| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 21, March 2023, pages 35-43
Gypenosides improved inflammatory status, insulin resistance and hepatic steatosis in obese C57BL/6J mice
Jie Liua, b, Yaqiong Zhangb, Holly Childsc, Ziyuan Wanga, Liangli (Lucy) Yuc, Boyan Gaob, Margaret Slavinc, *
aChina-Canada Joint Lab of Food Nutrition and Health (Beijing), Beijing Technology & Business University, Beijing 100048, China
bInstitute of Food and Nutraceutical Science, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China
cDepartment of Nutrition and Food Science, University of Maryland, College Park, MD, USA 20742
*Corresponding author: Margaret Slavin, Department of Nutrition and Food Science, University of Maryland, College Park, MD 20742, USA. Tel: +1 (301)405-4533; E-mail: mms@umd.edu
DOI: 10.31665/JFB.2023.18337
Received: February 15, 2023
Revised received & accepted: March 24, 2023
| Abstract | ▴Top |
This study investigated the effect of dietary gypenosides on inflammation, insulin resistance and hepatic steatosis in male obese C57BL/6J mice induced by feeding a high-fat diet (HFD) for 16 weeks. Treatment with 300 mg/kg BW/d gypenosides for eight weeks significantly reduced body weight gain, total plasma cholesterol and homeostasis model assessment-estimated insulin resistance (HOMA-IR) index in the obese mice compared with the control. Gypenosides also reduced the levels of tumor necrosis factor-α, monocyte chemoattractant protein-1, and interleukin-6 in both plasma and inguinal white adipocyte tissue. Moreover, gypenosides consumption alleviated hepatic steatosis and insulin resistance possibly by promoting energy expenditure through the AMPK signaling pathway and upregulating thermogenic genes in the brown and inguinal white adipocyte tissues. In addition, these metabolic changes were accompanied by an increased Akkermansia muciniphila abundance in the gut microbiota. The results suggest the health benefits of gypenosides intake in obese mice.
Keywords: Gypenosides; Obesity; Insulin resistance; Hepatic steatosis; Gut microbiota
| 1. Introduction | ▴Top |
Obesity is a serious health problem and has negative impacts on human quality of life and economic systems (Swinburn et al., 2015). Obesity may induce inflammation, which contributes to the development of type 2 diabetes, fatty liver disease and cardiovascular diseases (Kang et al., 2010). Thus, reducing inflammation (especially chronic inflammation) serves as an important strategy for the prevention of obesity-associated chronic metabolic diseases (Xie et al., 2018). Although a number of pharmacological treatments for acute inflammation are successful, few drugs are clinically available for chronic inflammation due to long-term efficacy and safety concerns (Nagata et al., 2017; Xie et al., 2018). Dietary interventions capable of reducing inflammation have attracted attention in recent years because of their lower cost and promising benefits (Chang et al., 2017; Saito et al., 2015; Zhang et al., 2016). Development of successful dietary interventions is further contingent upon identification of the bioactive dietary components which are effective in reducing the risk of obesity-induced inflammation and associated metabolic disorders, as well as characterization of the potential molecular mechanisms involved in their biological functions under different physiological conditions.
Gypenosides from Gynostemma pentaphyllum Makino (GP) have been shown to reduce the risk of obesity (Gauhar et al., 2012; Liu et al., 2017), diabetes (Hung et al., 2009) and cancer (Yan et al., 2014). Our previous study has illustrated the structure and the major composition of gypenosides isolated from tetraploid jiaogulan in China (Liu et al., 2016). We also found that oral gavage with 300 mg/kg BW/d gypenosides could prevent weight gain and associated insulin resistance through modulating adipose thermogenesis and the gut microbiota in C57BL/6J mice during a 12 weeks high-fat diet (HFD) (Liu et al., 2017). This prior study did not examine the potential beneficial effects of gypenosides in obese mice nor their potential application in treatment of existing obesity, as some previous studies did due to funding availability (Tong et al., 2019). Thus, whether gypenosides may suppress obesity-induced inflammation, which plays an important role in the development of obesity associated metabolic disorders, remains unknown.
This study was conducted to investigate the effect of oral gypenosides on body weight, inflammation, insulin resistance and hepatic steatosis in obese C57BL/6J mice induced by HFD. The potential effect of oral gypenosides on adenosine monophosphate (AMP)-activated protein kinase (AMPK) molecules in liver, and the thermogenic genes in the adipocyte tissues were also examined, along with their effects on gut microbiota. The results from this study may provide a scientific foundation for potential utilization of dietary gypenosides in reducing the risk of obesity-associated inflammation and other chronic disorders.
| 2. Materials and methods | ▴Top |
2.1. Reagents and materials
Gypenosides were prepared from tetraploid G. pentaphyllum (Thunb.) Makino leaves, collected in the Shanxi province of China. The extraction of gypenosides was carried out according to a previous reported method with identified chemical composition and structures, and also high purity (>95%) (Liu et al., 2016). Antibodies against Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α), PR domain containing 16 (PRDM16), uncoupling protein 1 (UCP-1), AMPK, phosphorylated AMPK (p-AMPK), acetyl-CoA carboxylase (ACC), phosphorylated ACC (p-ACC), AKT, p-AKT, Glucose transporter-4 (GLUT4), α-tubulin and β-actin were purchased from Abcam (Cambridge, Mass, UK). All the other chemicals were of analytical grade and used without further treatment.
