 171.1) at the same collision energy and scan ranges as mentioned before. The observed precursor ions of the peptides were further analyzed in the product ion scan mode.
2.5. Peptide synthesis
The Fmoc strategy was used to synthesize peptides using a PSSM-8 peptide synthesizer (Shimadzu) according to the manufacturer’s protocol, with slight modifications. Pyroglutamyl peptides were synthesized using the same protocol, except that L-pyroglutamic acid was used instead of the amino-terminal Fmoc amino acid. L-α-, L-β-, D-α-, and D-β-aspartyl peptides were synthesized using Fmoc-L-Asp-β-t-butyl-ester, Fmoc-L-Asp-α-t-butyl-ester, Fmoc-D-Asp-β-t-butyl-ester, and Fmoc-D-Asp-α-t-butyl-ester, respectively (Ejima et al., 2018). Diketopiperazines were synthesized as described by Nagao et al. (2024). L-γ-glutamyl peptides were synthesized by using Fmoc-L-Glu-α-t-butyl-ester. The synthesized peptides were purified using a Cosmosil 5C18-MS-II column (10 mm i.d. × 250 mm; Nacalai Tesque). The mobile phases were the same as those described previously. The gradient program was set up as follows: 0−20 min, 0−50% B; 20−30 min, 50−100% B; 30−35 min, 100% B; 35−35.1 min, 100−0% B; 35.1−45 min, 0% B at a flow rate of 2 mL/min. The column was kept at 40 °C. Absorbance at 214 and 254 nm was used to monitor peptide elution. The purity of the isolated peptides was determined by LC-MS/MS. The content of the purified peptides was quantified by amino acid analysis after HCl hydrolysis (Bidlingmeyer et al., 1984; Sato et al., 1992).
2.6. Quantification of peptides in fish and soy sauces
Aliquots of the sample filtrate (10 μL) was directly analyzed by the LC-MS/MS in the multi reaction monitoring (MRM) mode. An Inertsil ODS-3 column was used to resolve the peptides in the reversed phase mode. Synthetic peptides were used to optimize MRM conditions using LabSolutions LCMS Ver. 5.5 (Shimadzu) and were used as external standards for peptide quantification. The LC-MS/MS elution conditions were the same as those described above.
2.7. Animal experiments
Animal experiments were performed at the Louis Pasteur Center for Medical Research. Guidelines for animal studies from the National Institutes of Health (NIH) were followed for animal experiments. The Animal Care Committee of the Louis Pasteur Centre for Medical Research approved all experimental procedures (No. approval: 20223). Seven-week-old male Wistar rats (210−230 g) were obtained from Japan SLC (Shizuoka, Japan) and acclimatized to the environmental conditions for one week. The rats were kept in a 24–26 °C room, and 40–60% humidity under a 12-h light-dark cycle. The rats were fed a certified rodent diet (MF; Oriental Yeast, Tokyo, Japan) and had free access to water and food during the acclimatization period. Before the oral administration of the sample, all rats were fasted for 16 h and divided into two groups. The first group was administered 8% salt water (vehicle group, n = 3) at 222 μL/200 g body weight using a sonde, and the second group received two × dilutions of VFS (VFS group, n = 3). Under isoflurane anesthesia, blood was collected from the portal and abdominal veins using a heparinized syringe, 60 min after administration. The plasma was prepared by centrifugation at 800 × g for 10 min. The inner contents of the small intestine were flushed with 10 mL of PBS. The washed small intestine was cut into two parts (upper and lower) and used as the anterior and posterior parts, respectively. All samples were stored at −80 °C for further analyses.
2.8. Quantification of peptides in rats
Aliquots of plasma were mixed with three volumes of ethanol in 1.5 mL tubes. The precipitated proteins were removed by centrifugation at 12,000 × g for 10 min. The supernatant was used as the ethanol-soluble fraction of the blood plasma. The suspension of the inner contents of small intestine was mixed with three volumes of ethanol. Aliquots of the center of anterior and posterior parts of small intestine (100 mg) were homogenized with 100 μL PBS in the BioMasher II. The homogenate was then mixed with three volumes of ethanol. The ethanol-soluble fractions were prepared by centrifugation as described above. Aliquots of the ethanol-soluble fractions (100 μL) were dried under vacuum and kept at −80 °C until use. The residue was dissolved in 100 μL distilled water and then clarified by passing through the Cosmonice filter. The peptides in the filtrates were quantified by LC-MS/MS in the MRM mode under the same conditions mentioned above.
2.9. Statistical analysis
Data are shown as mean ± standard deviation (n = 3). Differences in the peptide content in the rat body between the vehicle and VFS groups were evaluated by Student’s t test using SPSS 22 (SPSS, Chicago, IL, USA). p < 0.05 was considered statistically significant.
| 3. Results | ▴Top |
3.1. Identification of peptides
Figure 1a shows MS chromatograms of AccQ-derivatives of amino compounds in VFS. Mass to charge ratios (m/z) of the main compounds are presented on each peak. These precursor ions were further analyzed by product ion scan mode. Based on the m/z of precursor ion and retention time, amino acids were identified and are indicated by one letter abbreviations on each peak. In addition to amino acids, monoamines, polyamines, and total of 43 normal linear peptides were identified in the VFS based on m/z of precursor and product ions as summarized in Table 1. Estimated peptide structure is also presented on each peak for example GP (Gly-Pro) in the Figures 1a and b. In addition to the peptides consisting of leucine and isoleucine, some glutamyl and aspartyl peptides generated multiple peaks with same m/z as indicated by arrows in Figure 1b. However, resolution of these peaks was not so good. To resolve these peaks, compounds in the water diluent of VFS were directly subjected to LC-MS analysis without the derivatization. MS chromatograms of the non-derivatives in the VFS in total ion scan mode are presented in Figure 2. Precursor ions in major peaks were analyzed by product ion scan mode. In addition to amino acids, acetyl, propionyl, and butyryl-amino acids were also identified (Figure 2) based on m/z of precursor and product ions (Table S1). Peaks of modified amino acids are indicated using prefixes such as acetyl, propionyl, and butyryl, and one letter abbreviation of amino acids on the peaks. Peaks assigned to peptides are numbered. Structures of these peptides were estimated based on m/z of precursor and product ions as summarized in Table 2. Peak numbers of diketopiperazines and pyroglutamyl peptides are highlighted in red and green, respectively (Figure 2). The peak numbers of the aspartyl and glutamyl peptides are highlighted in light blue and dark blue, respectively (Figure 2). Peaks sharing the same number had same m/z of precursor ions. Better resolution of the peaks sharing the same m/z was achieved compared to that of the AccQ derivatives. The precursor and product ion scan analyses revealed that peaks sharing same m/z consisted of peptides containing Ile or Leu (peaks 23 and 24) and dipeptides with aspartyl residues (peaks 26, 27, 28, and 29) and glutamyl residues (peaks 21, 22, 23, 24, and 25) at amino-terminus.
