| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 29, March 2025, pages 38-46
Anti-allergic effect of kefir from goat milk with Lactiplantibacillus plantarum Dad-13: In Vitro evaluation
Firarosa Asidaa, Yunika Mayangsaria, Dian Anggraini Surotoa, b, Tyas Utamia, b, Momoko Ishidac, d, e, Kosuke Nishic, d, e, Takuya Sugaharac, d, e, *
aDepartment of Food Science and Technology, Faculty of Agricultural Technology, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
bUniversity Center of Excellence for Integrated Research and Application for Probiotic Industry, and Center for Food and Nutrition Studies, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia
cDepartment of Applied Bioresource Science, The United Graduate School of Agricultural Sciences, Ehime University, Matsuyama, Ehime 790-8566, Japan
dDepartment of Bioscience, Graduate School of Agriculture, Ehime University, Matsuyama, Ehime 790-8566, Japan
eFood and Health Function Research Center, Ehime University, Matsuyama, Ehime 790-8566, Japan
*Corresponding author: Takuya Sugahara, Department of Food Science and Technology, Faculty of Agricultural Technology, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia. E-mail: sugahara.takuya.mz@ehime-u.ac.jp; Tel: +81 89 946 9863
DOI: 10.26599/JFB.2025.95029400
Received: January 14, 2025
Revised received & accepted: February 6, 2025
| Abstract | ▴Top |
Kefir is a type of fermented milk produced by a unique and complex mixture of bacteria and yeasts viable in a symbiotic relationship. Anti-allergic effect of goat milk kefir fermented with Lactiplantibacillus plantarum subsp. plantarum Dad-13 isolated from “Dadih” (curd made from buffalo milk from West Sumatera, Indonesia) was evaluated using rat basophilic leukemia cell line RBL-2H3 cells by identifying β-hexosaminidase release, intracellular calcium ion concentration ([Ca2+]i), microtubule formation and intracellular signal transduction. The results showed that goat milk kefir significantly inhibits degranulation of RBL-2H3 cells at 20 mg/mL without cytotoxicity by inhibiting the microtubule formation and the elevation in [Ca2+]i stimulated by antigens. Immunoblot analysis demonstrated that goat milk kefir significantly downregulated phosphorylation of Syk, Akt and PI3K in the signaling pathways activated by antigen-mediated stimulation. These results suggested that goat milk kefir fermented with Lactiplantibacillus plantarum subsp. plantarum Dad-13 has the potential to serve as a functional food with anti-allergic properties.
Keywords: Anti-allergy; Degranulation; Kefir; Goat milk; Lactiplantibacillus plantarum subsp. plantarum Dad-13
| 1. Introduction | ▴Top |
Food allergy impacts around 1 to 3% of adults and 5 to 8% of children (Liang et al., 2022). The mechanism of allergic reactions begins when IgE binds to high-affinity IgE receptor (FcεRI) on the surface of basophils and mast cells. This interaction leads to cross-linking of IgE with antigen, triggering the activation of intracellular signaling pathways, including an increase in intracellular calcium ion concentration ([Ca2+])i, which subsequently induces degranulation (Hiemori-Kondo et al., 2021). Degranulation involves the secretion of histamines inducing allergic symptoms (Refli et al., 2023) and the activation of further intracellular signaling pathways, such as the phosphorylation of proteins (Zorig et al., 2021). A cascade of tyrosine phosphorylation of degranulation signaling molecules in basophils/mast cells was triggered by antigen stimulation via IgE binding FcεRI, resulting an influx of Ca2+ into the cytoplasm and leading release of granules containing chemical mediators (Zorig et al., 2021). Type I allergy symptoms can be caused by chemical mediators such as histamine and leukotrienes (Hiemori-Kondo et al., 2021). β-Hexosaminidase, contained in granules along with chemical mediators serves as a biomarker for degranulation (Refli et al., 2023). Due to the interaction between IgE and antigen on FcεRI, RBL-2H3 cells are commonly used to study Type I allergy including food allergies (Yan et al., 2024).
The consumption of kefir on a regular basis has been demonstrated to improve digestion and tolerance to lactose, and to have anti-inflammatory, antioxidant activity, anti-allergenic activity (Rosa et al., 2017). Food allergy prevention can be achieved through the consumption of kefir, which reduces intestinal permeability to antigens and can increase mucosal defense. (Liu et al., 2006) showed that animals consuming cow milk and soymilk kefir had lower IgE and IgG levels, indicating reduced allergy markers. The impact of kefir is not limited to the gut, as its microorganisms and bioactive compounds positively influence the composition of the gut microbiota and overall immune health (Rosa et al., 2017).
Goat milk, one of the dairy products, is valued for its nutritional purposes (Sousa et al., 2019) that is characterized by the hypoallergenic activity; and easily digestible; and effective carrier for probiotics (Ren et al., 2024). Lactiplantibacillus plantarum subsp. plantarum Dad-13 was isolated from dadih (buffalo milk from West Sumatera Indonesia) (Purwandhani et al., 2018) and has been applied to products, such as yogurt and cheese, as a starter for fermented milk drinks which have proven to be favored by consumers (Luwidharto et al., 2022; Pamungkaningtyas et al., 2018a; Meidistria et al., 2020). A strain of Lactiplantibacillus plantarum subsp. plantarum Dad-13 can live in the digestive tract and is resistant to gastric juice and bile salt (Gunawan et al., 2021). A starter culture of freeze-dried Lactiplantibacillus plantarum subsp. plantarum Dad-13 was utilized to produce a fermented milk beverage (Utami et al., 2020a). Lactiplantibacillus plantarum subsp. plantarum Dad-13 may inhibit the growth of pathogenic Escherichia coli that cause diarrhea (Pamungkaningtyas et al., 2018b). Consumption of Lactiplantibacillus plantarum subsp. plantarum Dad-13 for 28 days has been shown to have deleterious effects on the body which makes these bacteria suitable to be called as probiotic (Purwandhani et al., 2018). Rahim et al., (2023) stated that the consumption of fermented milk enriched with probiotics and/or prebiotics has been demonstrated to ameliorate allergic conditions in human infants. However, there is no study on the application of Lactiplantibacillus plantarum subsp. plantarum Dad-13 in goat milk kefir products that has potential on anti-allergy. This study investigated the anti-allergic effect of goat milk kefir from fermented by Lactiplantibacillus plantarum subsp. plantarum Dad-13 on RBL-2H3 cells.