2.2. Obese mice model establishment and treatment
Male C57BL/6J mice (5 weeks old, Charles River Laboratories, Beijing, China) were fed a high-fat diet (45% calories from fat, D12451, Research Diets Inc., NJ, USA) for a total of 24 weeks. After 16 weeks feeding, the obese mice were randomly divided into two groups: a control group orally administrated with 0.5% CMC-Na aqueous solution (HFD, n=8) and a group orally administered with 300 mg/kg BW/d gypenosides dissolved in CMC-NA aqueous solution (HFD+Gypenosides, n = 8) for another 8 weeks. Mice body weight and food intake were monitored every week. All protocols and procedures were approved by Shanghai Jiao Tong University with the guidelines of the ethical committee of experimental animal care (Approval no. A2015024).
2.3. Plasma and tissue collections
All mice were fasted for 12 h and anesthetized with carbon dioxide. Blood was collected from the inferior vena cava, and plasma samples were obtained by centrifugation at 3,500 rpm, 4 °C for 10 min. Liver and adipocyte tissues were harvested, rinsed with physiological saline, weighed, and immediately frozen in liquid nitrogen or RNAlater (Invitrogen) for further analyses. Feces were collected for three consecutive days at week 24 and kept at −80 °C.
2.4. Oral glucose tolerance test (OGTT) and intraperitoneal insulin tolerance test (IPITT)
Blood glucose was measured through tail vein bleeding using a One-touch UltraEasy™ portable commercial glucose meter (Johnson Medical, Beijing, China) after 8 weeks of 300 mg/kg BW/d gypenosides intervention, following a laboratory protocol (Liu et al., 2017). For oral glucose tolerance tests (OGTT), mice fasted for 6 hr were gavaged with glucose (1.5 g/kg body weight), and the blood glucose level was determined at 0, 15, 30, 45, 60, 75 and 90 min post glucose administration. For intraperitoneal insulin tolerance tests (IPITT), mice fasted for 4 hr were intraperitoneally injected with insulin (0.75 unit/kg body weight), and the blood glucose level was determined at 0, 30, 60, 90 and 120 min post insulin injection.
2.5. Quantification of plasma pro-inflammatory cytokines, plasma insulin and homeostatic model assessment of insulin resistance (HOMA-IR)
Pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1) and anti-inflammatory cytokines interleukin-10 (IL-10) were measured using commercial mouse ELISA kits (eBioscience, CA, USA) following the manufacturer’s protocol. ELISA assay was used to measure the plasma insulin concentration (EZRMI-13K, Millipore, Germany). The HOMA-IR index was calculated following the equation: [fasting insulin concentration (mU/L) × fasting glucose concentration (mg/dL)]/22.5.
2.6. Histological analysis
Dissected liver tissues were fixed in 4% paraformaldehyde and embedded in paraffin according to standard procedures. Multiple sections were stained with haematoxylin and eosin (H&E) for general morphological observations (Liu et al., 2016).
2.7. Real-time PCR
Total RNA was extracted from adipose tissues with TRIzol (Invitrogen). 0.2 μg total RNA was reverse transcribed to cDNA with iScriptTM reverse transcriptase kit (Bio-Rad) followed by quantitative real time PCR analysis on ABI 7900 HT (Applied Biosystems, Carlsbad, CA) using SYBR green mix (Invitrogen) following a laboratory protocol (Liu et al., 2015). The primer sequences are supplied in the Supporting Materials, Table S1. The amplification parameters were performed according to our previously reported method, Ct values were normalized to β-actin and the relative gene expression was calculated using the 2−ΔΔCt method (Liu et al., 2016).
2.8. Western-blotting
Liver and adipose tissue proteins were homogenized with lysis buffer and quantified by a BCA protein assay kit according to the manufacturer protocol (Pierce, Rockford, IL, USA) (Liu et al., 2015). Protein samples (40 μg) were electrophoresed on 12% SDS-PAGE and then electro-transferred to PVDF membranes. After blocked with 5% non-fat milk, the PVDF membranes were incubated with specific primary antibody at 4 °C overnight according to manufacturer’s protocol. After placed with secondary antibodies at ambient temperature for 1.5 h, the blots were visualized using an ECL kit (Bio-Rad, Hercules, CA, USA). β-Actin and α-tubulin were used as internal controls.
2.9. Feces DNA extraction and amplification
Total DNA from the frozen feces samples were extracted by DNA Mini Kit (Qiagen, Valencia, CA, USA) and stored at −80 °C for further analysis. The concentration of isolated DNA was detected by Nano-Drop 1000 spectrophotometer (Nano-Drop Technologies, Wilmington, DE). Bacterial DNA content was PCR amplified with barcoded universal bacterial primers targeting the variable V3-V4 region of the 16S rRNA gene according to our previous report (Choe et al., 2018; Wan et al., 2017).
2.10. Bioinformatics analysis
Bioinformatics analysis was carried out according to the procedures described previously (Liu et al., 2015). Sequencing was conducted on the Illumina MiSeq platform from Shanghai Majorbio Bio-pharm Technology Co., Ltd. QIIME (version 1.9.1) quality trimming was performed with the following criteria: 1) truncate sequence reads which had a shorter length (<0 bp) and lower quality score (<20); 2) exact barcode matching, discard mismatches in primers and had no ambiguous base calls; and 3) minimum overlap sequence length of 10 bp was assembled and no assembled reads were discarded. Effective reads from all samples were selected from high-quality reads and clustered into operational taxonomic units (OTUs) based on a 97% sequence similarity. Ribosomal Database Project (RDP) Naive Bayes classifier was conducted to analyse the taxonomy of each 16S r RNA gene sequence (Cole, J.R. et al., 2014) compared with the silva (SSU123) 16S rRNA database using a confidence threshold of 70%.