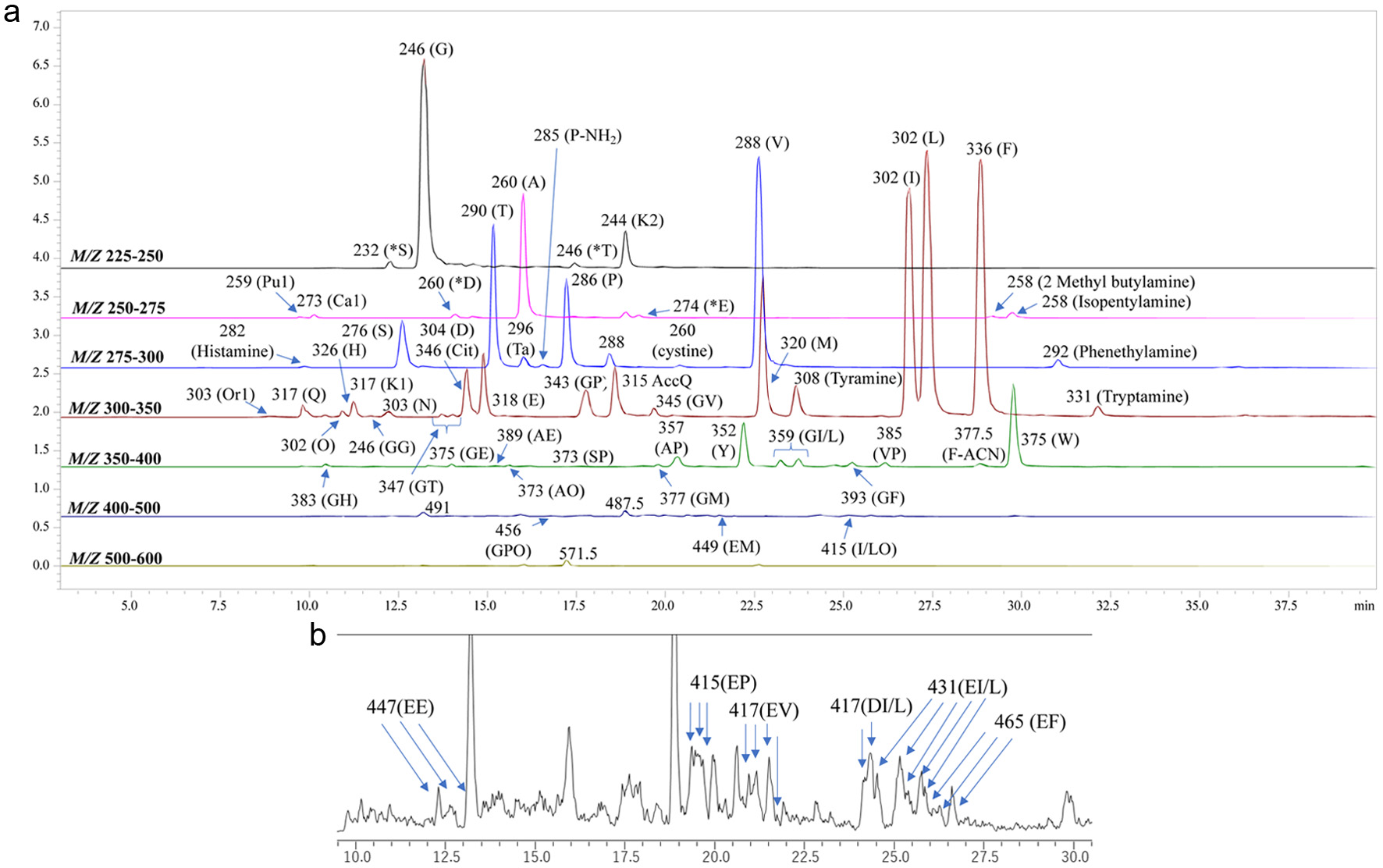 Click for large image | Figure 1. Mass spectrometry (MS) chromatograms of AccQ-derivatized compounds in the VFS. (a) Peaks were detected by precursor ion scan targeting the AccQ-derived product ion (m/z 171.1) in the positive mode across the m/z of 225–250, 250–275, 275–300, 300–350, 350–400, 400–500 and 500–600. Identified amino acids, peptides, and their metabolites are labelled using single-letter amino acid codes or full names. (b) Enlarged MS chromatogram at the scan range m/z 400–500. Other abbreviations: Hydroxyproline (O), β-alanine (*D), ethanolamine (*S), γ-aminobutyric acid (*E), isopropanolamine (*T), prolinamide (P-NH2), taurine (Ta), and citrulline (Cit). Lysine reacted with one and two AccQ are indicated as K1 and K2, respectively. Ornithine, putrescine, and cadaverine reacted with one AccQ are indicated as Or1, Pu1, and Ca1, respectively. Unlabelled peaks could not be assigned to amino acids, peptides, or their metabolites. |
 Click to view | Table 1. Estimated sequences of peptides in the derivatized derived with AccQ based on m/z of product ions and precursor ions |