| 2. Materials and methods | ▴Top |
2.1. Materials
Kefir grains as starter of fermentation were purchased from a local company (Tangerang, Indonesia), Lactiplantibacillus plantarum subsp. plantarum Dad-13 was obtained from PUI-PT Probiotics, PSPG University of Gadjah Mada, Indonesia. Goat milk was purchased from Bhumi Nararya Farm (Yogyakarta, Indonesia). Bovine serum albumin (BSA), Dulbecco’s Modified Eagle Medium (DMEM), penicillin, mouse anti-dinitrophenol (DNP) monoclonal IgE, DNP-human serum albumin (HSA), cell lysis buffer, and Triton X-100 were obtained from Sigma (St. Louis, MO, USA). A phosphatase inhibitor cocktail was purchased from Nacalai Tesque (Kyoto, Japan). Aprotinin, Pefabloc SC, and protease inhibitor cocktail were obtained from Roche Applied Science (Basel, Switzerland). Anti-phosphoinositide 3-kinase (PI3K) antibody, anti-phosphorylated PI3K antibody, anti-Syk antibody, anti-phosphorylated Syk antibody, anti-Akt antibody, anti-phosphorylated Akt antibody, and HRP-labeled anti-rabbit IgG antibody, were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.2. Sample preparation
In this study, kefir from goat milk fermented with Lactiplantibacillus plantarum subsp. plantarum Dad-13 (GK13) was compared with raw materials, such as goat milk (GM), Lactiplantibacillus plantarum subsp. plantarum Dad-13 (D13) and kefir grains (KG), by evaluating the degranulation activity. GK13, GM, D13, KG and were in freeze-dried form before extraction. The freeze-dried samples were suspended in distilled water (D.W.) and agitated for 12 h. Then the samples were centrifuged at 20,000 rpm for 30 min at 4°C to collect water-soluble extracts. The supernatant was collected, and the pH was adjusted at 7.4. After that, each supernatant was centrifuged again at 20,000 rpm for 10 min at 4°C, then freeze-dried again to measure the dry weight of the water-soluble extract. The dried form of the water-soluble extracts was dissolved in D.W. at 20 mg/mL. The sample solution was sterilized by using 0.22 μm or 0.45 μm of syringe filter under a sterile condition and stored at −30°C until further use. The extracts were further diluted into 20, 5, 1.25, and 0.31 mg/mL. For fractination of components in each extract by ethanol precipitation, ethanol was added to these extracts up to 50% and centrifuged to obtain supernatant (GK13 S50; 50% EtOH soluble fraction) and precipitate (GK13 P50; 50% EtOH insoluble fraction). In addition, samples were dialyzed using dialysis membrane with a molecular weight cut-off of 14 kDa, 6.8 kDa, and 3.5 kDa and then dialyzed samples were freeze-dried. Dialyzed and freeze-dried samples were redissolved in D.W. at 20 mg/mL.
2.3. Fermentation of goat milk using Lactiplantibacillus plantarum subsp. plantarum Dad-13
The fermentation was carried out according to Utami et al. (2020b) with modification. The freeze-dried starter culture of Lactiplantibacillus plantarum subsp. plantarum Dad-13 was pasteurized in goat milk and then incubated for 24 h at 37°C. The starter culture was prepared by inoculation of 1% culture of Lactiplantibacillus plantarum subsp. plantarum Dad-13 (107 CFU/mL), into goat milk. Finally, kefir samples were freeze-dried.
2.4. Viable cell of Lactobacillus plantarum
Lactic acid bacteria isolates were inoculated on LPSM (Lactobacillus plantarum specific medium) autoclaved at 121°C for 15 min. Next, it was incubated at 37°C for 24 h. The analysis was conducted by first dissolving 5 mL of kefir in 45 mL of 0.85% NaCl, followed by homogenization. A total of 0.1 mL of the resulting suspension was spread-plated on LPSM agar in a petri dish. LPSM media is specifically designed for the cultivation of Lactobacillus plantarum. Dilution and plating were performed in the final three dilution series. The dilutions used for Lactobacillus plantarum cell count were 104, 105, and 106. The formula used to calculate the number of cell colonies according to FDA (1998) is as follows:
where N = Number of colonies per ml or g of product
Σ c = Sum of all colonies on all plates counted
n1 = Number of plates in first dilution counted
n2 = Number of plates in second dilution counted
d = Dilution from which the first counts were obtained.
2.5. Cell and cell culture
Rat basophilic leukemia cell line RBL-2H3 cells were obtained from American Type Culture Collection (Rockville, MD, USA) and cultured in DMEM supplemented with 100 μg/mL of penicillin, 100 μg/mL of streptomycin, and 5% FBS at 37°C in a humidified incubator with a 5% CO2-95% air atmosphere. In this study, RBL-2H3 cells with less than 15 passages were used.
2.6. β-Hexosaminidase release assay
RBL-2H3 cells were pre-cultured for two days until 80% of confluence, and the cells were collected and inoculated in a 96-well culture plate at 4 × 105 cells/well. The cells were sensitized with anti-DNP IgE and incubated for 12 h at 37°C in a humidified 5% CO2 incubator. The cells were washed with modified Tyrode’s (MT) buffer (1.8 mM CaCl2, 5.6 mM glucose, 20 mM HEPES, 5 mM KCl, 1 mM MgCl2, 135 mM NaCl, and 0.05% BSA, pH 7.4) twice and treated with 120 μL of samples at different concentrations for 10 min. Degranulation was induced by adding 10 μL of DNP-HSA diluted in MT buffer, and the cells were incubated for 30 min.
2.7. Measurement of cell viability
RBL-2H3 cells were seeded, sensitized with anti-DNP IgE, and treated with DNP-HSA to trigger antigen-induced β-hexosaminidase release. The cells were then treated with the samples for 30 minutes, then the culture medium was removed, and the cells were rinsed with phosphate-buffered saline (PBS, pH 7.4). Fresh DMEM containing WST-8 reagent (Nacalai Tesque) was added to each well, then the cells were incubated at 37°C for 20 min. Viability was evaluated by measuring the absorbance at 450 nm using a microplate reader (Model 680; Bio-Rad Laboratories, Hercules, CA, USA).