2.11. Statistical analysis
Student’s t test was used for comparison between two groups. All statistical analyses were performed with SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered as statistically significant. Data are shown as mean ± SD.
| 3. Results and discussion | ▴Top |
3.1. Gypenosides reduced body weight gain and insulin resistance in the male obese C57BL/6J mice
The obese mice treated with 300 mg/kg BW/d gypenosides for 8 weeks had significantly less body weight gain and lower total plasma cholesterol in comparison to the control mice (p < 0.05, Figure 1a, b). This body weight gain reduction was not accompanied by a food intake reduction (Table S2). Moreover, 300 mg/kg BW/d gypenosides significantly decreased the concentration of fasting plasma insulin by 40.6% (p < 0.05, Figure 1c), and reduced the homeostatic model assessment-insulin resistance (HOMA-IR) index in comparison to the control group (p < 0.05, Figure 1d), suggesting a possible reduction in obesity-induced insulin resistance. As shown in Figure 1e and f, the gypenosides administration significantly reduced blood glucose levels and the area under the curve (AUC) value compared to the control group, indicating a possible improvement in insulin sensitivity (p < 0.05). Gypenosides did not improve the glucose tolerance under the experimental conditions (Figure 1g, h), and had no significant effect on fasting glucose (Figure 1i). Collectively, these findings indicate that dietary gypenosides might be used to reduce both weight gain and obesity-associated insulin intolerance in obese mice.
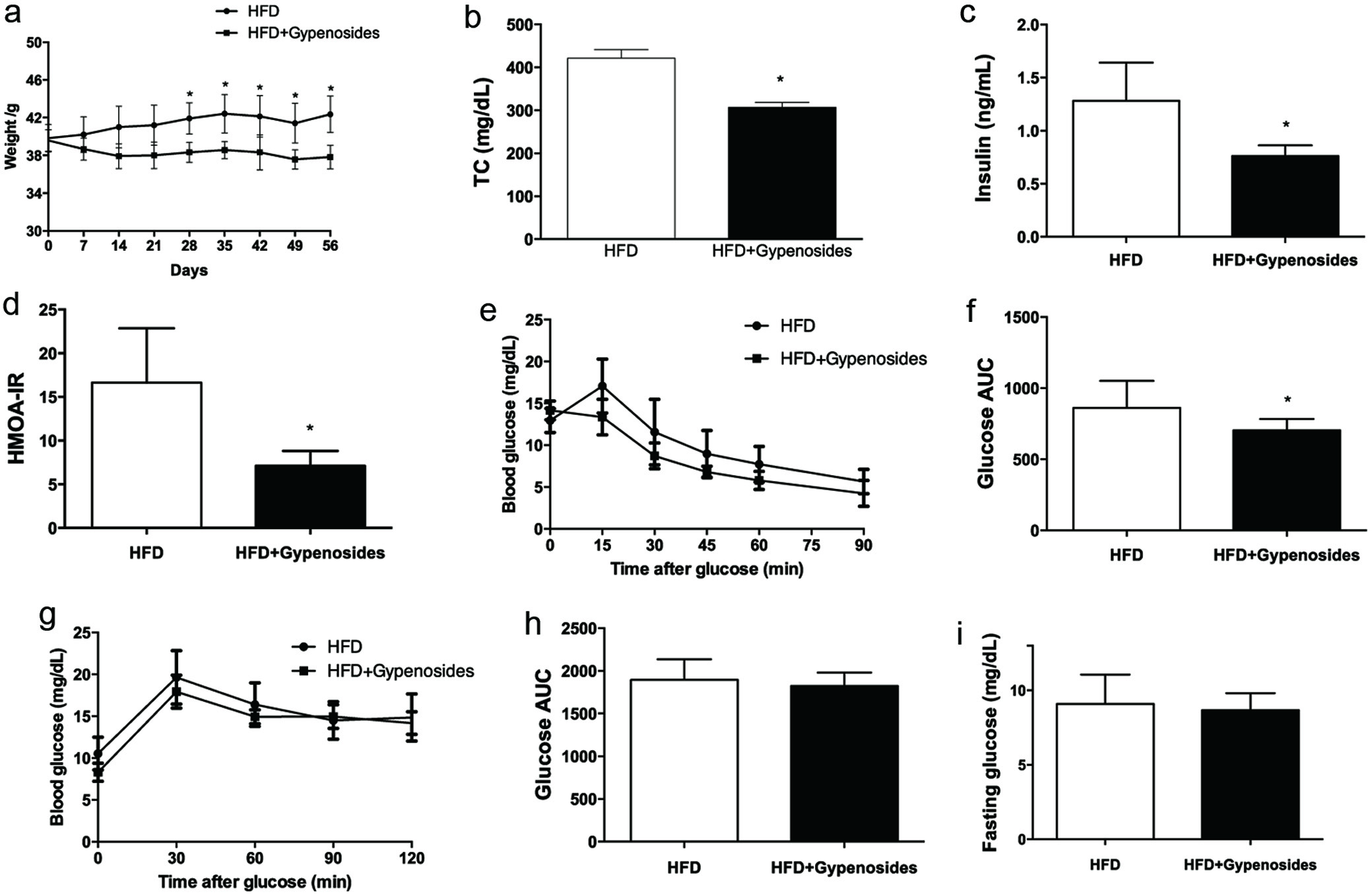 Click for large image | Figure 1. Effect of gypenosides on body weight gain (a), total cholesterol (b), fasting glucose (c), fasting insulin (d), HOMA-IR index (e), oral glucose tolerance test (OGTT, F-G) and intraperitoneal insulin tolerance test (IPITT, H-I). HFD represents the mice fed a high-fat diet and HFD+Gypenosides represent the mice fed HFD plus 300 mg/kg BW/d gypenosides. Values are means ± SD (n = 8). Values marked with * are significantly different from each other at p < 0.05. |
3.2. Gypenosides reduced adipocytokine dysregulation in the male obese C57BL/6J mice