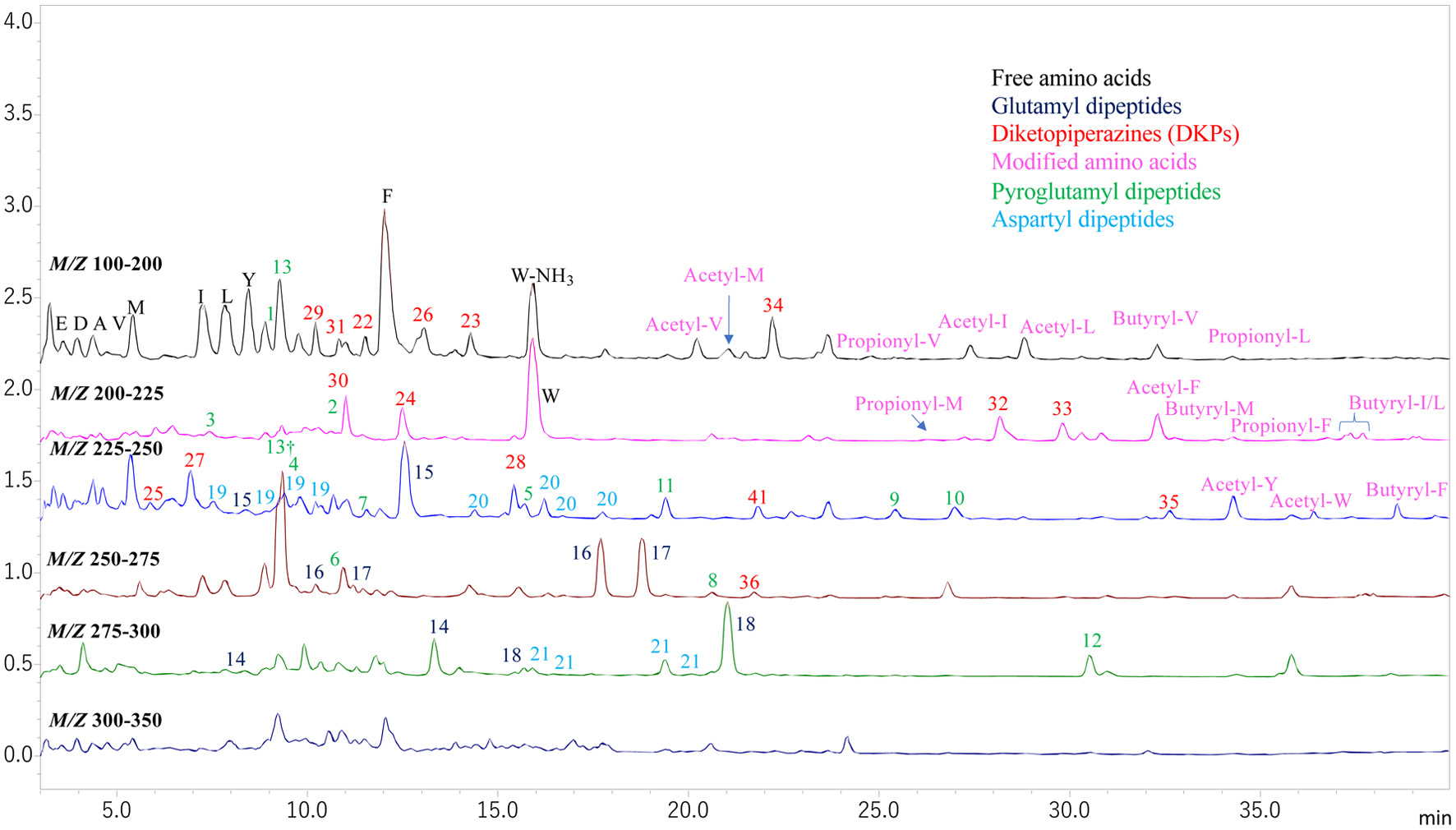 Click for large image | Figure 2. MS chromatograms of non-derivatized compounds in the VFS. Peaks were detected by total ion scan in the positive mode across the m/z of 100–200, 200–225, 225–250, 250–275, 275–300, and 300–350. Peaks of amino acids are presented by single-letter amino acid codes. Peaks of modified amino acids with organic acids are indicated using prefixes such as acetyl-, propionyl-, and butyryl- and single-letter amino acid codes. Peaks with numbers were identified as peptides. Peaks with same numbers indicated presence of peptide isomers with same precursor and product ions. Unlabelled peaks could not be assigned to amino acids, peptides, or their metabolites. *Indicates the ion consisting of two pyroglutamic acids and proton. |
 Click to view | Table 2. Estimated sequences of peptides in the VFS without any derivatization |
Presence of some α/γ-glutamyl peptides and α/β-L/D aspartyl peptides in some fermented foods has been reported (Yang et al., 2019; Nagao et al., 2024). Those isomers of glutamyl and aspartyl peptides in the VFS were identified by comparison of the retention time of synthetic standards in RP-HPLC. Representative mass chromatograms for glutamyl and aspartyl isomers in MRM mode are shown in Figures 3 and 4, respectively. These data indicate presence of α/γ-glutamyl peptides and α/β-L/D aspartyl peptides in the VFS. Figure 5 shows the structure of those peptides.