2.8. Measurement of intracellular calcium ion concentration ([Ca2+])
[Ca2+]i levels were measured using a fluorescent calcium indicator Fluo-3 AM (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions. RBL-2H3 cells were seeded in a black 96-well culture plate and treated with anti-DNP IgE as previously described. After IgE sensitization, the cells were rinsed with PBS and incubated with 100 μL of Fluo 3 AM for 1 h. The cells were washed with PBS and treated with 20 mg/mL of samples or 80% aqueous ethanol as a control at 37°C for 10 min. The cells were stimulated by adding 10 μL of DNP-HSA diluted in MT buffer to a final concentration of 0.625 μg/mL, and the fluorescence intensity was immediately recorded at an excitation wavelength of 490 nm and an emission wavelength of 530 nm using an SH-8000Lab microplate reader (Corona Electric, Ibaraki, Japan).
2.9. Immunofluorescent staining
RBL-2H3 cells were seeded in a 35 mm culture dish with a coverslip at a density of 6.0 × 105 cells per dish. Following sensitization with 50 ng/mL of anti-DNP IgE, the cells were treated with 20 mg/mL of GK13 for 10 min. The reagent was then removed, and the cells were stimulated with 50 ng/mL of DNP-HSA at 37°C for 10 min. After washing with PBS 2 times, the cells were fixed using 4% paraformaldehyde for 15 min. Subsequently, the cells were washed thrice with PBS and blocked with 5% BSA and 0.3% Triton X-100 in PBS for 1 h. After removing the blocking solution, the cells were incubated with an Alexa Fluor 488-labeled anti-α-tubulin antibody at 4°C for 12 h. After washing with PBS 3 times, images were captured using a fluorescence microscope (BX53, Olympus, Tokyo, Japan) (Nishi et al., 2024).
2.10. Immunoblot analysis
RBL-2H3 cells were inoculated at 2.5 × 105 cells/well in 24-well plate, treated with anti-DNP IgE, and incubated with 20 mg/mL of GK13 in MT buffer at 37°C for 10 min. Then, cells were stimulated with DNP-HSA at 2.5 µg/mL and further incubated for 10 min. After reagent removal, cell lysates were prepared using lysis buffer, centrifuged, and normalized for protein concentration. SDS-PAGE separated proteins, transferred onto PVDF membranes (GE Healthcare, Buckinghamshire, UK) using the Trans-Blot apparatus (Bio-Rad Laboratories). After blocking with Tris-buffered saline (TBS, pH 7.6) containing 5% skim milk for 1 h at room temperature, the membrane was incubated with primary antibodies overnight at 4°C. Membranes were incubated with HRP-conjugated secondary antibodies and developed with ImmunoStar LD chemiluminescence detection reagent (Fujifilm Wako Pure Chemical, Osaka, Japan). Bands were visualized with a ChemiDoc XPS Plus apparatus (Bio-Rad Laboratories). The chemiluminescent intensity was quantified using Quantity One software (Bio-Rad Laboratories).
2.11. Statistical analysis
Data obtained were shown as mean ± SD. The significance of these data was statistically analyzed using analysis of one-way variance (ANOVA) using SPSS (ver. 22). Tukey HSD was used as a post-hoc analysis. Values of p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) were regarded as statistically significant.
| 3. Results | ▴Top |
3.1. Fermentation process of GK13
The pH of GK13 and viable cell numbers of L. plantarum in GK13 were evaluated. As shown in Table S1, the pH of GK13 with three repetitions of analysis was around 4.0. The viable cell number of L. plantarum with two repetitions of analysis had a concentration 107.74 CFU/mL (Table S2). This result related to the required standards of fermented milk product that should contain a minimum concentration of 107 CFU/mL of microorganisms (Weltgesundheitsorganisation & FAO, 2011).
3.2. Effect of GK13 on degranulation of RBL-2H3 cells
β-Hexosaminidase release assay was conducted to examine the anti-degranulation effect of probiotic goat milk kefir (GK13) on RBL-2H3 cells. GK13 extracted in (D.W.) was added to the culture medium of RBL-2H3 cells sensitized with anti-DNP IgE at various concentrations. As shown in Figure 1a, degranulation of RBL-2H3 cells stimulated by antigen was significantly suppressed by GK13 in a dose-dependent manner. Goat milk (GM) also had anti-degranulation activity, but Dad-13 (D13) and kefir grains (KG) had no activity. A cell viability test was conducted to determine the cytotoxicity of all samples againts RBL-2H3 cells. As indicated in Figure 1b, all samples showed no significant cytotoxicity at any doses. These observations indicated that GK13 strongly inhibited degranulation without cytotoxicity at 20 mg/ml, hence, further experiments were conducted at this concentrations.