Gypenosides administration for 8 weeks significantly reduced the plasma pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) by 10.1%, 12.4% and 53.6 %, respectively (p < 0.05, Figure 2a–c) and significantly increased the level of an anti-inflammatory cytokine, interleukin-10 (IL-10) by 15.1% in comparison to the HFD control group (p < 0.05, Figure 2d). mRNA levels of TNFα, MCP-1 and IL-6 were also significantly decreased in the obese mice supplemented with dietary gypenosides in comparison to the control obese mice by 72.2%, 56.5 % and 78.8%, respectively in inguinal white adipocyte tissue (iWAT) (p < 0.05, Figure 2e). These results suggest that dietary gypenosides may reduce obesity-associated inflammatory responses. Moreover, macrophages in the adipose tissue from the mice may stimulate a vicious inflammatory cycle which is one of the important inducing factors in obesity-associated inflammation (Chang et al., 2018; Sawada et al., 2015). In the present study, gypenosides consumption also significantly reduced the mRNA expression of macrophage surface markers F4/80 and CD11c by 84.3% and 69.1% in the inguinal white adipocyte tissue (iWAT), respectively (p < 0.05, Figure 2e). These results suggest that dietary gypenosides may reduce the risk of the inflammatory cycle induced by the macrophages in adipose tissue.
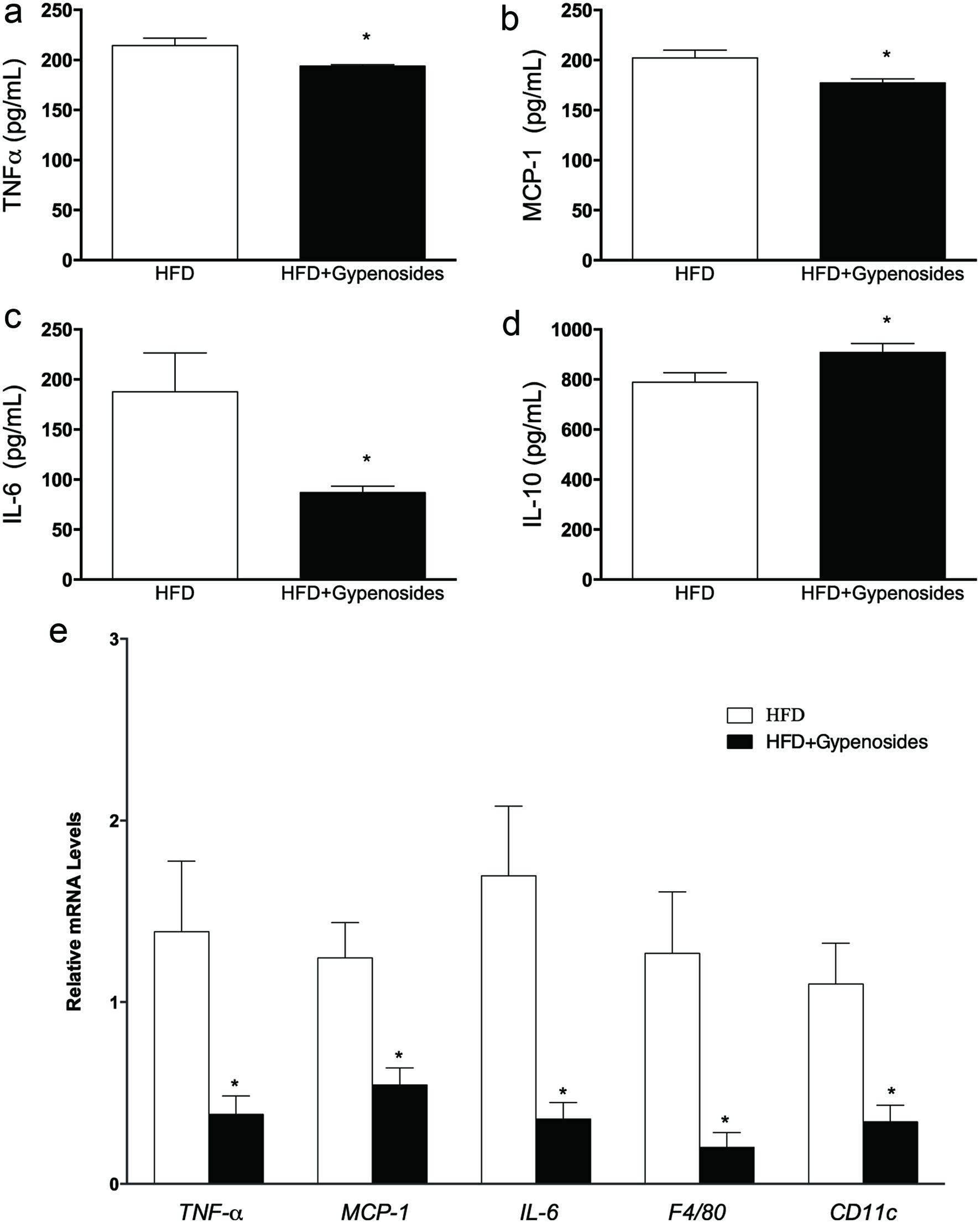 Click for large image | Figure 2. Effect of gypenosides on pro-inflammatory cytokines TNF-α (a), MCP-1 (b), IL-6 (c) and IL-10 (d) levels in the plasma, and related mRNA expression in white adipocyte tissue (e). HFD represents the mice fed a high-fat diet and HFD+Gypenosides represent the mice fed HFD plus 300 mg/kg BW/d gypenosides. Values are means ± SD (n = 8). Values marked with *are significantly different from each other at p < 0.05. |
3.3. Gypenosides alleviated hepatic steatosis and insulin resistance through AMPK pathway
Obesity-induced adipocytokine dysregulation could affect hepatic steatosis, glucose homeostasis and ultimately disturb lipid metabolism (Kang et al., 2010; Wu et al., 2016). In the present study, the inhibitory effect of dietary gypenosides on hepatic steatosis was examined in the liver tissues by histological analysis. Gypenoside treatment reduced hepatic steatosis with micro vesicles in comparison with the control mice based on hematoxylin and eosin (H&E) staining (Figure 3a). To further understand the possible mechanisms behind these beneficial effects of gypenosides, a group of proteins related to glucolipid metabolism were examined by Western-blotting. Gypenosides significantly up-regulated the gene expressions related to lipid oxidation and glucose transport, including AMP-activated protein kinase (AMPK), acetyl-CoA carboxylase (ACC), uncoupling protein 1 (UCP-1), AKT and glucose transporter-4 (GLUT4) (Figure 3b). The gypenosides