| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 29, March 2025, pages 29-37
Progress in research on flavor compounds in Gastrodia elata
Hongling Du, Chang Liu, Cheng Mei, Jianfeng Zhan*
College of Biology and Agricultural Resources, Huanggang Normal University, Huanggang 438000, China
*Corresponding author: Jianfeng Zhan, College of Biology and Agricultural Resources, Huanggang Normal University, Huanggang 438000, China. E-mail: zhanjianfeng2010@163.com
DOI: 10.26599/JFB.2025.95029404
Received: March 18, 2025
Revised received & accepted: March 29, 2025
| Abstract | ▴Top |
As a medicinal and edible plant, Gastrodia elata (G. elata) flavor compounds critically influence its sensory, nutritional, and medicinal properties. This review summarizes recent advances in G. elata flavor research. Volatile components, mainly comprising organic acids, aldehydes, alcohols, and esters, form its unique flavor profile, with 3-methylthiopropionaldehyde and 2,3,5,6-tetramethylpyrazine identified as key contributors to the characteristic “horse-urine odor.” Variations in volatile composition exist among cultivars, geographical origins, and harvest seasons. Non-volatile compounds, including phenolics (e.g., gastrodin, parishins), saccharides, and free amino acids, underpin pharmacological efficacy and nutritional value. Conventional extraction techniques (steam distillation, solvent extraction) face limitations in efficiency and eco-friendliness, while processing methods (steaming, fermentation) significantly alter flavor profiles. Future research should focus on advanced identification technologies, sustainable extraction methods, mechanistic elucidation, and industrial applications to optimize G. elata’s utilization in food and pharmaceuticals.
Keywords: Gastrodia elata; Flavor compounds; Volatile components; Extraction techniques; Processing methods
| 1. Introduction | ▴Top |
Gastrodia elata (G. elata) is a perennial, saprophytic herb of the Orchidaceae family, primarily distributed across Asian countries such as China, Japan, South Korea, India, and Nepal. In China, it is predominantly cultivated in the Yunnan, Guizhou, and Hubei provinces, thriving in cool, humid forest environments rich in humus. These ecological conditions promote optimal growth and accumulation of characteristic flavor compounds. As a dual-purpose medicinal and edible plant, G. elata exhibits sedative, anti-inflammatory, and antioxidant properties (Kwon et al., 2013). In provinces like Yunnan and Guizhou of China, it is traditionally consumed as a tonic food. Its flavor compounds,serve as critical links between sensory perception and functional value, guiding consumer preferences while also acting as key carriers of its nutritional and medicinal functions (ChP, 2020). Both the edible and medicinal qualities of G. elata are intrinsically linked to its flavor components (Ma et al., 2024), and processing techniques significantly influence the preservation of these flavor substances and bioactive constituents .
Recent advancements in analytical technologies have significantly accelerated research into the flavor chemistry of G. elata. Studies reveal that its distinctive flavor results from the synergistic interaction of volatile and non-volatile components. Volatile compounds are dominated by organic acids (accounting for up to 84.78%), aldehydes, and alcohols, with 3-methylthiopropionaldehyde and 2,3,5,6-tetramethylpyrazine (Figure 1) identified as key contributors to its characteristic “horse-urine odor” (Table 1). Non-volatile constituents, including gastrodin, parishins, polysaccharides, and free amino acids, play pivotal roles in pharmacological efficacy, textural properties, and nutritional functions. However, challenges persist in flavor research (Duan et al., 2023). On one hand, the chemical diversity of G. elata is strongly influenced by cultivar, geographical origin, harvest season, and processing methods. For instance, the volatile components differ significantly between red and green ecotypes, and the contents of gastrodin and palisin were dynamically changed by steaming and boiling processes (Ma et al., 2021). On the other hand, existing extraction techniques (e.g., steam distillation, supercritical CO2 extraction), despite their advantages, face limitations in trace compound identification, thermosensitive component protection, and eco-efficient extraction. Furthermore, the mechanisms linking flavor compounds to bioactivities, as well as the molecular basis of flavor evolution during processing, remain inadequately elucidated, hindering product innovation and industrial upgrading.