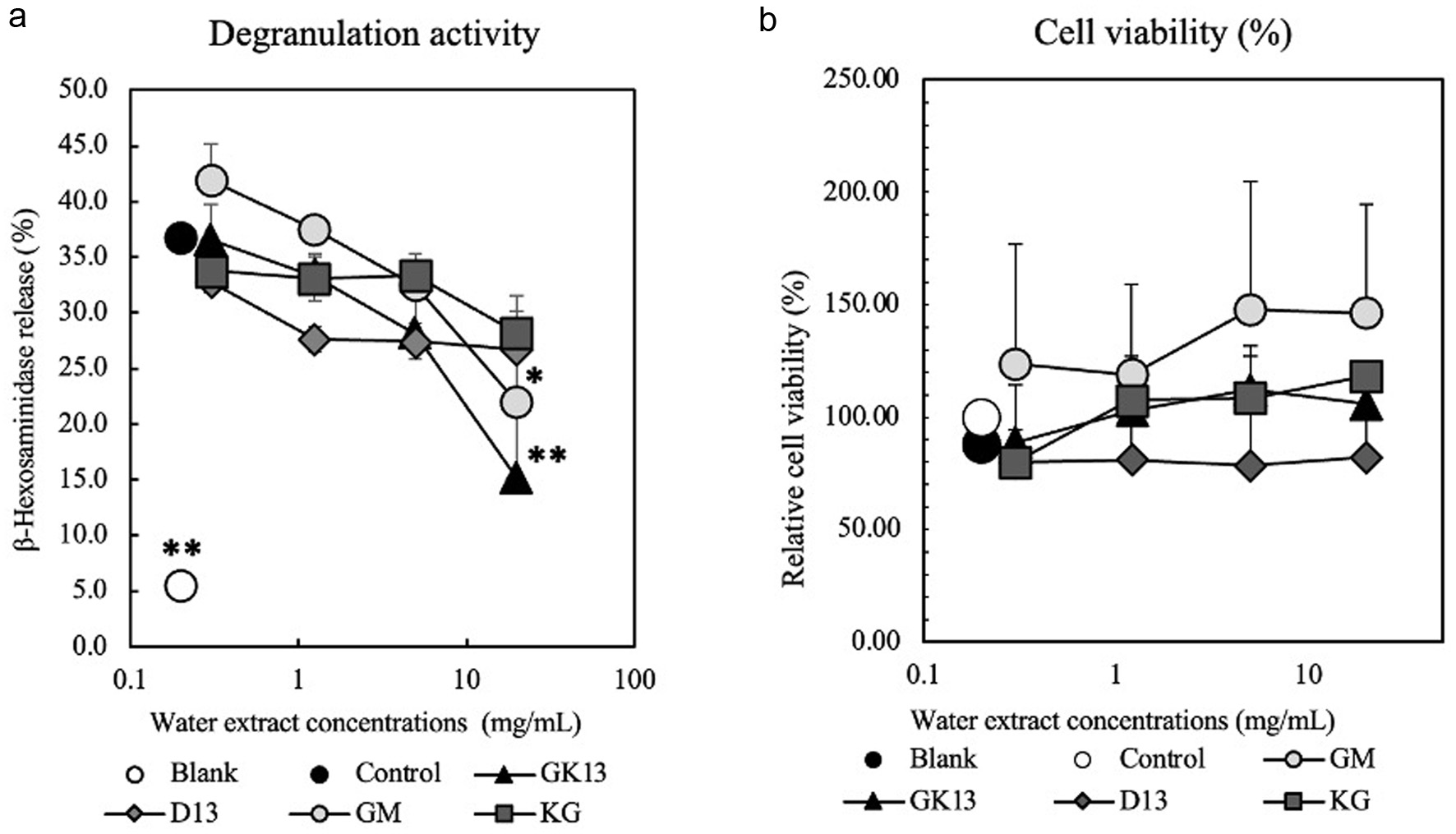 Click for large image | Figure 1. Effect of GK13 on degranulation of RBL-2H3 cells. (a) The release rate of β-hexosaminidase relative to control is presented as mean ± SD. (b) A cell viability of RBL-2H3 cells relative to control is presented as mean ± SD. Statistical significance compared with the control, analysed by Tukey's test, is represented as *p<0.05, **p<0.01, and ***p<0.001. (blank): cells not stimulated with DNP-HSA; (control): cells stimulated with DNP-HSA; (GK13, GM, D13, KG): GK13-, GM-, D13-, or KG-treated cells stimulated with DNP-HSA. |
3.3. Effect of GK13 on the ([Ca2+]i) in RBL-2H3 cells
One of the critical processes in mast cell degranulation is the increase in intracellular calcium ion concentration ([Ca2+]i) (Castellone et al., 2021). A high-affinity Ca2+ indicator Fluo-3 AM was used to examine the effect of GK13 on [Ca2+]i in RBL-2H3 cells induced by antigen stimulation. As shown in Figure 2, GK13 suppressed an increase in [Ca2+]i induced by antigen stimulation at 20 mg/mL. On the other hand, D13 and GM had no effect on [Ca2+]i. This result suggested that GK13 suppresses degranulation by inhibiting the elevation of [Ca2+]i.
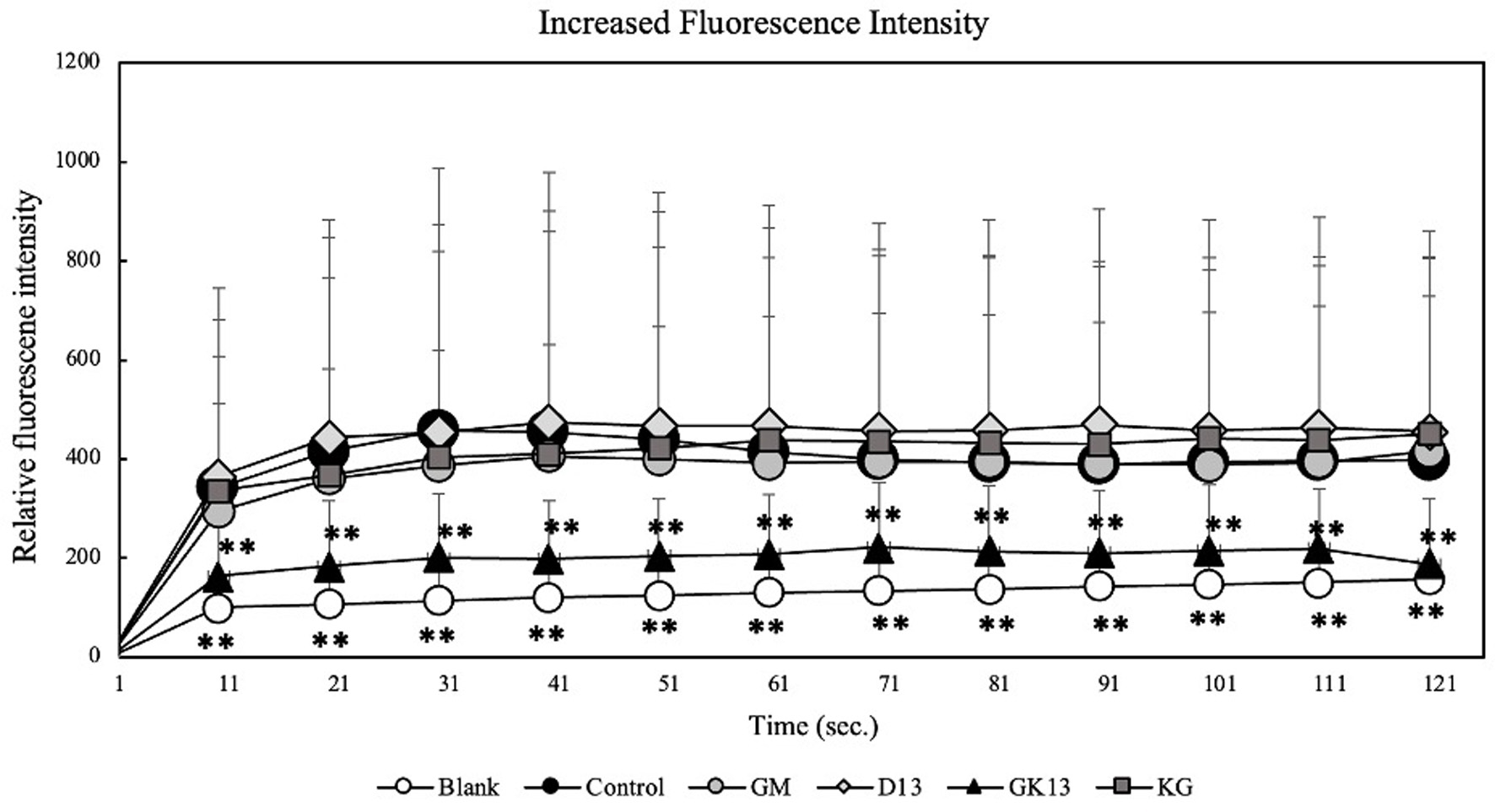 Click for large image | Figure 2. Effect of GK13 on [Ca2+]i in RBL-2H3 cells. [Ca2+]i was examined using Fluo-3 AM. (blank): cells not stimulated with DNP-HSA; (control): cells stimulated with DNP-HSA; (GK13, GM, D13): GK13-, GM-, or D13-treated cells stimulated with DNP-HSA. Statistical significance compared with the control, analysed by Tukey's test, represented as *p<0.05, **p<0.01, and ***p<0.001. |
3.4. Effect of GK13 on microtubule formation