treatment also significantly elevated the protein expression of phosphorylation of AMPK, ACC, AKT and GLUT4 in total cell extracts (p < 0.05, Figure 3b). AMPK is a key player in regulating energy metabolism, which also activates the hepatic PI3K/AKT signaling pathway and increases hepatic insulin sensitivity (Zheng et al., 2015). Activation of AMPK is one of the approaches for prevention and treatment of obesity and the related metabolic diseases, especially in the important metabolic organs such as liver and adipose tissue, and may serve as a more practical approach for screening and developing agents and functional foods that decrease the risk of and improve treatment for treatment of overweight or obesity, as well as obesity-associated chronic health problems (Zhang et al., 2014). For instance, berberine, curcumin and resveratrol have been shown to regulate organismal energy balance through AMPK activation, which may have potential in controlling body weight gain in obese animals (Lone et al., 2016; Wang et al., 2015; Zhang et al., 2014). The critical role of AMPK was also supported by findings from another study that oral administration of Gynostemma pentaphyllum extract to ob/ob mice for 8 weeks decreased body weight gain, liver weight and blood cholesterol levels through AMPK activation in the soleus muscle (Gauhar et al., 2012). In the present study, gypenosides treatment at 300 mg/kg BW/d for 8 weeks alleviated high-fat diet induced hepatic steatosis and insulin resistance through activating the AMPK signaling pathway. Taken together, AMPK activation may be the primary mechanism behind the beneficial effects of gypenosides in obese mice.
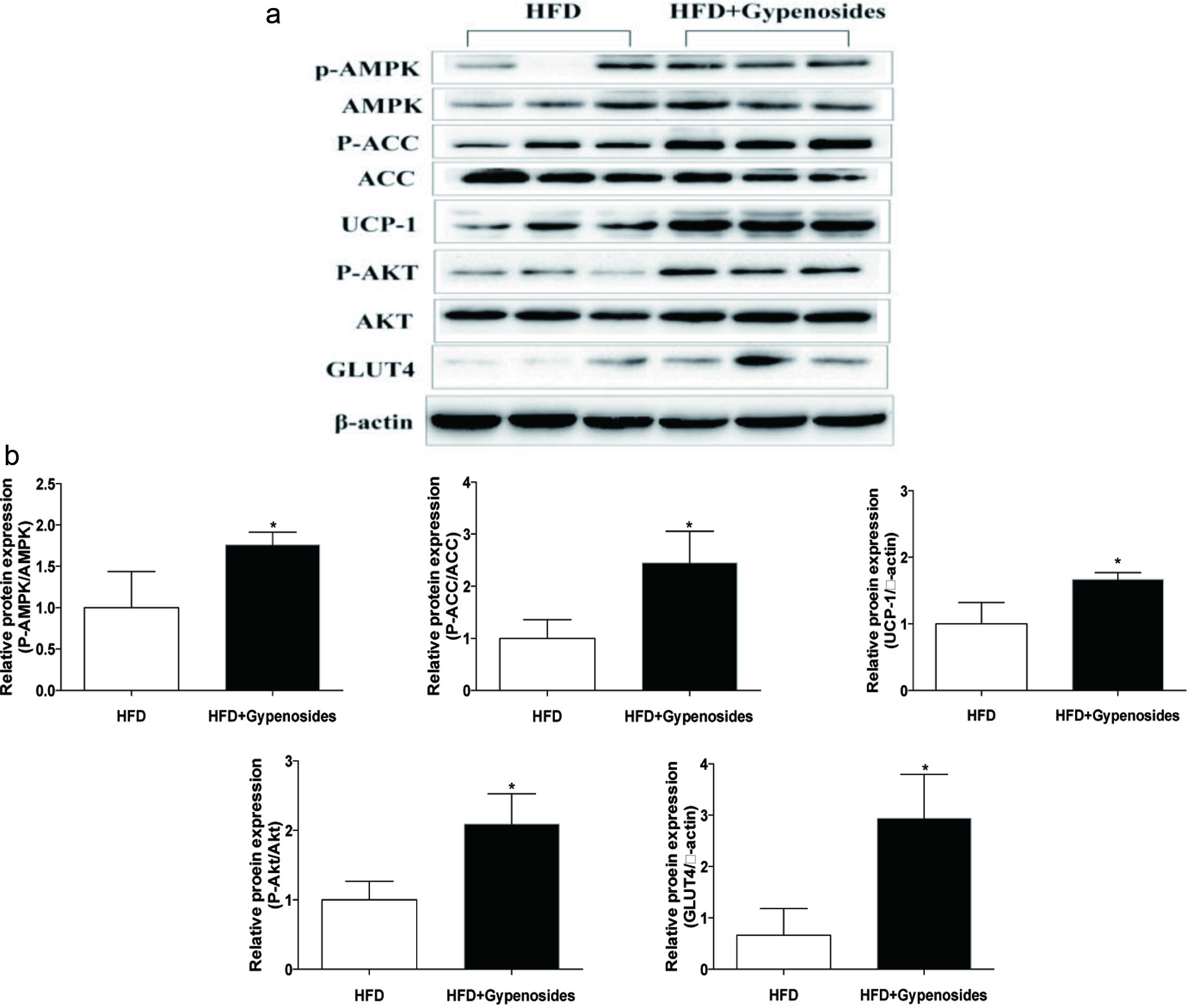 Click for large image | Figure 3. Effect of gypenosides on haematoxylin and eosin (H&E) staining of liver (a) and representative immunoblots and relative protein contents of total and phosphorylated ACC, AMPK AKT and total UCP-1, GLUT4 (b). HFD represents the mice fed a high-fat diet and HFD+Gypenosides represent the mice fed HFD plus 300 mg/kg BW/d gypenosides. Values are means ± SD (n = 8). Values marked with * are significantly different from each other at p < 0.05. |
3.4. Gypenosides increased BAT activity and iWAT browning