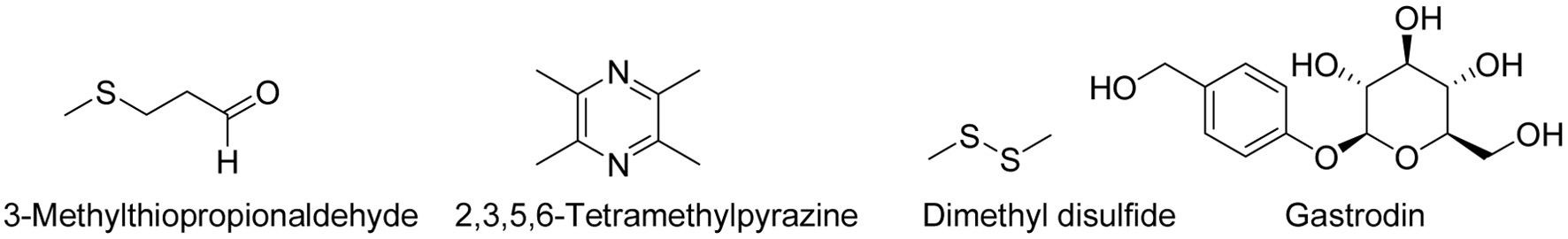 Click for large image | Figure 1. Structure of main compound associated with the horse-urine odor in Gastrodia elata. |
 Click to view | Table 1. Volatile Chemical Components of G. elata |
This paper systematically reviews research progress on G. elata flavor compounds, focusing on the classification, functions, and influencing factors of both volatile and non-volatile components. It also compares extraction technologies and processing methods, and proposes future research directions to advance theoretical foundations for deep resource utilization, flavor regulation, and value-added applications of G. elata.
| 2. Types and characteristics of flavor compounds | ▴Top |
The volatile components of G. elata constitute the foundational elements of its distinctive flavor profile. Common chemical constituents include phenolic glycosides, saccharides and their glycosides, steroids, organic acids and their esters, nucleosides, amino acids and derivatives among others. The unique flavor and bioactive functions of G. elata primarily originate from tuber-stored compounds, which are key secondary metabolites responsible for generating numerous potentially bioactive substances. Researchers have conducted comprehensive studies on its chemical composition, having isolated and identifyed over 200 compounds from G. elata tubers. These compounds are primarily categorized into phenolic compounds, parishins, phenol-amino acid conjugates, phenol-nucleotide derivatives, and carbohydrate derivatives (Zhu et al., 2021).
The sensory perception of food typically initiates with olfactory stimulation triggered by volatile substances that carry characteristic aromas. In G. elata, its distinctive fragrance has been specifically attributed to volatile organic compounds (Sun et al., 2020). While non-volatile compounds primarily mediate its taste-active properties, they also demonstrate significant biological functions and pharmacological efficacy.
2.1. Volatile flavor compounds
Supercritical CO2 extraction was utilized for the selective isolation of volatile components from G. elata, with subsequent compositional analysis conducted via gas chromatography-mass spectrometry (GC-MS). The volatile compounds idebtified in the study mainly consist of aldehydes, phytols, esters, alkanes, alkenes, phenols, organic acids, and nitrogen-containing compounds (Table 1). Among these, organic acids demonstrated the most prominent abundance and structural diversity, accounting for 84.78% of the total identified components. The synergistic interplay of these components likely underlies the distinctive sensory characteristics associated with G. elata rhizomes (Han et al., 2018).
A comprehensive review of recent literature on G. elata volatile oils reveals its rich chemical composition, as summarized in Table 1. Key components include aldehydes, alcohols, esters, acids, phenols, ketones, alkanes, aromatic compounds, and other volatile substances. These collectively define its unique sensory attributes. Through GC-MS analysis, critical constituents such as β-sitosterol, linoleic acid, n-hexadecanoic acid, and stigmasta-3,5-diene have been identified. However, discrepancies in reported volatile compositions and relative abundances persist across studies, primarily attributed to variations in analytical techniques, equipment specifications, G. elata cultivars, and geographical origins. Notably,a significant proportion of compounds remain uncharacterized, highlighting the need for methodological refinements and advanced analytical technologies to achieve comprehensive analysis and comprehensive characterization of G. elata volatile oils.
