| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 29, March 2025, pages 20-28
Structure characteristics related to biological properties and application of chitooligosaccharides: Mini-Review
Liping Fana, b, Nichakorn Khondeec, Monthana Weerawatanakorna, *
aDepartment of Agro-Industry, Faculty of Agriculture, Natural Resources and Environment Naresuan University, 99 Moo 9, Tha Pho, Phitsanulok 65000, Thailand
bSchool of Life Science, Jiaying University, No. 100, Meisong Road, Meizhou, Guangdong province 514015, China
cDepartment of Natural Resources and Environment, Faculty of Agriculture, Natural Resources and Environment, Naresuan University, 99 Moo 9, Tha Pho, Phitsanulok, Thailand
*Corresponding author: Monthana Weerawatanakorn, Department of Agro-Industry, Faculty of Agriculture, Natural Resources and Environment Naresuan University, 99 Moo 9, Tha Pho, Phitsanulok 65000, Thailand. Tel: +66 0629514194; E-mail: monthanac@nu.ac.th
DOI: 10.26599/JFB.2025.95029402
Received: March 14, 2025
Revised received & accepted: March 29, 2025
| Abstract | ▴Top |
Chitooligosaccharides (COS) are degradation products after cleavage of β-1,4-glycosidic bonds from chitosan, which are converted and extracted from chitin in the shells of shrimp, crabs and other crustacean creatures. Degradation methods to obtain chitooligosaccharides include acids hydrolysis, oxidative cleavage, ultrasound oscillation, enzymatic catalysis, and the combination of two or more methodologies. Due to the low degree of polymerization of glucosamine units, chitooligosaccharides possess superior characteristics to chitosan and chitin, such as a lower molecular weight and higher aqueous solubility, translating more applicability in food and related products in particular. The primary amino groups at C-2 position of chitooligosaccharides are positively charged in weak acidic conditions and also readily derivatized with acylation, alkylation, carboxylation, esterification, Maillard reaction, protonation forming salts, and sulfation. Exploration of chitooligosaccharides biological activities revealed that they possess antibacterial, antioxidant, anti-inflammatory, and immunomodulatory effects. The major applications of chitooligosaccharides are in food packaging, functional films, hydrogels, emulsions and drug-loaded complex. This review aims to summarize chitooligosaccharide properties and their application in food related products.
Keywords: Chitooligosaccharides; Biological activities; Functional properties; Chitosan; Food packaging material
| 1. Introduction | ▴Top |
Chitosan extracted from chitin in the shells of shrimp, crabs and other crustacean creatures, can be degraded chemically or enzymatically by cleaving glycosidic bonds to generate small polymers called chitooligosaccharides (COS). COS exhibits both excellent antimicrobial and antioxidant activities, and it also has superior characteristics to chitin, such as a lower molecular weight and higher aqueous solubility, translating more applicability in food and related products in particular (Jeon et al., 2001; Jeon and Kim, 2000a; Shahidi and Ambigaipalan, 2015). Materials prepared from COS, including hydrogels, emulsions, and drug-loaded systems, have garnered considerable attention due to their biocompatibility, biodegradability, and inherent antibacterial properties (Ma et al., 2024; Tabassum et al., 2021). Due to the distinctive structure and special characteristics, COS application is of great significance especially in food science, particularly in food packaging as an essential ingredient. The functional properties of COS, including antimicrobial activity and the ability to extend shelf life, are especially critical in this domain (Fang et al., 2024; Liu et al., 2022). Given the rising demand for sustainable packaging solutions, there is an increasing interest in utilizing natural, bio-based materials of COS to develop environmentally friendly alternatives to conventional synthetic films (Fernández-de Castro et al., 2016). This review is a brief summary of chitooligosaccharides properties and their application in food related products.
| 2. Preparative methods of chitooligosaccharides application | ▴Top |
The preparation methods of COS are closely associated with their physical, chemical and other properties. β-1,4-glycosidic bonds are the linkers of glucosamine units in chitosan (Xing et al., 2017). There are several methods to break these glycosidic bonds, majorly including chemical and enzymatic methods.