The microtubule is a cytoskeleton component that has a function as a transport pathway of granules containing histamine from the cytoplasm to the cell membrane to trigger the allergic symptoms by releasing granules. The α-tubulin immunofluorescent staining was conducted to examine the effect of GK13 on microtubule formation induced by antigen stimulation. As shown in Figure 3, the cells stimulated by the antigen had thick elongation and long, branched fibrous structures. On the other side, the cells treated with GK13 at 20 mg/mL had less fibrous structures similar to non-stimulated cells indicating shorter microtubule length. Therefore, it suggested that GK13 suppresses the degranulation by inhibition of microtubule formation along with inhibition of [Ca2+]i.
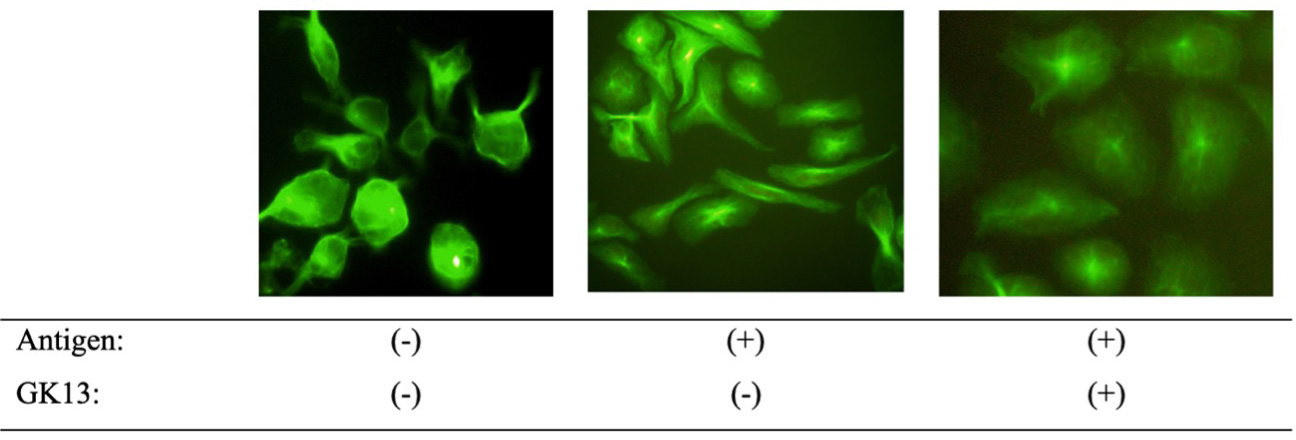 Click for large image | Figure 3. The effect of GK13 on microtubule formation. Microtubules in RBL-2H3 cells were stained with an Alexa Fluor 488-labeled anti-α-tubulin antibody. Images were captured using a fluorescence microscope. |
3.5. Effect of GK13 on the intracellular signaling pathways involved in degranulation
Two signaling pathways are involved in degranulation such as a calcium-dependent pathway (Lyn-Syk-LAT) and a calcium-independent pathway (Fyn-PI3K-Akt). The effect of GK13 on these signaling pathways was examined. As indicated in Figures 4 and 5, GK13 significantly decreased the phosphorylation level of Syk, Akt, and PI3K compared with control, while no significant effect on the phosphorylation of Lyn was observed. This result indicated that GK13 suppresses degranulation by inhibiting both signaling pathways followed by suppression of microtubule formation and an increase in [Ca2+].