Aldehydes, typically formed through fatty acid oxidation, impart unique aromas at low concentrations but exhibit rancid or pungent odors when exceeding critical thresholds (Wang et al., 2021). Their relative content ranges from 35.60% to 57.06%, collectively representing a significant proportion of G. elata volatile oils (Sun et al., 2022). Specific aldehydes contribute distinct sensory attributes: nonanal delivers fatty-grassy notes with citrus undertones; phenylethanal and hexanal enhance floral and fruity nuances; hexadecanal introduces cardboard-like olfactory characteristics; 2-methylbutanal evokes cocoa and almond aromas; and 3-methylbutanal imparts chocolate and peach-like fragrances (Li et al., 2025). Notably, 3-methylthiopropionaldehyde generates a sharp, pungent odor, strongly associated with the characteristic “horse-urine odor” of G. elata (Martínez-Arellano et al., 2016).
Alcohols, derived from microbial metabolism of protein-derived amino acids and lipid peroxidation (Domínguez et al., 2014), predominantly contribute pleasant floral-fruity notes to the flavor profile (Sansenya et al., 2018; Huang and Li, 2018) identified alcohols as the second-largest volatile group (relative content: 39.07%) using simultaneous distillation extraction coupled with GC-MS (SDE-GC-MS). Esters, accounting for 20.00–23.92% of volatile oils, include methyl palmitate with waxy-greasy tones, which significantly shape the organoleptic profile (Sun et al., 2022; Huang and Li, 2018).
Organic acids, comparable to aldehydes in diversity, are characterized by linoleic acid (mild fatty note) and α-linolenic acid (dominant at 52.29%) as key components (Han et al., 2018). Hexanal, hexanoic acid, and phenethyl alcohol exert synergistic modulation of the aroma: hexanoic acid, with sweaty undertones, may partially explain G. elata’s distinct odor . 4-Methylphenol, contributing narcissus-acacia-mimosa aromatic profiles, comprises up to 20.41% in G. elata volatiles (Huang and Li, 2018). The aromatic characteristics of eugenol is more complex, with clove flavor, wood flavor and rich caryophylla musk smell, which belongs to the edible spices classified as an approved food additive in China.
Ketones demonstrate chain-length-dependent aromas: short-chain variants emit fatty-toasty notes, while long-chain counterparts produce floral aromatic tones. Phenethyl alcohol and acetophenone further enrich the sensory profile with buttery-floral-fruity nuances accompanied by honeyed-cheesy accents (Tan et al., 2025). Alkanes (7.97–16.63%) comprise tetradecane/hexadecane (mild waxiness) and isopentane (mildly pleasant olfactory characteristics) (Huang and Li, 2018; Ma et al., 2024). Aromatic compounds like naphthalene contribute spice-like and tar-like characteristics.
Notably, 2,3,5,6-tetramethylpyrazine (musty odor) and dimethyl disulfide (sulfuric rancidity), combined with hexanoic acid’s sweat-like note, are implicated in G. elata’s off-flavors (Tan et al., 2025; Cao et al., 2018). The chemical structure of dimethyl disulfide is depicted in Figure 1. Studies demonstrate that alkanes, aromatic compounds, and sulfur -containing compounds collectively account for 81.42% of the distinctive aroma in black ecotype (Guizhou Bijie population) through integrated electronic nose and GC-MS analyses.
Critical bioactive constituents such as hexadecanoic acid, α-linolenic acid, and trans-squalene may underlie G. elata’s therapeutic efficacy in against neoplastic, neuropsychiatric, and metabolic disorders. The synergistic interactions of these compounds underpin both its organoleptic properties and therapeutic value (Han et al., 2018; Shu et al., 2013).
Significant variations in flavor profiles are observed among different cultivars and provenances of G. elata. The volatile organic components of the green ecotype are predominantly ethyl linoleate and styrene, whereas the red ecotype is characterized by 2-pentylfuran and E,E-2,4-decadienal. These two ecotypes exhibit significant quantitative differences in both the chemical diversity and concentration gradients of volatile compounds (Ma et al., 2024; Wang et al., 2025). Pharmacognostic studies have revealed distinct variations in volatile content and composition among raw medicinal materials harvested seasonally. Similarly, Cinnamomum spp. of varying ages demonstrate phytochemical divergence in the constituent profiles of volatile oil constituents (Cheng et al., 2024; Li et al., 2013), suggesting that disparities in volatile composition may originate from microenvironmental conditions, microclimate variations, physiological states, and morphological differences during plant growth.