2.1. Chemical hydrolysis
Chemical degradation offers a cost-effective approach for large production of chitooligosaccharides (COS) (Lodhi et al., 2014). Acid hydrolysis and oxidative cleavage are the two most popular reactions of chemical methods. In acid hydrolysis, hydrochloric, nitric, phosphoric, hydrofluoric and other inorganic acids are widely used with or without addition of electrolytes. Organic acids including formic, acetic, trichloroacetic, or dehydroascorbic acid were also used (Mourya et al., 2011; Nguyen et al., 2023; Suhaimi et al., 2025; Vårum et al., 2001). In acid hydrolysis, protonation of oxygen at the β-1,4-linkage followed by cleavage is commonly recognized acid-catalyzed mechanism.
Oxidative hydrolysis is also routinely used to cleavage the β-1,4-glycosidic bonds to get COS. Hydrogen peroxide is normally employed as an oxidizing agent. Hydroxy radicals formed from hydrogen peroxide attack and further break the β-1,4-glycosidic bonds, yielding depolymerized COS (Mittal et al., 2023; Nguyen et al., 2023). Catalyzes including ascorbic acid, ozone, and transition metals are also added to assist the hydrogen peroxide-mediated oxidative hydrolysis of chitin or chitosan to accelerate the oxidation reaction rate and yield of COS (Tan et al., 2022; Xia et al., 2013; Yang et al., 2025; Yue et al., 2009).
2.2. Enzymatic hydrolysis
Both specific and non-specific enzymes are used in the enzymatic cleavage of the chitosan β-1,4-glycosidic bonds (Hellmann et al., 2025; Shinya and Fukamizo, 2017). The specific enzyme of chitosanase (EC3.2.1.132) is applied in the fermentation process to produce COS, while non-specific enzymes such as lipase, protease, carbohydrase and other proteolytic enzymes are practically used with low yield of COS compared to chitosanase (Poshina et al., 2020; Shinya and Fukamizo, 2017; Vishu Kumar and Tharanathan 2004). Using chitosanase, Jeon and Kim (2000b) employed a dual-reactor system consisting of an ultrafiltration membrane reactor and an immobilized enzyme column reactor to achieve continuous production of chitooligosaccharides from chitosan (Jeon and Kim, 2000b). Different enzymatic catalyzed cleavage of chitosan yields COS with different molecular weights and physical/chemical properties because of different reaction conditions contributed to different pH and temperature, required by selected enzymes satisfying the maximum enzyme activities (Koumentakou et al., 2025).
2.3. Other methods
In addition to chemical and enzymatic cleavage of the chitosan β-1,4-glycosidic bonds in the depolymerization process of chitosan, physical methods are also employed to break the glycosidic bonds producing COS. Microwave induce molecules’ oscillation, thermal cleavage , ultrasound treatment with lambda radiation are examples of other physical methods for breaking down chitosan β-1,4-glycosidic bonds to produce COS (Li et al., 2023; Wasikiewicz and Yeates, 2013; Xing et al., 2017).
However, due to limitations like reaction yield, efficiency, or other necessary properties of COS, a combination of various methods is commonly used for the large-scale production of COS. Table 1 lists common preparation methods of chitooligosaccharides and their advantages and limitations.
 Click to view | Table 1. Preparative methods of COS |
| 3. Physical and chemical properties of chitooligosaccharides | ▴Top |
COS are linear oligosaccharides with a low molecular weight (<3.9 kDa), typically comprising a degree of polymerization ranging from 2 to 20 (Figure 1). These oligosaccharides consist of either glucosamine or acetyl glucosamine units linked by β-1,4-glycosidic bonds (Leal et al., 2025; Liang et al., 2018). COS are positively charged cationic oligosaccharides, non-toxic and easily degradable, with good water solubility, biocompatibility and low viscosity (Yuan et al., 2019).