The possible effect of gypenosides intake on both white adipose tissue (WAT) and brown adipose tissue (BAT) were also examined in the present study, because of their roles in obesity and its associated inflammation and insulin resistance (Stanford et al., 2013). Under the experimental conditions, gypenosides consumption was able to significantly increase the expression of key proteins UCP-1, PGC-1α and PRDM16 at both protein and mRNA levels in brown adipose tissues (p < 0.05, Figure 4a, b), suggesting the elevated BAT activity, which transforms the stored chemical energy into heat and protects mice against overfeeding and cold. Furthermore, gypenosides administration also significantly increased the mRNA expression of genes important for mitochondrial activity and fatty acid oxidation reactions including cytochrome complex (CytoC), elongase of very long chain fatty-acids protein 3 (Elvol3), medium-chain acyl-CoA dehydrogenase (Mcad) and carnitine palmitoyl transterase-1β (CPT1β) in the brown adipose tissue (p <0.05, Figure 4b). Under the experimental conditions, gypenosides administration increased the gene expression of UCP-1 and PGC-1α at both protein and mRNA levels in the white adipose tissue, but had no significant effect on PRDM16 gene expression (p < 0.05, Figure 4c, d). In addition, gypenosides significantly increased the expressions of genes important for mitochondrial activity and β-oxidation reaction including CytoC, Elvol3, Mcad and CPT1β in the white adipose tissue (p <0.05, Figure 4d). These results suggest that gypenoside may increase white adipocyte transformation to beige (brown-in-white) adipocytes in obese mice, which could function in a similar way of “classical” brown adipocytes. Increasing BAT activity and iWAT browning have been proven as an effective strategy for prevention and treatment of obesity and its associated metabolic disorders (Garcia-Alonso & Claria, 2014; Stanford et al., 2013). Stanford et al. reported that BAT transplantation into the abdominal cavity was able to improve the glucose intolerance in obese mice, lower their body weight, and completely reverse their insulin resistance induced by high-fat diet (Stanford et al., 2013). Zhang et al. showed that white adipocyte browning could increase the expression of lipid oxidation genes, and promote respiration and energy expenditure (Zhang et al., 2014). In the present study, gypenosides significantly improved the expression of key genes related to BAT function and WAT browning. This observation is consistent with findings from our previous obesity prevention study and from several previous studies by other research groups that natural compounds with very diverse structures can play a critical role in controlling obesity by increasing body thermogenesis (Zhang et al., 2016; Zhang et al., 2014).