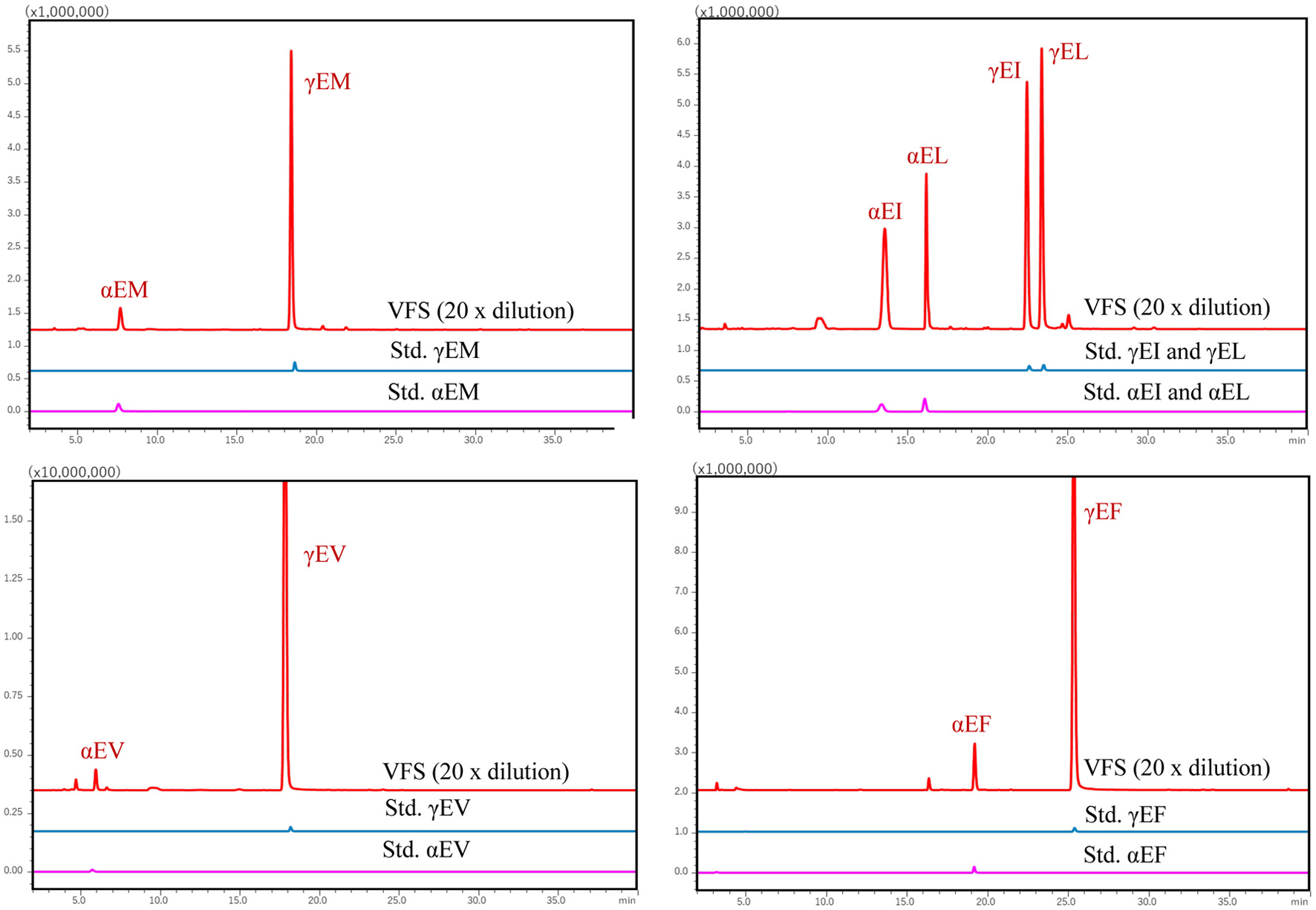 Click for large image | Figure 3. Representative MS chromatograms of two isomers of the underivatized α− and γ-glutamyl-dipeptides in the VFS. Samples and the synthetic standard peptides were analysed using RP-HPLC-MS/MS in MRM mode. αEM and γEM represent α-Glu-Met and γ-Glu-Met, respectively. For other glutamyl dipeptides are shown to be similar. |
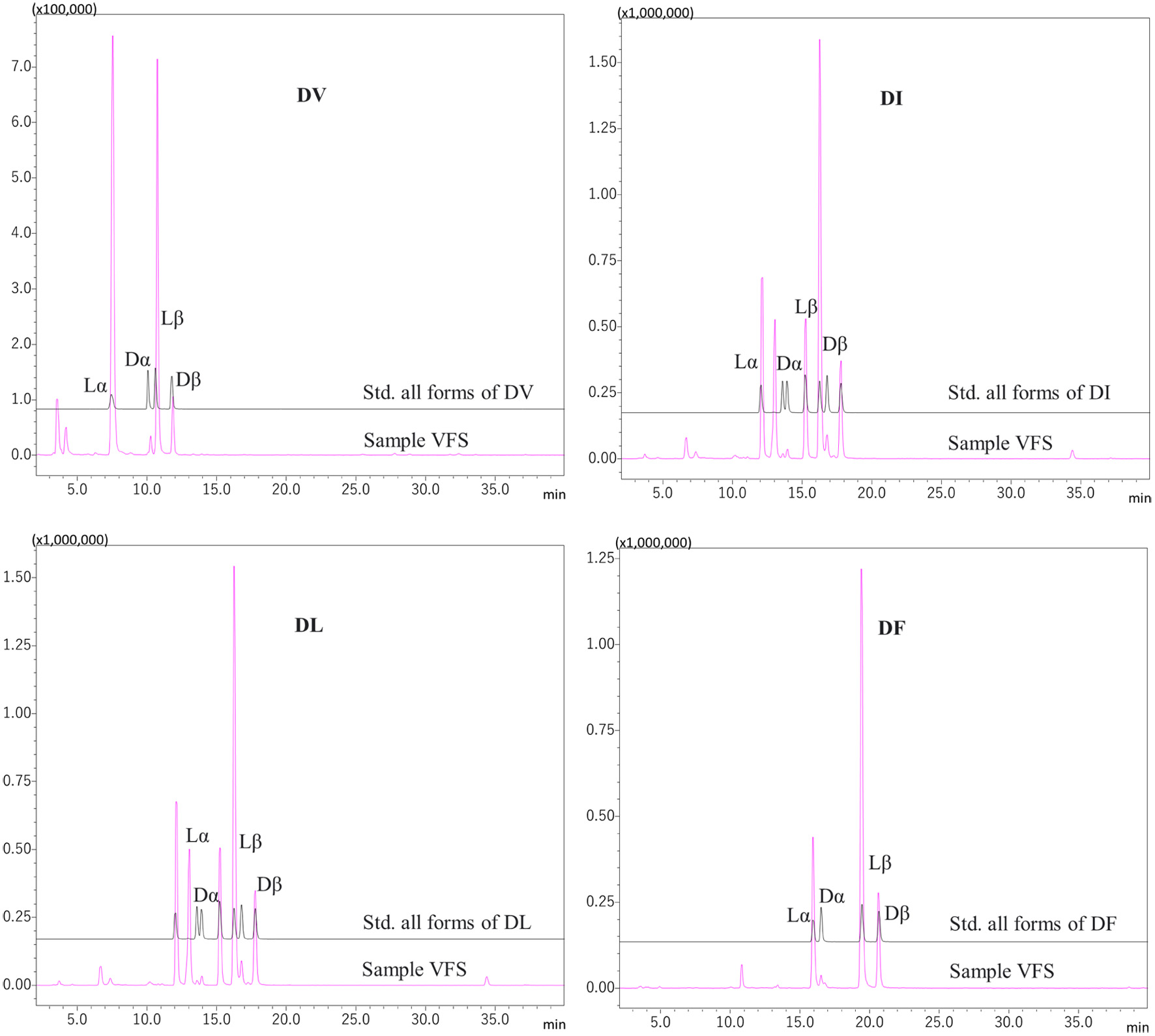 Click for large image | Figure 4. Representative MS chromatograms of four isomers of underivatized aspartyl-dipeptide (Lα-Asp-X, Lβ-Asp-X, Dα-Asp-X, and Dβ-Asp-X) in the VFS. X is occupied by Val (V), Ile (I), Leu (L) and Phe (F). Samples and synthetic standard peptides were analysed using RP-HPLC-MS/MS in MRM mode. |
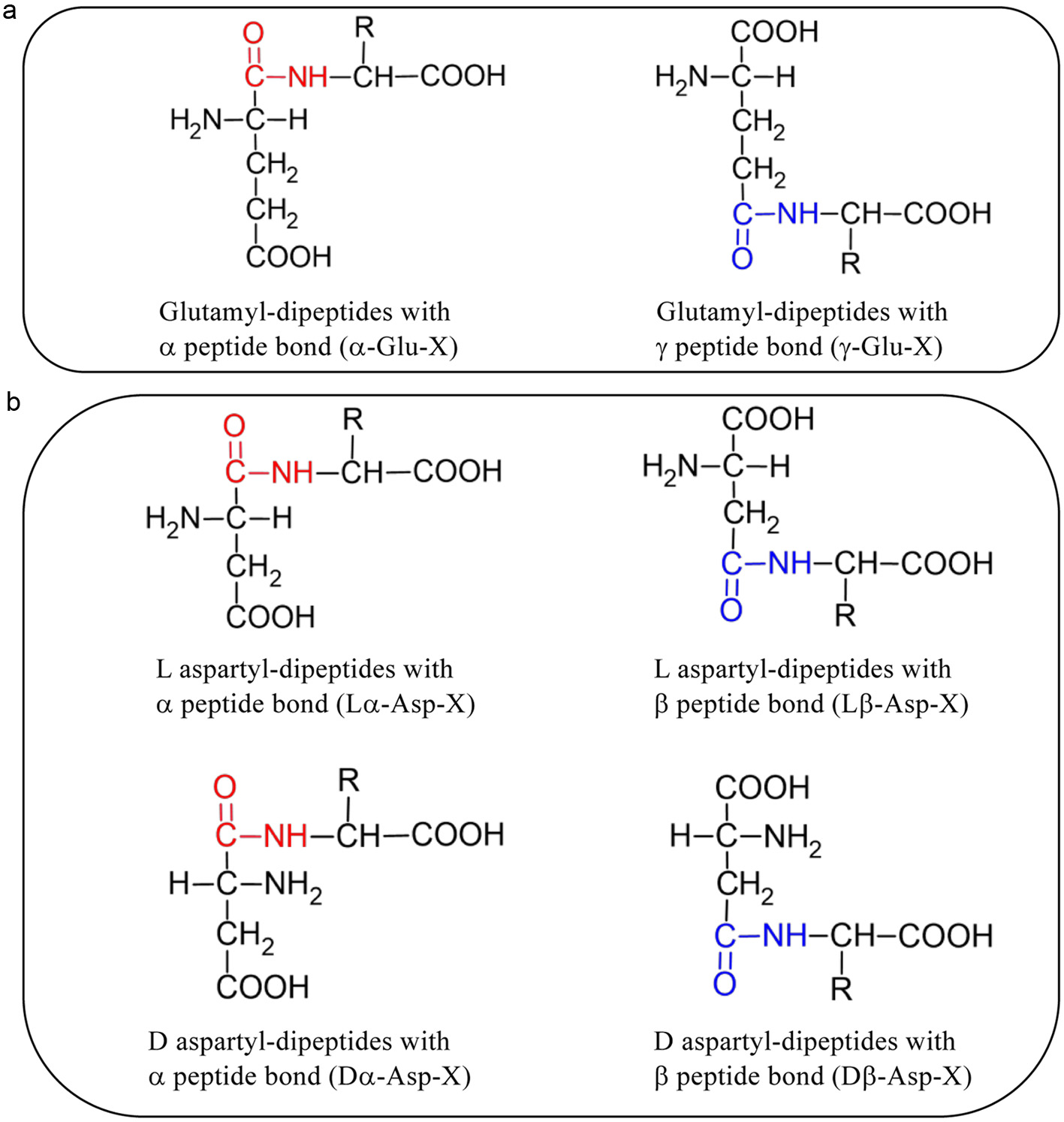 Click for large image | Figure 5. Structure of α- and γ-glutamyl dipeptides (a) and α- and β- L/D aspartyl dipeptides (b). X is occupied by amino acid residues. |
Consequently, we identified 12 pyroglutamyl peptides, 5 glutamyl dipeptides with α and γ peptide bonds, 4 aspartyl dipeptides with L/D aspartyl residues and α/β peptide bonds, and 15 diketopiperazines (Table 2) and 43 unmodified dipeptides and tripeptides (Table 1). Total of 96 peptides were identified.