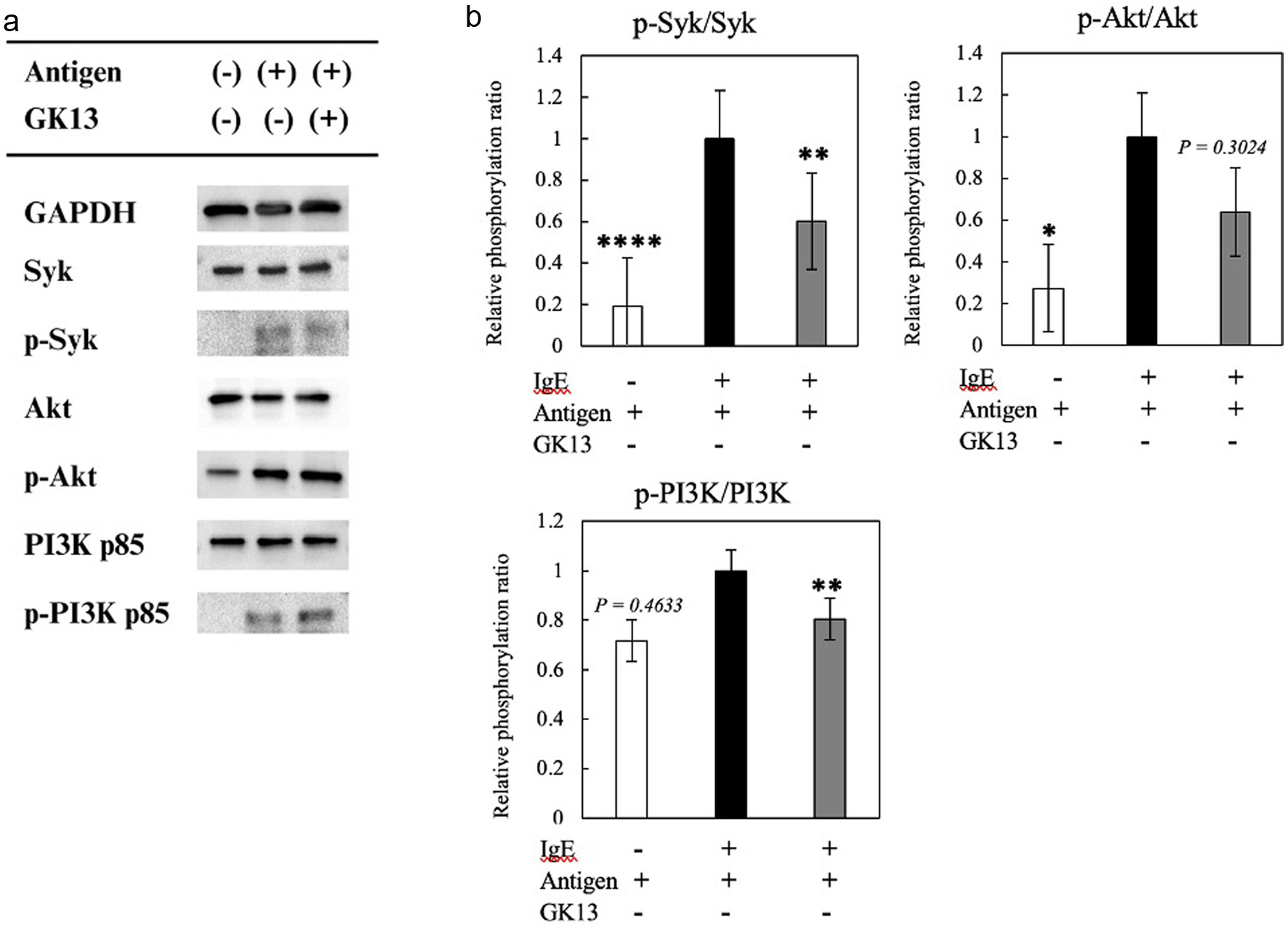 Click for large image | Figure 4. Effect of GK13 on degranulation signaling pathways in RBL-2H3 cells. (a) The phosphorylated protein levels of GAPDH, Syk, Akt, and PI3K p85 were evaluated using immunoblot analysis. (b) The phosphorylation ratios of Syk, Akt, PI3K p85. Statistical significance compared with the control, analysed by Tukey's test, is represented as *p<0.05, **p<0.01, and ***p<0.001. |
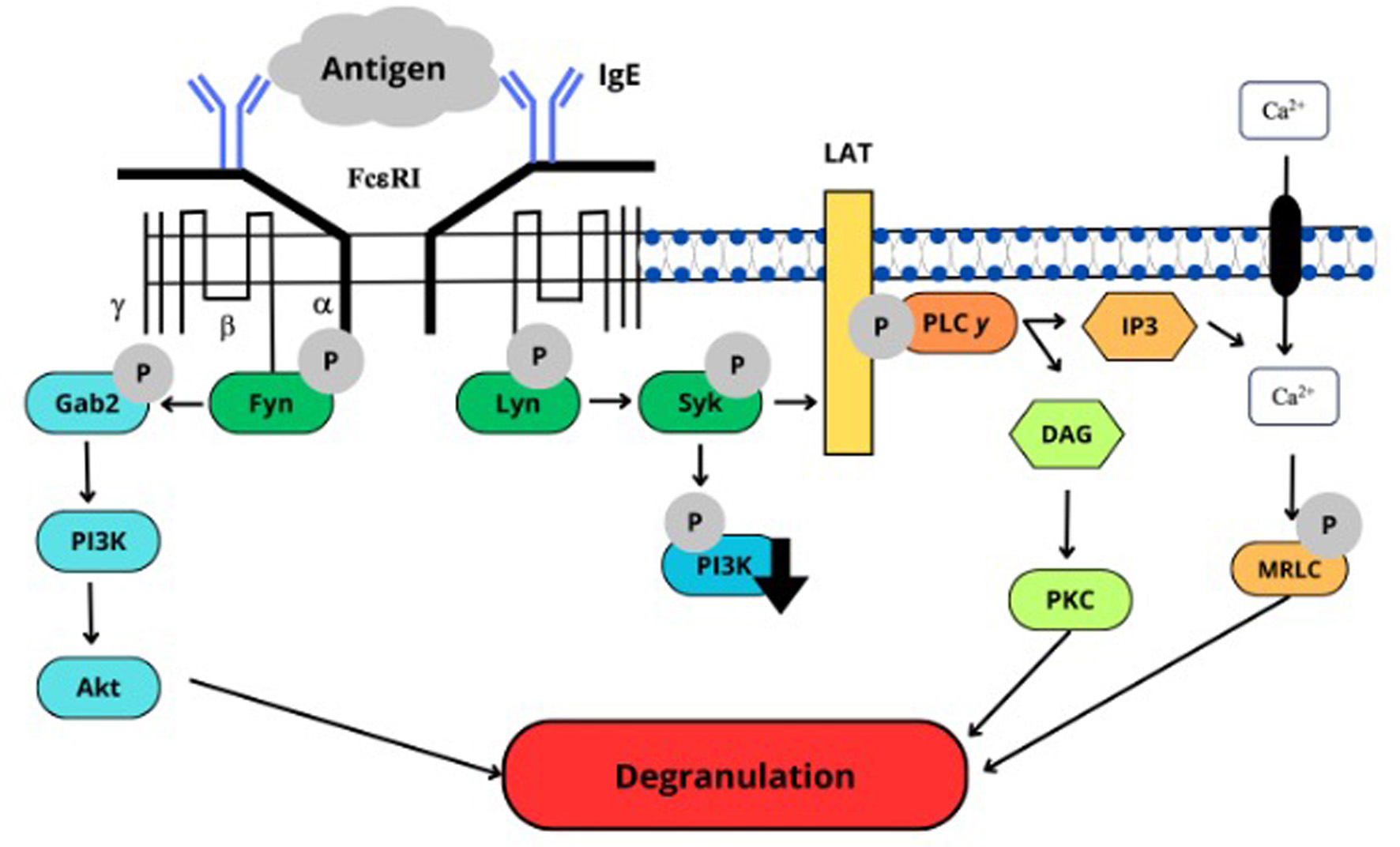 Click for large image | Figure 5. The mode of action of GK13. |
3.6. Characteristic of active substances in GK13
In this study, GK13 was dialyzed and dissolved in 50% EtOH to evaluate the characteristics of the anti-degranulation active substances in GK13. GK13 was dialyzed by dialysis membrane with a molecular weight cut-off of 14, 6.8, and 3.5 kDa and then compared the anti-degranulation activity with non-dialyzed GK13. Figure 6a showed that GK13 dialyzed by 3.5 kDa cut-off membrane retained anti-degranulation activity as intact GK13. In contrast, GK13 dialyzed by 14 and 6.8 kDa membranes completely lost its activity. GK13 was fractionated by 50% of EtOH precipitation. As indicated in Figure 6b, GK13 S50 (50% EtOH supernatant) strongly inhibited degranulation, as well as intact GK13. On the other hand, GK P50 (50% EtOH precipitate) had no activity. This result suggested that the active substance in GK13 has a molecular weight between 3.5 and 6.8 kDa and is soluble in 50% EtOH.