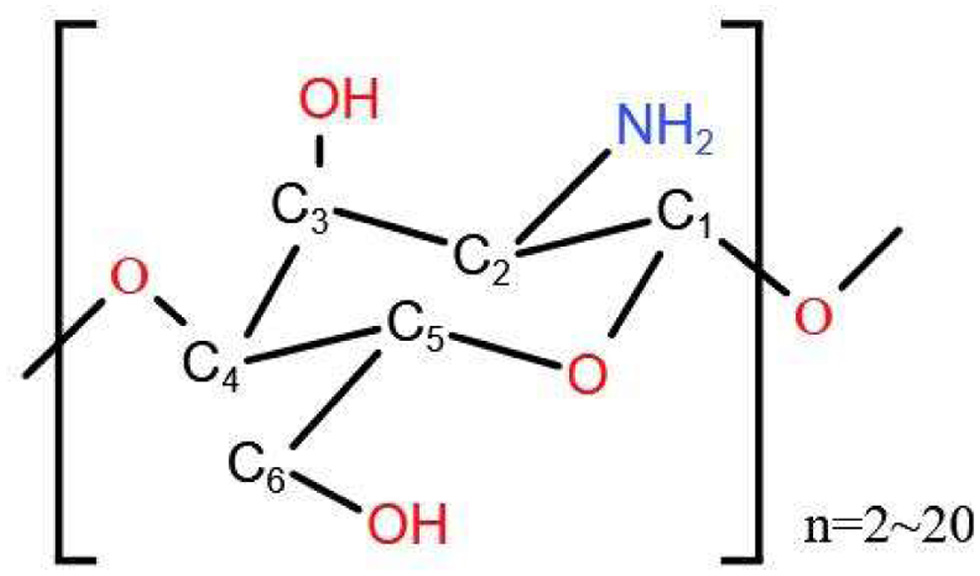 Click for large image | Figure 1. Structure of chitooligosaccharides. |
The primary amino groups at C-2 position together with C-3 and C-6 hydroxyl groups of COS are capable of being derivatized with other functional groups such as carboxylates, esters, sulfates, and functional alkyl groups, leading to COS derivatives by corresponding reactions like carboxylation, acylation, phosphorylation, sulfation and alkylation reaction (Liu et al., 2019; Ngo et al., 2019). Thus, certain COS derivates were synthesized for different functionalities of COS through targeted design to achieve desired biological activities.
With the widespread recognition of their functional properties, COS have increasingly been explored for the development of new biomaterials. In particular, research has focused on the use of COS complexes in drug delivery systems, molecular modification, and the function enhancement of composite materials through the introduction of active COS groups (Li et al., 2018; Tabassum et al., 2021; Wang et al., 2021). These applications highlight the growing value of COS in advancing material science and biotechnology. COS contains chemically reactive groups including -NH2 at C-2 position and -OH at C-3 and C-6 positions (Figure 2), enabling them to form complexes with various substances. These substances normally include metal ions, proteins, polysaccharides, lipids, and flavonoids, and they are able to form complexes with COS through coordination complexation, electrostatic interactions, transamination, Maillard reactions, amidation, esterification, carboxylation, sulation, cylation, and alkylation (Huang et al., 2005; Lang et al., 2023; Liu et al., 2024; Liu et al., 2022; Yu et al., 2016). The relevant mechanisms are illustrated in Figure 3. The complexes formed via these methods mentioned above modify the activities of functional ingredients or serve as innovative biomaterials for stabilizing, cross-linking, and loading sensitive functional compounds. As a result, COS are found to have broad applications across medicine, agriculture, and food industries.