2.2. Non-volatile flavor compounds
As a traditional medicinal herb, the chemical composition of G. elata was first investigated in the 1930s (Mar, 1936). Non-volatile chemical constituents constitute the pharmacologically active basis, exerting therapeutic effects including endogenous wind suppression (anticonvulsant activity), liver yang hyperactivation inhibition, meridian obstruction relief, and sedative-hypnotic regulation (ChP, 2020). Additionally, G. elata demonstrate significant neuroprotective efficacy in cognitive enhancement, antiepileptic activity, cardiovascular homeostasis modulation, and symptomatic alleviation of cephalalgia and peripheral neuropathy (He et al., 2023; Sun et al., 2023). Its primary non-volatile flavor-active compounds primarily include phenolic glycosides, heteropolysaccharides, phytosterols, and organic acids.
2.2.1. Phenolic compounds
Gastrodin (4-(hydroxymethyl)phenyl-β-D-glucopyranoside), a phenolic glycoside, serves as both a primary bioactive constituent and a key flavor determinant in G. elata . Its chemical structures is illustrated in Figure 1. As the chemical marker for quality standardization, gastrodin constitutes the principal criterion in G. elata product authentication (Li et al., 2019). Phytochemical investigations further reveal G. elata also contains phenolic compounds such as p-hydroxybenzyl alcohol and Parishins, which concurrently mediate therapeutic efficacy and organoleptic characteristics (Sun et al., 2022).
p-Hydroxybenzyl alcohol, the aglycone of gastrodin, along with gastrodin itself, constitutes the primary pharmacological basis for G. elata’s therapeutic efficacy in synergy with gastrodin. These compounds demonstrate significant bioactivities, including sedation, anticonvulsant action, analgesia, neuroprotective, cardioprotective, antihypertensive effects, and anti-thrombotic/antiplatelet aggregation properties (Lin et al., 2006).
Parishins (synonym: Balishanglycosides) are phenolic ester glucosides isolated from G. elata. Structurally, they structurally characterized by citric acid derivatives esterified with 4-hydroxybenzyl alcohol or its derivatives via ester bonds. Parishins act as biotransformation precursors of gastrodin and are typically present in higher concentrations than gastrodin itself in raw G. elata (Zhang et al., 2022). However, Parishins are thermally unstable and prone to degradation. During processing methods involving heat (e.g., steaming or boiling), the ester bonds in Parishins were hydrolyzed, leading to a marked increase in gastrodin content post-processing. The 2020 edition of the Chinese Pharmacopoeia established a quality control markers for G. elata using gastrodin, p-hydroxybenzyl alcohol, and Parishins A, B, C, and E as marker peaks (ChP, 2020). To date, 33 Parishin analogs have been structurally elucidated in Gastrodia elata, with nine Parishins A, B, C, D, E, J, K, G, and I documented. Their chemical structures are illustrated in Figure 2.