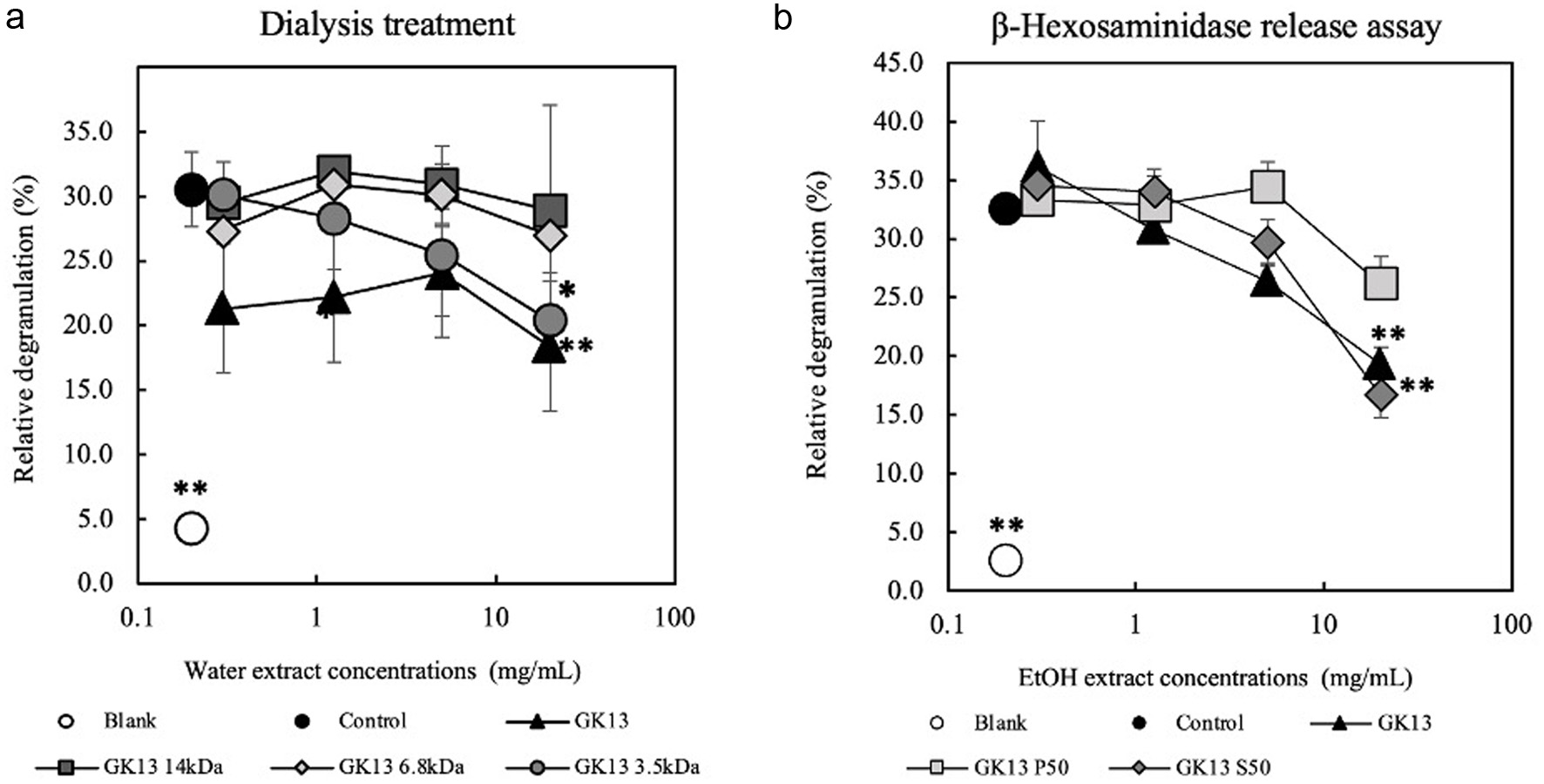 Click for large image | Figure 6. Characteristics of active substance in GK13 by dialysis and 50% EtOH precipitation. (a) Anti-degranulation activities of dialyzed GK13 with various molecular weight cut-off dialysis membranes. (b) Fractionation of the active substance in GK13 by 50% EtOH. Statistical significance compared with the control, analysed by Tukey's test, is represented as *p<0.05, **p<0.01, and ***p<0.001. (blank): cells not stimulated with DNP-HSA; (control): cells stimulated with DNP-HSA; (GK13): GK13-treated cells stimulated with DNP-HSA. |
| 4. Discussion | ▴Top |
This present study evaluated the anti-degranulation activity of GK13 containing acetic acid bacteria, lactic acid bacteria, and yeast within a matrix composed of exopolysaccharides (EPS) and proteins. Lactiplantibacillus plantarum subsp. plantarum Dad-13 is an indigenous bacteriuma isolated from “Dadih” (curd from buffalo milk of West Sumatera, Indonesia). Previous studies showed that fermentation of kefir is conducted at a temperature of 20–25°C for 24 h with a pH around 4 (Hanum et al., 2021). Viable and active bacteria are found in many fermented foods, with the requirements of viable probiotic bacteria must be at least 106–107 CFU/g (Garcia-Gonzalez et al., 2021; Marinova et al., 2019). Probiotics can provide health benefits by modulating the commensal microbiota, inhibiting pathogens, modulating the immune system, and producing bioactive molecules. Probiotic bacteria have already been part of the human routine for good gut microbiota maintenance (Palanivelu et al., 2022).