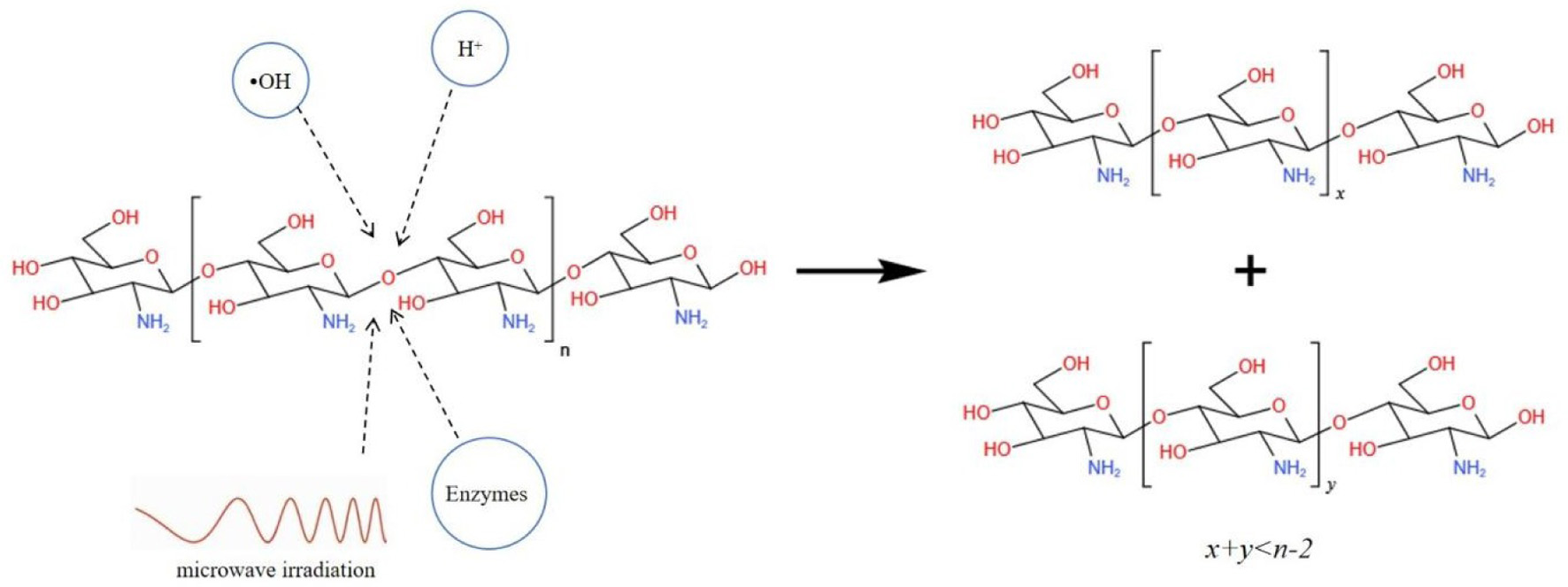 Click for large image | Figure 2. Preparative methods of chitooligosaccharides. |
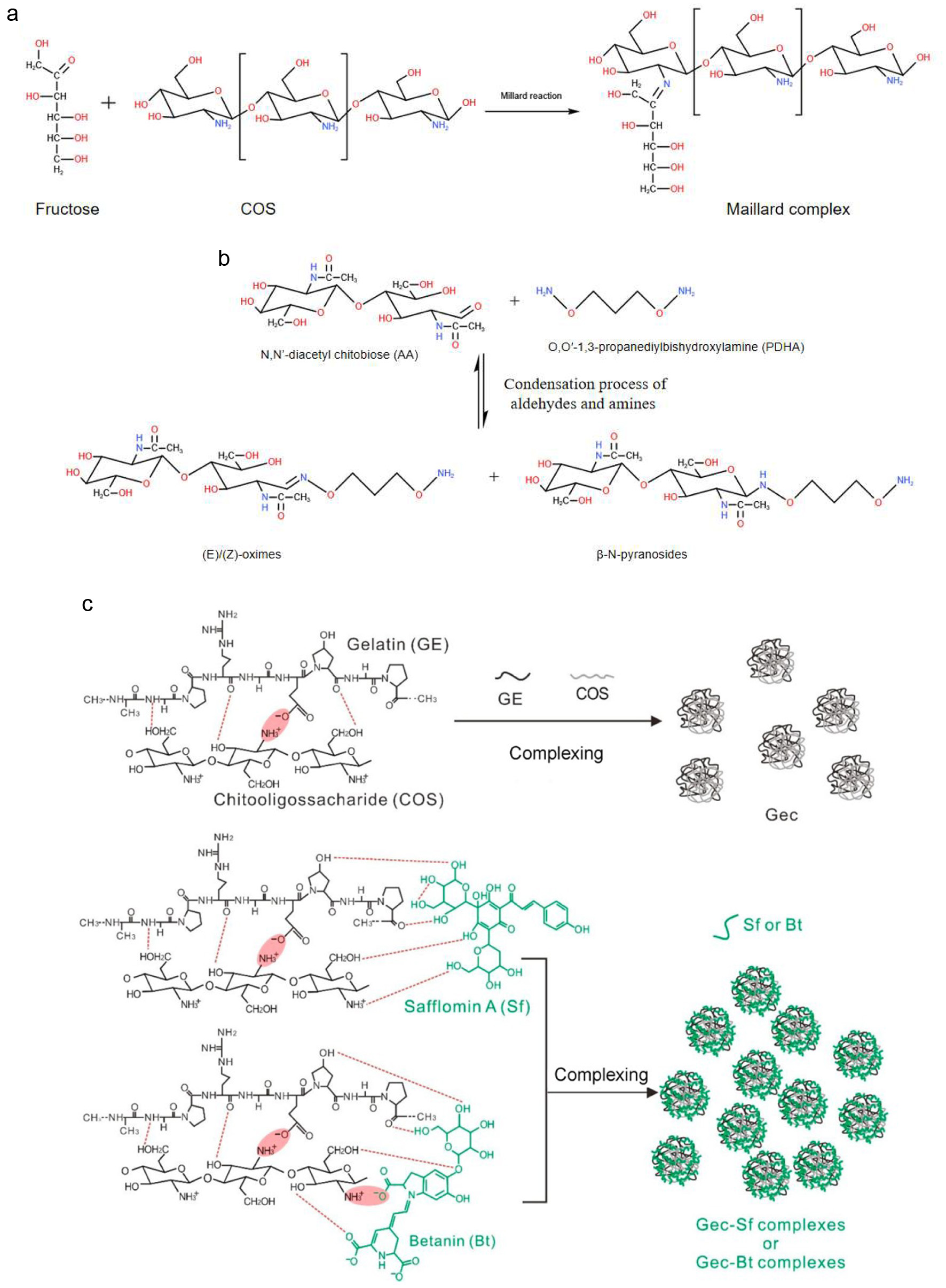 Click for large image | Figure 3. Chitooligosaccharide binding mechanism. (a) Maillard reaction; (b) Condensation process of aldehydes and amines; (c) Electrostatic self-assembly process (Vikøren Mo et al., 2020; Wang et al., 2023; Zhang et al., 2016). |
| 4. Bioactivities of chitooligosaccharides | ▴Top |
COS exhibit effective biological activities including antibacterial, antioxidant, anti-inflammatory, and immunomodulatory effects (Fang et al., 2024; Muanprasat and Chatsudthipong, 2017). It has been demonstrated that COS exhibit inhibitory effects against a variety of fungi and bacteria. Fernandes et al. (2008) revealed that COS possess strong antibacterial activity in fruit juice matrices, but their effectiveness is diminished in milk matrices (Fernandes et al., 2008). This suggests that food matrix and chemical component such as protein and pH levels play a significant role in modulating the antibacterial properties of COS. Moreover, some studies using Trichophyton rubrum as research subjects have indicated that COS are effective in inhibiting fungal growth (Mei et al., 2015). In addition to their antibacterial and antifungal effects, COS also possess antiviral activity and anticancer activity. Artan et al. (2010) found that COS with a molecular weight of 3–5 kDa exhibit anti-HIV activity when sulfated, highlighting their potential property as antiviral agents (Artan et al., 2010). Kim et al. (2012) found that COS with higher degree of deacetylation and lower molecular weights showed higher anticancer activity (Kim et al., 2012).