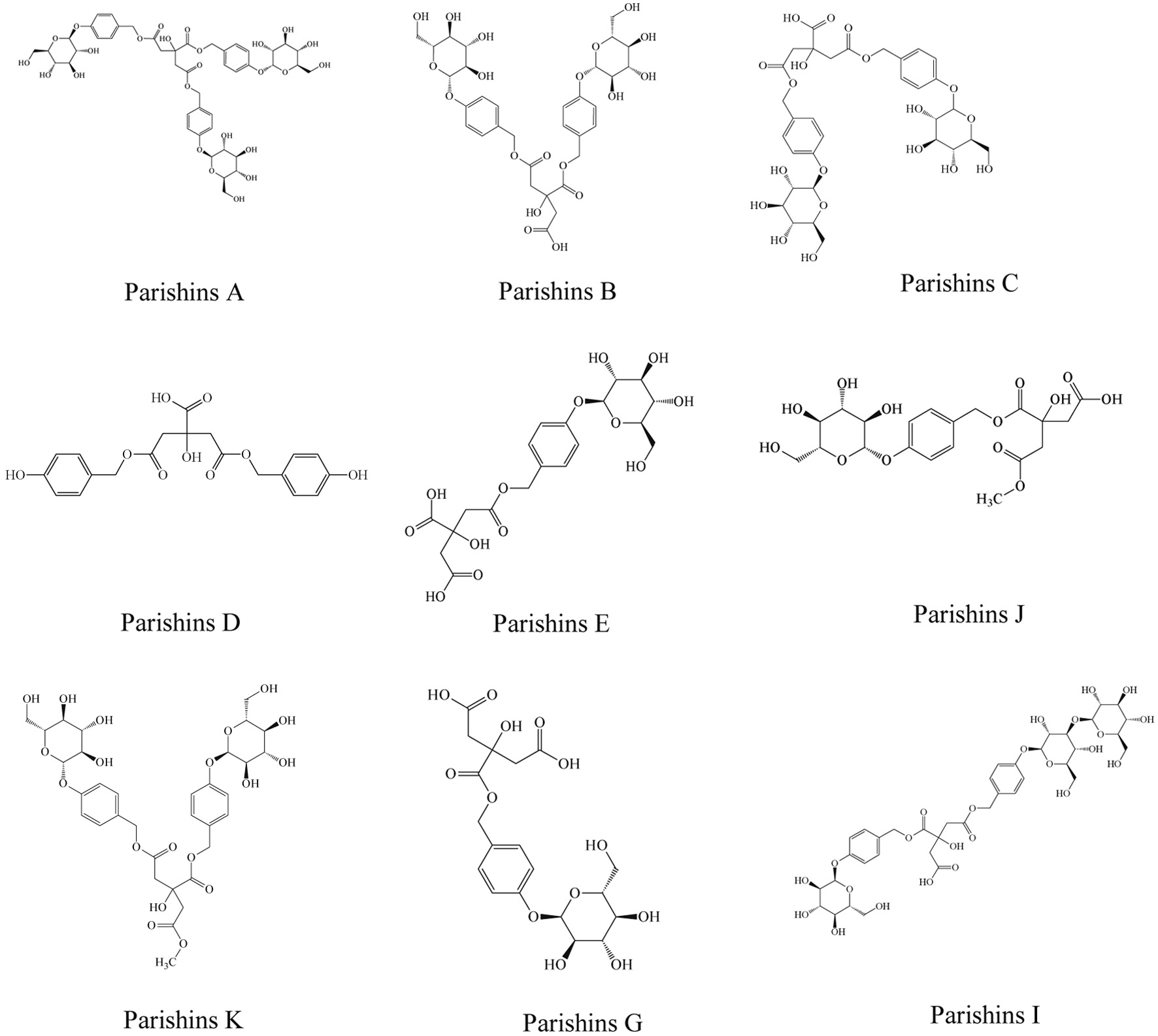 Click for large image | Figure 2. Structures of the nine most studied parishin-type compounds. |
G. elata is chemically characterized by diverse polyphenolic constituents beyond gastrodin, p-hydroxybenzyl alcohol, and parishins, including phenolic derivatives (p-hydroxybenzaldehyde, vanillyl alcohol, vanillin, syringic acid), flavanols (catechin, epicatechin), phenylpropanoids (caffeic acid, cinnamic acid), and specialized metabolites (gastrodophenols A/B, quercetin) (Zhan et al., 2016; Wang et al., 2007; Li et al., 2007).