3.2. Contents of peptides in samples
In addition to the seven major normal peptides, the aspartyl and glutamyl dipeptides were quantified in all samples, and their levels, including all their all isomers, are displayed as a heat map in Figure 6. Contents of all γ-glutamyl peptides (γ-Glu-Met, γ-Glu-Val, γ-Glu-Ile, γ-Glu-Leu, and γ-Glu-Phe) in soy and fish sauces (0.1–1.8 mM) were higher than those of their α-glutamyl forms (0.01–0.1 mM). In particular, γ-Glu-Val and γ-Glu-Phe were abundantly present in the VFS (>1.4 mM). Only negligible amounts of γ-glutamyl peptides were present in lightly fermented foods, such as salami and cheese, which were used as references. All aspartyl dipeptides (Asp-Val, Asp-Ile, Asp-Leu, and Asp-Phe) and their isomers were detected in all fish and soy sauces samples. Contents of these peptides were distributed between 0.01–0.9 mM in soy and fish sauces depending on the sequences. The contents of Dα-Asp-Leu and Dα-Asp-Phe were lower than those of the other aspartyl peptides. Only negligible amounts of β-Asp dipeptides were present in the reference foods (salami and cheese).
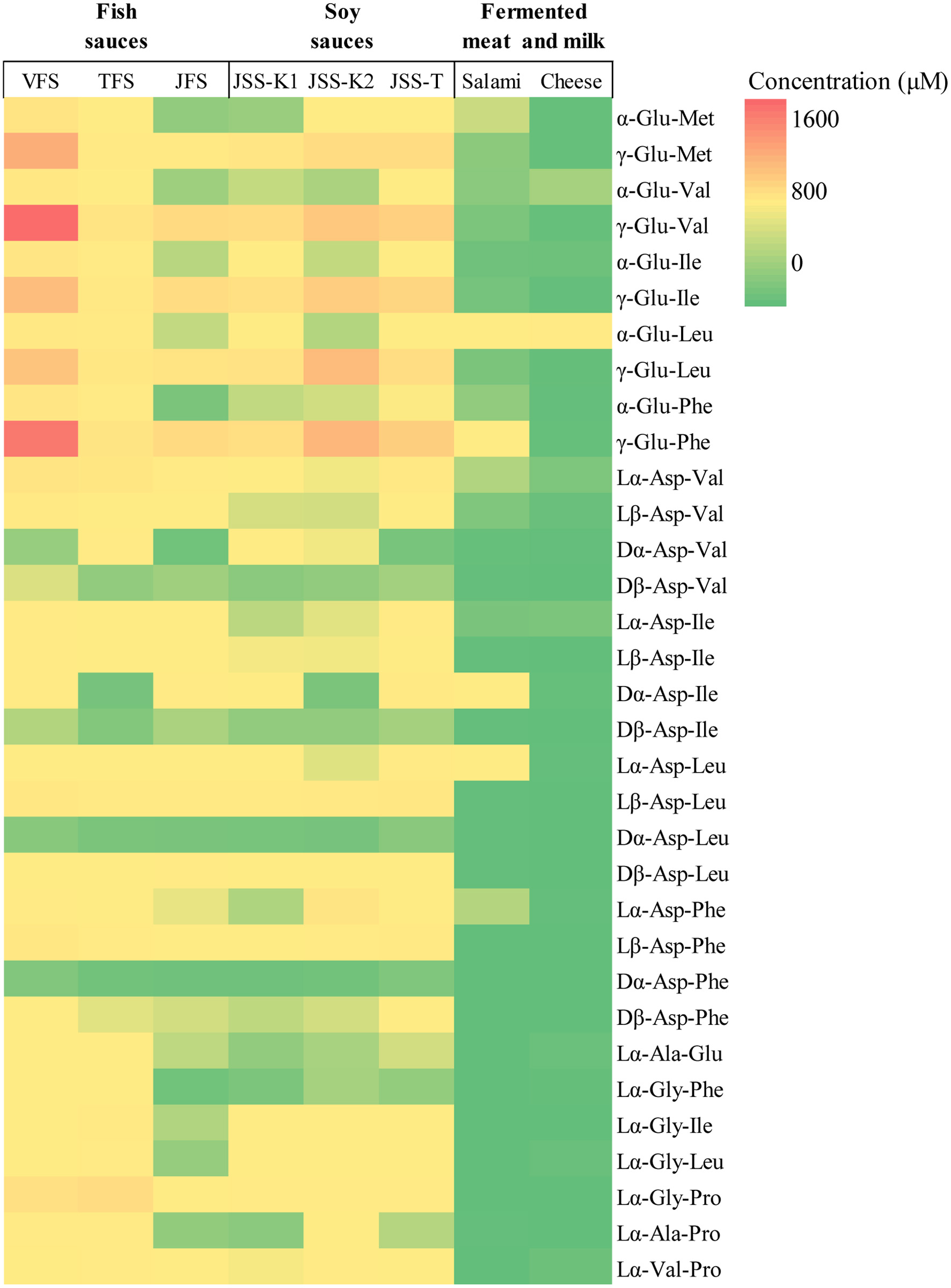 Click for large image | Figure 6. Heatmap of contents of all forms of 10 glutamyl dipeptides,16 aspartyl dipeptides, and 7 normal major peptides in the samples. Refer Figure 5 for structures of isomers. |
Levels of pyroglutamyl peptides and diketopiperazines are displayed as heat map in Figure 7. Levels of pyroglutamyl dipeptides were much abundant (0.01–1.3 mM) compared to longer pyroglutamyl peptides in all samples. Lower but significant levels of pyroglutamyl peptides were present in salami but not in cheese.