Recently, many researches indicated that primary amino groups of COS are responsible for COS antibacterial properties. In acidic or neutral solutions, protonated COS bind to the surface of microbial cell membranes. This interaction either prevents transmembrane transport, disrupting microbial activity, or alters membrane permeability, leading to microbial cell death (Sahariah and Masson, 2017). Furthermore, the free amino groups of chitosan and COS have a strong ability to chelate metal ions on bacterial cell surfaces, forming complexes followed by inhibiting bacterial growth through disrupting the supply of essential minerals (Rabea et al., 2003). Additionally, due to their low molecular weight and low degree of polymerization (DP), COS can penetrate bacterial cells, and interfere with replication and gene expression, ultimately disrupting intracellular bacterial metabolism (Kim et al., 2003).
Kamil et al. (2002) investigated the antioxidant effects of chitosan with different viscosities (14 cP, 57 cP, and 360 cP) added to minced cooked herring meat. The results showed that low-viscosity (14 cP) chitosan exhibited the strongest antioxidant activity, suggesting that low-viscosity (low molecular weight) chitosan could be applied as a functional antioxidant ingredient in the food industry (Kamil et al., 2002). Qu and Han (2016) revealed that COS exhibit potent antioxidant activity and protect mice from oxidative stress by COS scavenging free radicals both in vitro and in vivo using a high-fat diet mouse model (Qu and Han, 2016). The activities of catalase, superoxide dismutase, and glutathione peroxidase were significantly increased in the stomach, liver, and serum of mice on a high-fat diet after the administration of COS, suggesting that COS have antioxidant property that restores the reduced enzyme activity affected by a high-fat diet. Similar results were observed in the study conducted by Tonphu et al. (2025). In addition, Yang et al. (2017) applied COS to beer and found that COS with a molecular weight of 2 or 3 kDa (concentrations of 0.001–0.01%) inhibited the formation of aging compounds in aged beer, improving free radical scavenging activity which played an important role in preventing flavor deterioration (Yang et al., 2017).
The action mechanism of COS has been widely studied by various researchers (Chen et al., 2022; Coreta-Gomes et al., 2023; Fang et al., 2024; Leal et al., 2025), suggesting that the amino or hydroxyl groups of COS have ability to react with unstable free radicals to form stable free radicals, thereby producing antioxidant capacity (Huang et al., 2012; Tomida et al., 2009). Moreover, the –NH2 groups of COS bind to protons in solution to form ammonium ions (NH3+), which then react with free radicals through addition reactions (Park et al., 2004; Zhao et al., 2013). These mechanisms indicate that the antioxidant activity of COS are primarily determined by the number of reactive hydroxyl and amino groups. The antioxidant activity of COS is influenced by many factors including the degree of deacetylation and molecular weight. Generally, antioxidant activity increases with a higher degree of deacetylation due to more exposure of amino groups. Furthermore, antioxidant activity tends to increase as the molecular weight decreases. Specifically, COS with a lower molecular weight exhibit stronger antioxidant activity. The reason is that COS with low-degree polymerization of COS has better hydroxyl radical scavenging ability than chitosan and chitin (Li et al., 2012; Mengíbar et al., 2013).
| 5. Application of chitooligosaccharide complex | ▴Top |
COS are known for their remarkable biological activities (Mittal et al., 2023). A systematically evaluation for the safety of chitooligomers, and a 30-day rat feeding study showed that rats found no abnormal symptoms and clinical signs or death during the test (Qin et al., 2006), and similar reports were also found in the same result (Vela et al., 2015). Consequently, COS are considered as a safe raw material for use in the food industry.