2.2.2. Carbohydrates
Carbohydrates in G. elata comprise of small-molecule sugars (monosaccharides, disaccharides, and glycosides) and polysaccharides. G. elata polysaccharides (GEPs) are key bioactive components with immune-regulatory and anti-neoplastic effects, while also influencing the herb’s texture and mouthfeel. Structurally, GEPs are predominantly contain glucose monomers, absence of uronic acid residues, proteoglycans, or ketoses, and are classified as neutral homopolysaccharides polysaccharides. Researchers isolated water-extracted (WGEW) and alkali-extracted (AGEW) polysaccharides, while Li et al. characterized two distinct polysaccharides, GBP-I and GBP-II (Qiu et al., 2007; Zhang et al., 2023). Studies have demonstrated that GEPs exhibit therapeutic multifunctionality, including immune regulation, senescence retardation effects, and antihypertensive properties (Zhao et al., 2025). Structurally modified GEPs demonstrate superior targeted efficacy in reactive oxygen species scavenging and malignant cell proliferation inhibition (Guan et al., 2025). These biochemical attributes underscore the potential of G. elata in pharmaceutical and nutraceutical industries. Glycosides, with their structural stability and higher bioavailability compared to polysaccharides, also hold significant promise for development (Liu et al., 2016).
2.2.3. Organic acids
G. elata is chemically characterized by small-molecule organic acids such as oxalic acid, malic acid, citric acid, and succinic acid, which mediate pH regulation and contribute to its organoleptic characteristics. These acids serve pivotal roles in defining the herb’s organoleptic properties (Su et al., 2023). Pharmacological investigations demonstrate that non-volatile organic acids in traditional Chinese medicine exhibit antioxidant, hepatoprotective, immunomodulatory, antimicrobial, and antiviral bioactivities (Abdel-SalamOmar et al., 2014). Notably, citric acid is essential to the biosynthesis of Parishins, indicating metabolic coordination with phenolic glycosides in G. elata. Consequently, the diversity and concentration of organic acids are critical evaluation metrics for evaluating the herb’s quality, medicinal efficacy, and phytochemical applications.
2.2.4. Free amino acids
Free amino acids, the fundamental proteinogenic precursors and essential components of human physiology, serve pivotal roles in regulating nitrogen balance, immune potentiation, and promoting metabolic vitality (Neinast et al., 2019; Solon-Biet et al., 2019). As a dual-purpose medicinal and edible resource, the free amino acids in G. elata reflect its nutritional value. The herb contains a comprehensive profile of amino acids with high nutritionally complete, categorized as follows: sweet-tasting amino acids: threonine (Thr), serine (Ser), glycine (Gly), and alanine (Ala) (4 types); bitter-tasting amino acids: valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), histidine (His), and arginine (Arg) (8 types); umami-tasting amino acids: aspartic acid (Asp) and glutamic acid (Glu) (2 types); and non-flavor-active amino acids: cysteine (Cys), lysine (Lys),and proline (Pro) (3 types).
Processing methods significantly influence amino acid content. Immersion in water leads to partial leaching, while heat treatment induces decomposition (Kong et al., 2017). Techniques such as steaming, boiling, and lactic acid fermentation reduce bitter amino acids by 72.56–87.51%, effectively mitigating bitterness and improving palatability (Yang et al., 2024). Furthermore, post-harvest processing methods (e.g., drying protocols) and morphological variations of G. elata markedly alter amino acid composition and concentration. Consequently, processing strategies are pivotal for flavor modulation and sensory optimization in both culinary and medicinal applications.
| 3. Extraction techniques for flavor compounds in G. elata | ▴Top |
The isolation and identification of chemical constituents in G. elata require specialized extraction and analytical methods. Thus, extracting flavor compounds is the methodological foundation in studying its flavor profile. Common extraction techniques are summarized in Table 2.
 Click to view | Table 2. Flavor Extraction Techniques for G. elata |
Steam distillation is a classical method for extracting volatile components, it has simple operational requirements with minimal equipment requirements. However, risks thermal degradation thermolabile compounds through isomerization or decomposition, compromising flavor integrity. Solvent extraction uses organic solvents for compound isolation. While straightforward, it risks solvent residue contamination. Supercritical CO2 extraction is noted for preserving volatile compound integrity, this method was employed by Han et al. to extract G. elata volatiles. Thirty compounds were identified via GC-MS (Han et al., 2018). Headspace-Gas Chromatography-Mass Spectrometry (HS-GC-MS) is a rapid, precise method requiring minimal sample volume, and this method was directly used to analyze volatile components in powdered G. elata. Solid-Phase Microextraction (SPME) enriches volatiles using fiber-coated adsorbents, followed by GC-MS analysis. This method is demonstrating particular efficacy for rapid volatile profiling in dried samples. Microwave-Assisted Extraction (MAE) accelerates compound dissolution via microwave irradiation, offering high-efficiency and reduced solvent use. Zuo et al. optimized MAE for extracting gastrodin and p-hydroxybenzyl alcohol, validated by HPLC. Results showed HPLC-optimized protocol with minimal processing time (Zuo et al., 2018).Simultaneous Distillation-Extraction (SDE) concurrently heats aqueous and organic phases, enabling volatile concentration with minimal solvent. Coupled with GC-MS (SDE-GC-MS), it supports qualitative and quantitative analysis but carries solvent residue risks (Huang and Li, 2018).