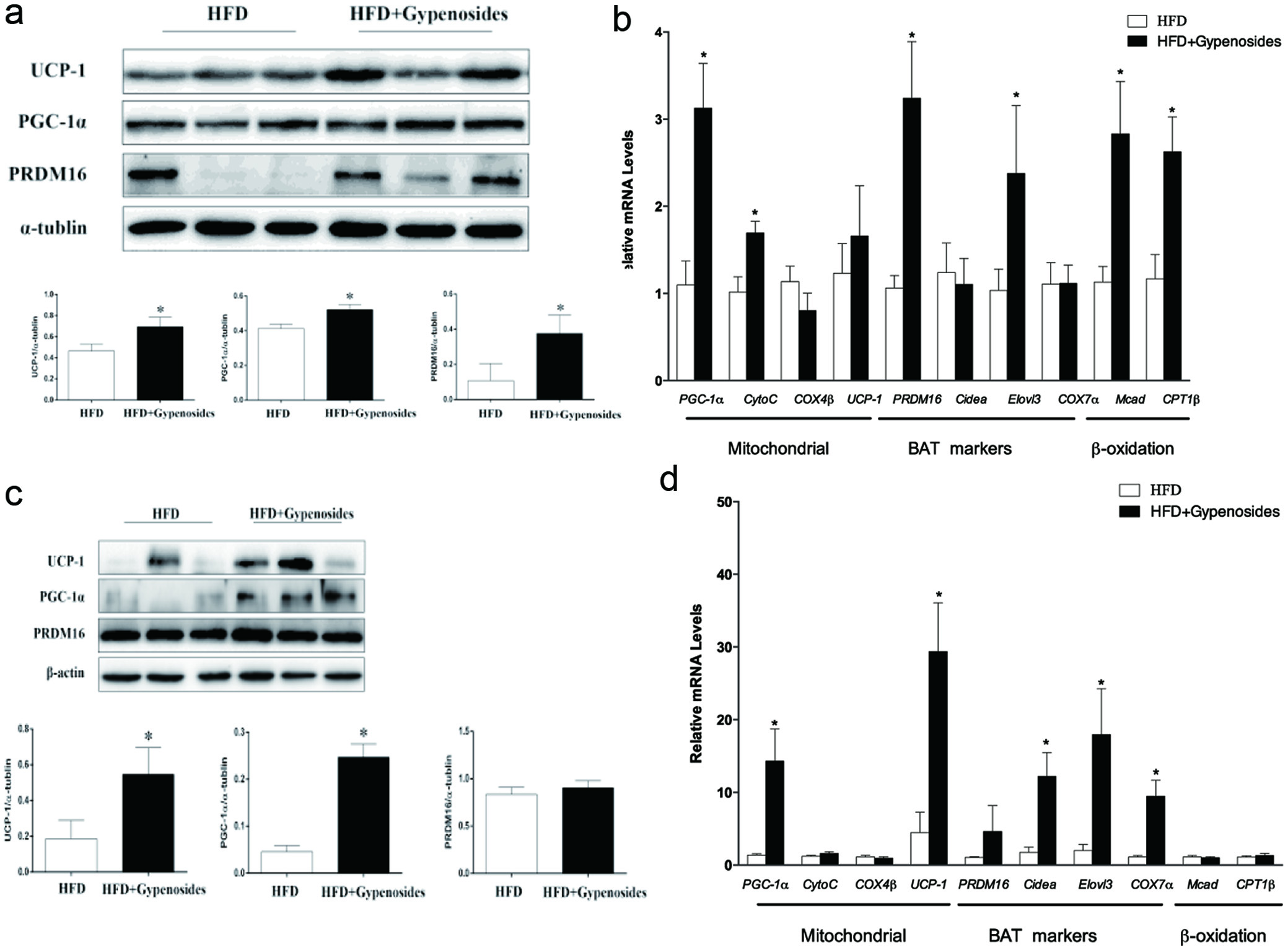 Click for large image | Figure 4. Effect of gypenosides on molecular markers of lipid metabolism in brown adipocyte tissue (a–b) and white adipocyte tissue (c–d) at both protein and mRNA levels. HFD represents the mice fed a high-fat diet and HFD+Gypenosides represent the mice fed HFD plus 300 mg/kg BW/d gypenosides. Values are means ± SD (n = 8). Values marked with * are significantly different from each other at p < 0.05. |
3.5. Gypenosides modulated gut microbiota and induced the detectable Akkermansia muciniphila in the obese C57BL/6J mice
Gypenosides consumption significantly increased Bacteroidetes abundance and decreased Firmicutes abundance at a phylum level, resulting in a decrease in the ratio of Firmicutes to Bacteroidetes in comparison with that of the obese control mice (p < 0.05, Figure 5a). Gypenosides treatment also enhanced the abundance of Verrucomicrobia (p < 0.05, Figure 5a). Interestingly, gypenosides induced detectable and significant levels of Akkermancia muciniphila at a genus level, which was negligible in the obese control mice (p <0.05, Figure 5b). Akkermancia muciniphila belongs to the phylum Verrucomicrobia, and has been found more abundant in the mucosa of healthy subjects than that of diabetic patients or animals (Shin et al., 2014). Akkermancia muciniphila has also been associated with a possible improvement in glucose homoeostasis and blood lipids, and reduced body weight (Plovier et al., 2017; Shin et al., 2014). Gypenosides consumption significantly increased the relative abundance of Akkermansia muciniphila, a mucin-degrading bacterium, which has been inversely associated with inflammation, body fat mass and glucose intolerance in mice (Shin et al., 2014; Zhao et al., 2017). This observation is supported by a previous report that saponins extracted from Agave salmiana attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice (Maria Leal-Diaz et al., 2016). Moreover, Akkermansia muciniphila was considered as a key factor orchestrating the overall energy homeostasis and enabling positive energy balance (Chevalier et al., 2015). Previous reports have suggested that the abundance of Akkermansia muciniphila was positively related to thermogenesis, such as hepatic lipogenesis and WAT browning in high-fat diet induced obese mice (Maria Leal-Diaz et al., 2016; Zhao et al., 2017). Taken together, the microbiota data suggested a prebiotic activity of the gypenosides in the obese mice, and its potential utilization in obesity treatment.