Building on this safety profile, Zhao et al. (2020) and Zhu et al. (2020) developed COS-desalted duck egg white and COS-casein phosphopeptide complexes based on egg white peptide-COS nano-delivery systems and casein phosphopeptide-COS carrier systems. These complexes significantly improved the binding capacity and stability of soluble calcium and facilitated enhanced calcium absorption in the human body, demonstrating that COS complexes play an important role in the field of nutritional fortification (Zhao et al., 2020; Zhu et al., 2020). Seong et al. (2018) utilized layer-by-layer self-assembly technology, which leverages the electrostatic interactions of COS, to develop a COS-lipid complex carrier. This carrier was capable of encapsulating hydrophobic flavonoids, demonstrating stability and a favorable skin compatibility (Seong et al., 2018). COS-lipid complex also effectively enhanced the release and skin penetration of quercetin. Similarly, Jing et al. (2023) designed a colon-targeted quercetin delivery system known as COS-CaP-QT, where pectin (PEC) and Ca2+ microspheres were cross-linked with COS. The release profile of COS-CaP-QT was pH-dependent and responsive to the colonic microenvironment, allowing preferential distribution of the system in the colon (Jing et al., 2023). Further study revealed that yeast protein-COS and ovomucin-COS effectively stabilized betain and lutein, respectively. These compounds enhanced the stability, biological act of betain and lutein within the system. These results suggest that chitooligosaccharide-protein complex system can protect betain and lutein from degradation and facilitate controlled release in simulated gastrointestinal conditions (Xu et al., 2024; Yang et al., 2024). Yin et al. (2020) explored the electrostatic interaction between COS and bacterial cellulose to fabricate COS-bacterial cellulose films. These films exhibited strong mechanical properties and improved antibacterial and antioxidant capabilities (Yin et al., 2020). Both Yan and Yu groups successfully prepared COS-glycine and COS-lysine complexes through the Maillard reaction between COS and amino acids. These complexes exhibited excellent antioxidant property during the storage of fruit juices and fresh-cut fruits (Yan et al.,2018; Yu et al., 2019). Based on the Maillard reaction characteristics of COS with reducing sugars, related studies have confirmed that these complexes effectively inhibit the formation of acrylamide in foods containing amino acids (particularly aspartic acid) during heating, thereby enhancing the safety of baked goods (Chang, Sung, Chen, 2016; Shyu et al., 2019).
In summary, the charged nature of COS and their ability to form complexes with other molecular structures enable various applications of COS. COS interact with protein, polysaccharide, and polyanionic compounds leading to adsorb and stabilize functional components. Consequently, they were employed in creating food-grade preservation materials with specific structures and functions. Table 1 illustrates the types and applications of COS-related complexes. The preparation of the complexes and their numerous applications in food science and technology, especially on hydrogels, emulsions, drug-loaded and food preservation systems are summarized in Table 2.
 Click to view | Table 2. Application of COS complex |
| 6. Conclusion | ▴Top |
Prepared by the cleavage of β-1,4-glycosidic bonds of chitosan, COS, positively charged cations with primary amino groups, are the distinctive characteristics of in terms of natural resources, low degree of polymerization, and high water solubility. COS exhibit various bioactivities of antioxidant, antimicrobial, and antiinflammation activities. Due to the charged nature of COS and active functional groups, their ability to form complexes with other molecular structures enable their use in various applications. Therefore, COS have vast application potential in food related products, including bio-functional films to extend food shelf life, emulsions, complex with proteins to increase bioavailability, to enhance stability and homogeneity, and to protect product from discolor, disflavoring and other microbial and oxidative damage.
Acknowledgments
This work was supported by Naresuan University (NU), and National Science and Innovation Fund (NSRF) with grant NO R2568B046 and the Teaching quality and teaching reform project of Jiaying university with Grant No. ZLGC2024301. The funding was allocated to the establishment of the Modern Industrial College of Characteristic Agricultural Product Processing.
Conflict of interest
The authors declare no conflict of interests.
| References | ▴Top |