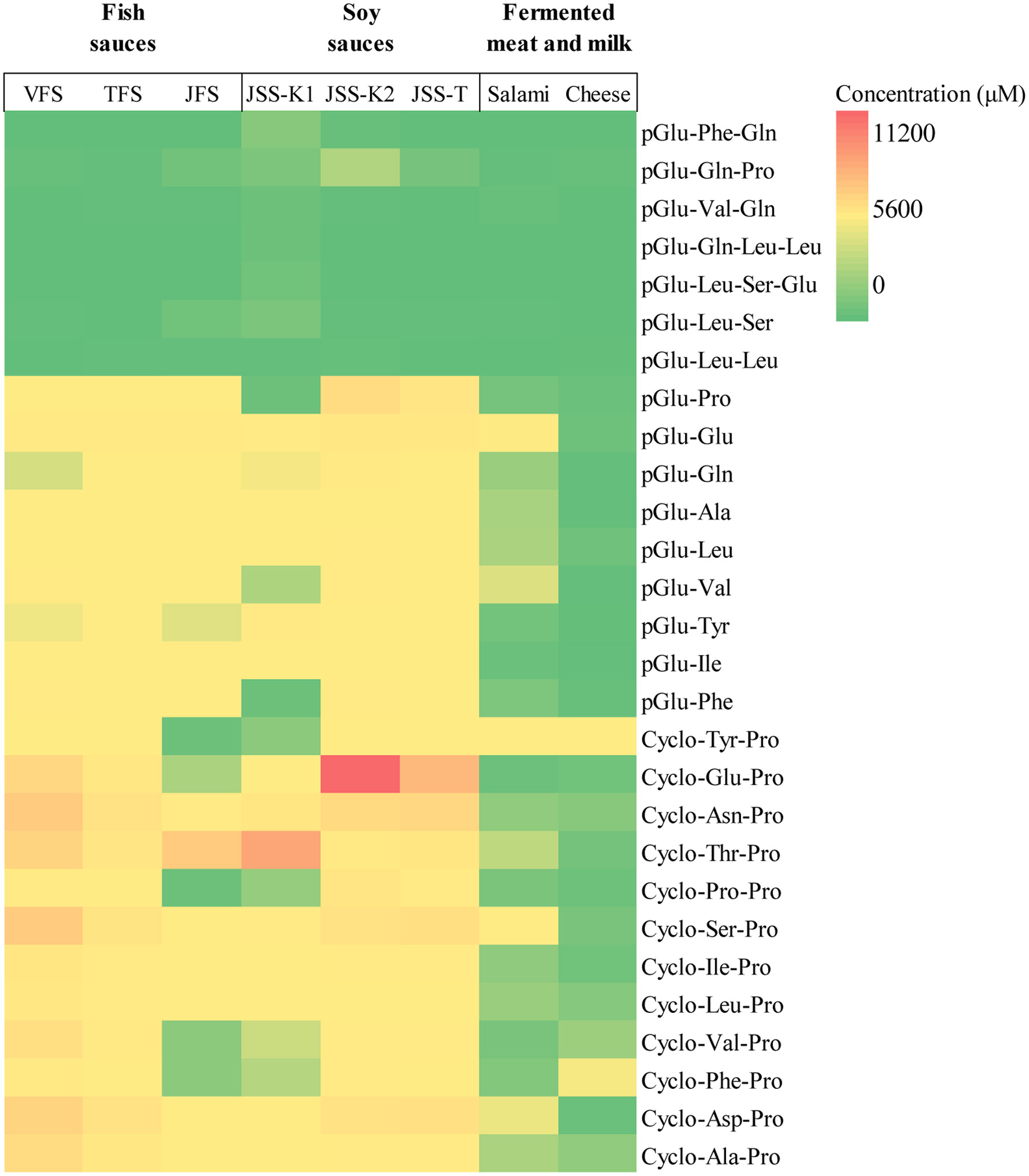 Click for large image | Figure 7. Heatmap of contents of 16 pyroglutamyl peptides and 12 diketopiperazines in the samples. pGlu represents pyroglutamyl residue. The Cyclo-Tyr-Pro is a diketopiperazine consisting of Tyr and Pro residues. |
All diketopiperazines identified in the VFS were present in all fish and soy sauces samples at different levels (0.1−11 mM). Among them, cyclo-Glu-Pro in the soy sauce (JSS-K2) showed the highest content (>10 mM) followed by cyclo-Thr-Pro in JSS-K2 (>5 mM). A fish sauce (JFS) contained smaller levels of cyclo-Tyr-Pro, cyclo-Pro-Pro, cyclo-Val-Pro, and cyclo-Phe-Pro compared to other fish sauces. Some diketopiperazines were also present in the reference foods (salami and cheese).
3.3. Bioavailability of peptides
3.3.1. Small intestinal lumen
As shown in Table 3, the seven major normal peptides except for the aspartyl and glutamyl dipeptides did not significantly increase in the lumen of the small intestine 60 min after the administration of VFS compared to the administration of vehicle, whereas some of them (Gly-Phe, Ala-Glu, Gly, Ile, Gly-Leu, Gly-Pro/Pro-Gly, Ala-Pro, and Val-Pro) were present in the lumen even after administration of vehicle after overnight fasting. As shown in Tables 4 and 5, most modified peptides except for some tri- and tetra-pyroglutamyl peptides were present in the small intestinal lumen of the vehicle group ranging from 0.00 to 0.50 μM in the effluent (10 mL). These peptides are produced from endogenous and microbial proteins. After the oral administration of VFS, only one of α-glutamyl (α-Glu-Leu) and most γ-glutamyl dipeptides except for γ-Glu-Ile significantly increased in the lumen (0.10 −5.60 μM). γ-Glu-Val and γ-Glu-Phe were two highest γ-glutamyl isopeptides in the lumen after administration of VFS and then followed by γ-Glu-Ile and γ-Glu-Leu. Most aspartyl dipeptides were significantly increased by the administration of VFS. Among them, Lβ-form of Asp-Ile, Asp-Leu, and Asp-Phe increased 10−15 times higher than the vehicle group and reached until 0.6 µM in the effluent (10 mL).
 Click to view | Table 3. Contents of some normal major dipeptides in the inner content and washed tissue of small intestine and blood plasma after oral administration of vehicle and Vietnamese fish sauce (VFS) |
 Click to view | Table 4. Contents of glutamyl dipeptides and aspartyl dipeptides in the inner content and washed tissue of small intestine and blood plasma after oral administration of vehicle and Vietnamese fish sauce (VFS) |
 Click to view | Table 5. Contents of diketopiperazines and pyroglutamyl dipeptides in the inner content and washed tissue of small intestine and blood plasma after oral administration of vehicle and Vietnamese fish sauce (VFS) |
Most diketopiperazines, except for cyclo-Phe-Pro, significantly increased in the lumen after the administration of VFS (Table 5). Levels of the diketopiperazines in the effluent of lumen were approximately 0.01−0.40 and 0.02−9.30 μM of the effluent (10 mL) after the administration of vehicle and VFS, respectively. Cyclo-Asp-Pro increased most abundantly and followed by cyclo-Asn-Pro, cyclo-Glu-Pro, and cyclo-Ser-Pro.