Farag et al., (2020) stated that kefir contains many macro- and micronutrients, such as vitamins, proteins, lipids, and amino acids. These ingredients explain why persons with lactose intolerance can take kefir and why it has antibacterial, immunological, chemopreventive, and hypocholesterolemic properties. While kefir can be prepared from various milk types, the antioxidant activity of cow milk kefir was higher than that of other milk types (M’hir et al., 2024). Sunarti et al., (2015) reported that goat milk kefir and a combination of goat milk and soybean milk kefir considerably lowered plasma glucose levels in diabetic Wistar rats. Furthermore, soybean and goat milk have anti-inflammatory components. For example, oligosaccharides in goat milk decrease intestinal and colon inflammation. From these findings, kefir has great potential in improving health, but using which type of milk can also affect the health benefit to be achieved because each type of milk contains different active components.
In Type I allergy, granules containing chemical mediators such as histamine are released from basophils and mast cells activated by allergens (Refli et al., 2023). Degranulation is induced when the antigen binds with IgE on basophils and mast cells via FcεRI, triggering intracellular signaling pathways for degranulation (Hiemori-Kondo et al., 2021). β-Hexosaminidase is also stored in granules (Matsuda et al., 2016) and released by degranulation (Guo et al., 2018). Therefore, β-hexosaminidase release assay can be used to quantify the degranulation of basophils and mast cells (Figure 1a). As indicated in Figure 2, GK13 inhibited the elevating of [Ca2+]i levels stimulated by antigen stimulation. The suppression of phosphorylation of Syk in degranulation signaling pathway supported this result. We also investigated microtubule formation. Cells treated with GK13 exhibited shorter and unbranched fibrous structures, similar to those non-stimulated cells (Figure 3). Therefore, this result indicated that GK13 inhibited microtubule formation. This finding was supported by the suppression of the phosphorylation of Akt and PI3K in degranulation signaling pathway. Two signaling pathways are involved in degranulation: calcium-dependent and calcium-independent pathways (Hada et al., 2019). The calcium-dependent involves the Lyn-Syk-LAT cascade, while the calcium-independent involves Fyn-Gab2-PI3K cascade (Nishida et al., 2005 and Kopeć et al., 2006). The tyrosine kinase pathway links two protein tyrosine kinases, Lyn and Syk, which are involved in degranulation. FcεRI aggregation induced by IgE-antigen complex activated the phosphorylation of Lyn kinase, which subsequently facilitates the recruitment and activation of Syk kinase in response to antigen triggering of IgE-sensitized basophils and mast cells. Syk plays a crucial role in allergic responses downstream of FcεRI signaling (Refli et al., 2023). The PI3K pathway is critical in managing cell viability, growth, and the production of inflammation mediators. PI3K directly activates Akt through the production of PIP3. When PI3K is activated (e.g., by FcεRI receptors on mast cells), it catalyzes the formation of PIP3 at the plasma membrane so that Akt can be activated (Hada et al., 2019).
GK13 suppressed the degranulation induced by antigen (Figure 1a). As indicated in Figure 2, GK13 inhibited the elevation of [Ca2+]i levels stimulated by antigen stimulation. The suppression of phosphorylation of Syk in the calcium-dependent degranulation signaling pathway supported this result. We also investigated microtubule formation. Cells treated with GK13 exhibited shorter and unbranched fibrous structures than those of control cells treated with antigen, indicating that GK13 inhibited microtubule formation (Figure 3). This finding was supported by the suppression of the phosphorylation of PI3K and Akt in the calcium-independent degranulation signaling pathway.
Active substances in kefir that inhibit degranulation had molecular weight between 3.5 and 6.8 kDa and were soluble in 50% EtOH as shown in Figure 6. The molecular weight of peptides in milk has been determined, revealing 16 bioactive peptides with molecular weights range of 3.5 kDa, including β-casein, α-S2-casein, α-lactalbumin (Wang et al., 2024). Since GK13 is known to contain components with anti-allergic effects, further chemical analyses, such as HPLC, GC-MS, and LC-MS, are necessary to identify the specific active substances responsible for these effects.
| 5. Conclusion | ▴Top |
The overall result, GK13 significantly inhibited the degranulation activity of RBL-2H3 cells via two pathways: suppression of the elevation of intracellular calcium ion levels and inhibition of microtubule formation. GK13 significantly inhibited the degranulation of RBL-2H3 cells without cytotoxicity by suppressing the elevation of [Ca2+]i, mediated by downregulation of phosphorylation of the molecules in degranulation signaling pathways, particularly Syk. Additionally, GK13 inhibited microtubule formation by downregulation of PI3K and Akt activation. We also observed that goat milk suppressed the degranulation of RBL-2H3 cells, although the effect of goat milk was lower than that of GK13. The molecular weight range of the active substances in GK13 was between 3.5 and 6.8 kDa. Further studies are required to identify the specific active substances in kefir and goat milk, and the anti-allergic effect in vivo needs to be evaluated.
| Supplementary material | ▴Top |
Table S1. Average pH of GK13.
Table S2. Viable cell number of Lactobacillus plantarum.
Acknowledgments
The authors thank the SUIJI JP master’s degree program for financial support and thank to Endang Sutriwasti Rahayu for allowing to use Lactiplantibacillus plantarum subsp. plantarum Dad-13 from PUI-PT Probiotics, PSPG of Gadjah Mada University, Indonesia. The animal experiment was conducted at the Division of Genetic Research Support of the Advanced Research Support Center (ADRES), Ehime University.
Conflict of interest
The authors declare no conflict of interest.
Conceptualization and design, F.A., T.S., Y.M., D.A.S., T.U; Methodology, F.A.; Software, F.A., K.N; Validation, F.A.; Formal Analysis, F.A., M.I., K.N. and T.S.; Investigation, F.A; Resources, T.S; Data Curation, F.A. M.I.; Writing – Original Draft Preparation, F.A.; Writing – Review & Editing, T.S., K.N., Y.M; Visualization, F.A.; Supervision, K.N., T.S., Y.M.
| References | ▴Top |