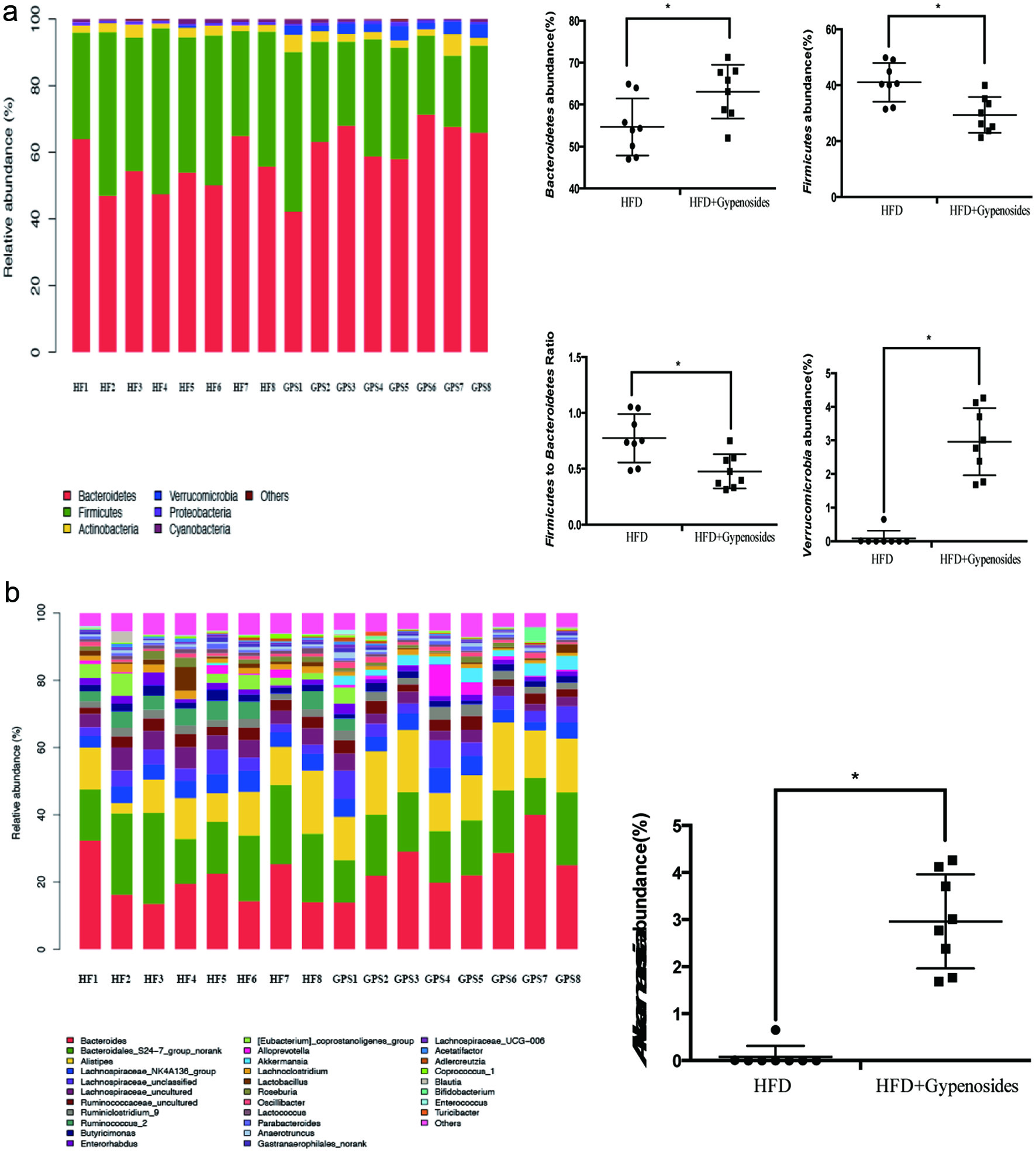 Click for large image | Figure 5. Fecal bacterial community at the phylum level (a) and genus level (b). HFD represents the mice fed a high-fat diet and HFD+Gypenosides represent the mice fed HFD plus 300 mg/kg BW/d gypenosides. Values are means ± SD (n = 8). Values marked with * are significantly different from each other at p < 0.05. |
| 4. Conclusions | ▴Top |
In summary, dietary gypenosides may help in controlling the the body weight of obese mice which have already become obese, and reduce the risk of the obesity-associated inflammation, insulin resistance and hepatic steatosis through activating energy expenditure and gut microbiota content and profile. The results from this study support the potential utilization of gypenosides in mitigating obesity and obesity-associated metabolic abnormalities. Additional studies are needed to investigate the potential relationship between Akkermansia muciniphila, BAT function and WAT browning.
| Supplementary material | ▴Top |
Table S1. Primer sequences for RT-PCR.
Table S2. Organs weight and food intake of different treatment group. HFD represents mice fed a high-fat diet and HFD+Gypenosides represent mice fed HFD plus 300 mg/kg gypenosides. Values are means ± SD (n = 8). Values marked with different letters are significantly different from each other at p < 0.05.
Acknowledgments
This research was supported by grants from The National Key Research and Development Program of China (Grant No. 2017YFD0400205), the Chinese National Natural Science Foundation (Grant No. 31501479), and grants from the Beijing Advanced Innovation Center for Food Nutrition and Human Health.
Conflict of interest
The authors declare that there are no conflicts of interest and otherwise to this work.
| References | ▴Top |