Most pyroglutamyl dipeptides significantly increased or tended to increase after the administration of VFS, while larger pyroglutamyl peptides did not significantly change except for pGlu-Phe-Gln.
3.3.2. Washed small intestinal tissue
We also measured the levels of VFS-derived peptides in the anterior and posterior parts of the washed small intestines of the rats. In the anterior part, the levels of indigenous glutamyl and aspartyl dipeptides were 166–6,764 and 2–117 nmol/kg, respectively. In the posterior part, the levels of indigenous glutamyl and aspartyl dipeptides were 172–3,556 and 2–102 nmol/kg, respectively. After oral administration of VFS, unexpectedly, some normal peptides in VFS except for Lα-Asp-Val, Lα-Asp-Ile and Val-Pro significantly increased in the intestinal tissue (Tables 3 and 4). Most of β and γ isomers of aspartyl and glutamyl dipeptides, except for γ-Glu-Met, γ-Glu-Ile, γ-Glu-Leu, γ-Glu-Phe in the anterior part and Dα-Asp-Ile, Dα-Asp-Leu and Dα-Asp-Phe in both parts, significantly increased by administration of VFS compared to the vehicle. The small intestinal tissue contained high levels of γ-Glu-Val, γ-Glu-Ile, γ-Glu-Leu and γ-Glu-Phe compared to other isopeptides after administration of VFS. Generally, levels of the glutamyl dipeptides were higher compared to those of the aspartyl dipeptides (Table 4).
As shown in Table 5, all diketopiperazines and most pyroglutamyl dipeptides except, for pGlu-Tyr and the long-chain pyroglutamyl peptides, were significantly increased in the both small intestinal tissues, while these peptides were also present in the vehicle group. Levels of the diketopiperazines in the both parts of the small intestine tissue were distributed between 7–378 and 21−3,203 nmol/kg after administration of vehicle and VFS, respectively. The pyroglutamyl dipeptides were distributed between 0–344 nmol/kg and 0–1,448 nmol/kg after administration of vehicle and VFS, respectively. Cyclo-Asp-Pro and pGlu-Val were the most abundant diketopiperazine and pyroglutamyl peptide in the both parts of intestinal tissue after ingestion of VFS. These data indicate that the dipeptides found in VFS including some normal dipeptides were absorbed by the small intestine.
3.3.3. Portal and peripheral blood
Blood plasma levels of glutamyl and aspartyl dipeptides, pyroglutamyl peptides, diketopiperazines, and the seven major normal peptides were examined by using LC-MS/MS in MRM mode. The seven major normal peptides including Lα forms of glutamyl and aspartyl dipeptides did not significantly increase in blood plasma after administration of VFS. All forms of glutamyl dipeptides found in VFS were present in portal and abdominal blood plasma of rats receiving the vehicle after overnight fasting ranging from 0.8 to 307 nM. Nonetheless, γ-Glu-Val, γ-Glu-Ile, and γ-Glu-Phe increased slightly but significantly increased to 228, 90 and 271 nM in the plasma from abdominal vein blood after the administration of VFS, respectively. Detectable but small amounts of all forms of aspartyl dipeptides found in VFS were present in the portal and abdominal blood plasma of rats received vehicle ranging from 0.0 to 8.0 nM. Except for the normal Lα- forms, most aspartyl isopeptides significantly increased in both bloods after administration of VFS (0.4−14.9 nM). In addition, concentrations of γ-glutamyl dipeptides in the blood plasma from vehicle group were higher compared to those of α-glutamyl dipeptides in blood and all isomers of aspartyl dipeptides.
Most diketopiperazines, except the cyclo-Tyr-Pro, and some pyroglutamyl dipeptides, such as pGlu-Ile, pGlu-Tyr, pGlu-Leu, and pGlu-Pro, were significantly increased in the both bloods, while these peptides were also present in the vehicle group. After administration of vehicle and VFS, levels of the diketopiperazines in the portal and abdominal blood were 28.3–459.3 and 47.7−5,175 nM and pyroglutamyl dipeptides were 0.0–28.6 and 0.0–53.6 nM, respectively. Cyclo-Thr-Pro and pGlu-Pro were the most abundant diketopiperazine and pyroglutmyl peptide in the blood after administration of VFS.
| 4. Discussion | ▴Top |
Fish and soy sauces are made from fish and soybean, respectively. Roasted wheat grain is generally used as fungi starter (Aspergillus sojae) for most Japanese-style soy sauce (koikuchi-type) Proteins in these ingredients are degraded by endogenous and also fungal, bacterial, and yeast proteases into amino acids and small peptides, which provides umami (Hakimi et al., 2022; Lioe et al., 2010; Zhao et al., 2016) and kokumi (Phewpan et al., 2019; Chen et al., 2023) on these products. The present study revealed presence of some modified amino acids such as N-acetyl, N-propionyl, and N-butyryl amino acids in addition to free amino acids in the fish and soy sauces. Furthermore, presence of some modified peptides such as γ-glutamyl dipeptides, β-aspartyl dipeptides, D-aspartyl dipeptides and amino terminal blocked peptides such as pyroglutamyl peptides and diketopiperazines (cyclic dipeptides) were detected in these samples.
It has been reported that pyroglutamyl peptides are widely present in many enzymatic digests of food proteins (Ejima et al., 2018; Miyauchi et al., 2022; Sato et al., 1998; Wijanarti et al., 2024) and fermented foods (Kiyono et al., 2016; Nagao et al., 2024; Shirako et al., 2020). The present study also demonstrates presence of pyroglutamyl peptides in fish and soy sauces. Presence of γ-glutamyl peptides in fermented foods such as cheese (Kuroda et al., 2020) and some non-fermented foods such as legumes (Dunkel et al., 2007; Taylor et al., 2008) has been reported. In addition, glutathione, γ-glutamyl-cysteinyl-glycine, and related compounds such as γ-glutamyl-S-methyl-cysteine are widely distributed in many organisms (Lu et al., 2021). The present study shows that some fish sauces contain high levels (0.5−2 mM) of γ-glutamyl peptides such as γ-Glu-Val (1.58 mM) and γ-Glu-Phe (1.42 mM) than other foods. The α- and γ-glutamyl peptides were well resolved by reversed phase-high performance liquid chromatography without derivatization, while separation of AccQ-derivatives of α- and γ-glutamyl peptides were not easy. Nagao et al. (2024) used AccQ derivatization for detection of peptides in miso. There is, therefore, a possibility that γ-glutamyl dipeptides may be present in miso, while the presence of γ-glutamyl dipeptides in miso was not reported (Nagao et al., 2024). Some studies have reported that diketopiperazines are present in non-fermented foods, such as roasted cocoa (Stark and Hofmann, 2005) and chicken soup broth (Chen et al., 2004) and fermented foods, miso (Nagao et al., 2024). However, their contents are not so high compared to those in the fish and soy sauces (0.1−11 mM). Only a limited number of studies have reported the presence of aspartyl isopeptides in enzymatic hydrolysates of porcine liver proteins (Ejima et al., 2018) and miso (Nagao et al., 2024). Long-aged miso contains higher levels of aspartyl isopeptides than fish and soy sauce. Taken together, fish sauces are characterized by high contents of some γ-glutamyl peptides such as γ-Glu-Val and γ-Glu-Phe, while soy sauces are characterized with high contents of some diketopiperazines, such as cyclo-Glu-Pro and cyclo-Thr-Pro.