The extraction and identification of G. elata flavor compounds involve diverse techniques, each with unique advantages and limitations. Selecting the optimal method based on research objectives ensures efficient and accurate outcomes.
| 4. Processing techniques of G. elata | ▴Top |
The processing of G. elata is a critical determinant of its flavor and bioactive components. Commonly used methods such as steaming, boiling, and fermentation alter its physicochemical properties and significantly influence the composition and concentration of flavor compounds.
4.1. Steaming process
Steaming, a traditional method, involves treating G. elata with high-temperature steam to induce physical and chemical changes. This process achieves enzyme inactivation and glycoside preservation, effectively halting enzymatic degradation of key compounds. Upon post-steaming, gastrodin content increases, while p-hydroxybenzyl alcohol levels decrease (Yang et al., 2019). Fresh G. elata is rich in adenosine, but steaming reduces its content by approximately 50%. Subsequent drying methods (e.g., hot-air or freeze-drying) partially restore adenosine levels, with hot-air drying increasing adenosine by 2.6-2.7-fold increases compared to steamed samples (Wu et al., 2022). Additionally, steaming softens the herb’s texture, facilitating further processing and consumption.
4.2. Boiling process
Boiling involves immersing G. elata in boiling water to solubilize internal components. This method extracts water-soluble compounds such as polysaccharides and amino acids into the aqueous phase. Compared to steaming, boiling significantly reduces polysaccharide content but increases water-soluble protein levels (Yang et al., 2024). It also lowers organic acids and bitter-taste amino acids, thereby improving sensory acceptability.
4.3. Fermentation process
Fermentation, an emerging technique, leverages microbial activity to transform G. elata’s chemical profile. After fermentation, polysaccharide and water-soluble protein contents rise, while organic acid and free amino acid compositions shift dynamically (Yang et al., 2024). Notably, fermentation mitigates undesirable odors (e.g., “horse-urine-like off-flavor”)and enhances sensory acceptance (Tan et al., 2025).
| 5. Concluding remarks | ▴Top |
Although significant progress has been made in the study of flavor compounds in G. elata, several challenges remain unresolved. Future research should focus on leveraging advanced analytical technologies to conduct an in-depth exploration of flavor profiles, particularly the identification of trace flavor components and comprehensively elucidating their composition.
The development of high-efficiency, eco-friendly, and cost-effective extraction techniques is essential to improve the yield and purity of flavor compounds while minimizing environmental impact. Synergistic applications of multiple extraction methods should also be explored to harness their complementary advantages.
Further investigation into the mechanistic roles of G. elata flavor compounds in food and pharmaceutical systems is critical to establish a robust molecular basis for their rational utilization. Building on these insights, translational product innovation in functional foods, nutraceuticals, and pharmaceuticals should be prioritized to meet consumer demands, expand market applications, and promote the sustainable growth of the Gastrodia elata industry.
In summary, G. elata possesses a rich diversity of flavor compounds, encompassing both volatile and non-volatile constituents, which collectively define its unique sensory attributes. While various extraction technologies and processing strategies have been established, ongoing research holds promise for unlocking the full potential of these compounds, paving the way for new opportunities in the industrial transformation of G. elata.
Hubei Provincial Department of Education “100 Schools and 100 Counties - Colleges and Universities Serving Rural Revitalization Science and Technology Support Action Plan” (Grant No. BXLBX0842) Development and Utilization of Special Functional Foods in Dabie Mountains, 2021-2025.
Conflict of interest
All authors declare that there is no conflict of interest.
| References | ▴Top |