It is well-known that glutathione, one of γ-glutamyl peptides, is synthesized by γ-glutamyl cysteine ligase and glutathione synthetase. Other γ-glutamyl peptides are generated from glutathione by γ-glutamyl transpeptidases in mammalian, bacteria, yeast, and fungi. Sofyanovich et al. (2019) reported that Saccharomyces cerevisiae is able to produce γ-Glu-Val. Lactobacillus reuteri in certain fermented foods is capable of producing γ-glutamyl dipeptides like γ-Glu-Ile and γ-Glu-Cys, which possess an exclusive kokumi sensation (Yang et al., 2019). These facts suggest that γ-glutamyl peptides in fish and soy sauces are synthesized by enzymes from microorganisms involved in fermentation. Miso is fermented without stirring, whereas the fish and soy sauce are fermented with stirring. Therefore, fermentation for the production of fish and soy sauces is considered to be more aerobic than that of miso. This difference may be related to the higher γ-glutamyl peptide content in the fish and soy sauces.
Diketopiperazines are generated from linear peptides through nonenzymatic processes, such as heating (Otsuka et al., 2019; Shimamura et al., 2017). Japanese soy sauce is generally heated during the final process. Soy sauce is darker in color than fish sauce, indicating that soy sauce undergoes a stronger Maillard reaction during heating. Although the detailed manufacturing conditions for the production of fish sauce are not available, the heating process may be responsible for the higher levels of diketopiperazines in soy sauce.
Some modified peptides, such as pyroglutamyl peptides, L/D-β-aspartyl peptides, and diketopiperazines, were increased in the luminal content and small intestinal tissue after oral administration. In blood, diketopiperazines and hydrophobic L/D-aspartyl peptides were significantly increased, while only a portion of pyroglutamyl peptides showed slight increase. The present study reveals that administration of VFS significantly increased γ-glutamyl dipeptides in luminal content and small intestinal tissue. However, increase of γ-glutamyl dipeptides in blood was not so high compared to diketopiperazines and β-aspartyl peptides but higher than pyroglutamyl peptides. Possibly, part of γ-glutamyl dipeptides may be degraded or modified by some enzymes after absorbance into enterocytes. Miyauchi et al. (2022) have reported that normal peptide levels do not increase in the small intestine and blood after ingestion of rice protein hydrolysate. Surprisingly, in the present study, some normal dipeptides were increased in the small intestine, but not in the blood. This suggests that the modified peptides may inhibit exopeptidase activity in the intestine. However, once these normal peptides enter the bloodstream, they may be completely degraded by exopeptidases into amino acids.
It has been reported that γ-glutamyl dipeptides are involved in enhancing kokumi sensation (Ohsu et al., 2010). In addition, γ-glutamyl dipeptides such as γ-Glu-Val have been reported to ameliorate TNF-α-induced vascular inflammation using a cell culture model (Guha et al., 2020) and lipopolysaccharide-induced sepsis in mice model (Chee et al., 2017). Furthermore, it has been reported that γ-Glu-Cys reduced postprandial hyperglycemia (Muramatsu et al., 2014). Recent in vitro study has demonstrated that these γ-glutamyl dipeptides act as an allosteric ligand of calcium sensing receptor (CaSR), which modulates intracellular signaling, consequently suppresses inflammatory response (Guha and Majumder, 2022). Daily consumption of East Asian fish and soy sauces has potential benefits based on the activation of the CaSR mentioned above. However, these studies used relatively high doses of γ-glutamyl dipeptide (0.01–1 mM in culture medium and 20–50 mg/kg body weight orally). Therefore, the effectiveness of daily consumption of these products via CaSR activation by γ-glutamyl dipeptides needs to be examined.
Pyroglutamyl peptides, particularly pGlu-Leu and pGlu-Asn-Ile, have been reported to improve hepatitis (Sato et al., 2013), colitis (Kiyono et al., 2016), high-fat diet-induced obesity (Shirako et al., 2020), and gut microbiota dysbiosis (Shirako et al., 2019) in animal models at relatively low doses (0.1–1 mg/kg). Pyroglutamyl peptides are also widely distributed in other East Asian fermented foods, such as miso (Nagao et al., 2024) and Japanese rice wine, sake (Kiyono et al., 2016). Therefore, pyroglutamyl peptides in fish and soy sauces along with other East Asian fermented seasonings may contribute to the beneficial effects in humans in these areas. Further epidemiological, observational, and interventional studies are required to confirm this finding. It has been demonstrated that some β-aspartyl peptides exert anti-fatigue activity in animal model (Nakagawasai et al., 2021) and have inhibitory activity against angiotensin converting enzyme (Nagao et al., 2024). Unlike other ACE inhibitor peptides, present study and Nagao et al (2024) showed that β-aspartyl peptides have high bioavailability and, therefore, have potential for the antihypertensive and anti-inflammatory effects of consuming these fermented foods. Diketopiperazines have been reported to exert antioxidant, antidepressant, antimicrobial, and other beneficial effects (Taga et al., 2017). However, efficacy of diketopiperazines in doses obtained by consumption of the fermented foods remains to be examined.
| 5. Conclusion | ▴Top |
The present study identified short-chain modified peptides such as γ-glutamyl dipeptides, β-aspartyl dipeptides, diketopiperazines (cyclic dipeptides), pyroglutamyl peptides and modified amino acids in fish and soy sauces in higher amounts compared to those in the reference foods (cheese and salami). Among them, γ-glutamyl dipeptides (0.5–2 mM) were abundantly presents in the Vietnamese fish sauce (VFS). These modified peptides showed higher bioavailability compared with the normal peptides. Taken together with previously reported data, these modified peptides in fish and soy sauces can be candidates of bioactive peptides with health-promoting activities. Further research is necessary to elucidate their effects of the doses obtained by actual consumption of these fermented foods.
| Supplementary material | ▴Top |
Table S1. Modified amino acids binding to acyl group (RC(O)–) and hydrocarbyl group in fermented foods.
Acknowledgments
The authors thank to the Ministry of Education, Culture, Sports, Science and Technology (Monbu-kagaku-shō) for the financial support (Mext Scholarship) and the Kyoto Louis Pasteur Center for Medical Research’s for their permission to conduct animal experiments in their facility.
Conflict of interest
All authors declare that there is no conflict of interest related to this paper.
| References | ▴Top |