| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 20, December 2022, pages 90-97
Reactivity comparison of Amadori rearrangement product formation of glycine, diglycine, and triglycine in a competing Maillard system
Yue Luoa, Baojun Xub, *, Shiming Lia, Chi-Tang Hoa, *
aDepartment of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
bFood Science and Technology Program, BNU-HKBU United International College, Zhuhai, Guangdong 519087, China
*Corresponding author: Baojun Xu, Food Science and Technology Program, BNU-HKBU United International College, Zhuhai, Guangdong 519087, China. E-mail: baojunxu@uic.edu.cn; Chi-Tang Ho, Department of Food Science, Rutgers University, New Brunswick, NJ 08901, USA. E-mail: ctho@sebs.rutgers.edu
DOI: 10.31665/JFB.2022.18332
Received: December 3, 2022
Revised received & accepted: December 28, 2022
| Abstract | ▴Top |
Although a certain number of amino acid-Amadori rearrangement products (ARPs) have been studied, there is still a lack of knowledge of small peptide-ARPs. Filling the gap should be a great step in the potential usage of ARP as future flavor additives. This study illustrated that small peptides (diglycine and triglycine) exhibited better relative reactivity of ARP formation than an amino acid (glycine) at relatively low temperature such as 80 and 100 °C and in a wide range of pH from acidic to neutral conditions, but the result reversed at high temperatures for severer instability of small peptide-ARPs. The relative reactivity of ARP formation of amino compounds in a competing Maillard systems results from dynamic systems with various factors including the chemical characterization and composition of intrinsic reactants, and parameters of matrix conditions like pH, temperature and thermal treatment time among others. Further research should be conducted to investigate peptide-ARPs, for which are ubiquitous in real food systems and worth to pay more attention.
Keywords: Amadori rearrangement product; Relative reactivity; Amino acid; Small peptides
| 1. Introduction | ▴Top |
Since first discovery by French chemist Louis-Camille Maillard in 1912, Maillard reaction has been one of the most important reactions in the field of flavor generation (Maillard, 1912). Occurring ubiquitously in food processing and storage, Maillard reaction provides attractive and complex flavors especially for thermally processed food such as bread, cereal, coffee, and roasted meat. These flavors mainly come from formation or addition of Maillard reaction products (MRPs), including pyrazines, pyrroles, alkylpyridines, acylpyridines, furanones, furans, oxazoles, thiazoles, and thiophenes among others (Deblander et al., 2015; Ruan et al., 2018).
However, these Maillard reaction-derived flavors are often unstable. The loss of attractive flavor compounds such as acetaldehyde, furfural and butanal, has been a major concern regarding flavor quality and consumer acceptance (Hansen and Arora, 1990). Pyranone, commonly found in glucose-rich or lactose-rich foods, and 3,4-dihydroxy-3-hexen-2,5-dione with a caramel odor, are significantly prone to degradation during short-term storage (Andrewes, 2012). For instance, furaneol (2,5-dimethyl-4-hydroxy-3(2H)-furanone), also known as strawberry furanone, is heat-labile and rapidly degraded particularly at low pH (Shu et al., 1985).
Enormous efforts have been made for the stabilization of flavor compounds, but not until recently, people started to pay attention to the flavorless intermediates named Amadori rearrangement products (ARPs). A big advantage of ARPs is of their superior stability during storage and synchronous production of fresh and desirable flavors during thermal treatment, which makes ARPs potential flavor enhancers and food additives. Up to now, studies on the potential application of ARPs in food flavor industry are still conducted sporadically though reviewed by a few groups (Cui et al., 2021; Luo et al., 2021). Among the published papers of ARPs, only a few of them is related to peptides which take a dominant position in real food systems (Tang et al., 2019; Xia et al., 2022). There is an obvious lack of systematic comparison on ARP formation of amino acids and peptides. Glycine, as the simplest amino acid and good start point, was compared separately with diglycine and triglycine on the activation energy of ARP formation in individual Maillard models with glucose (Xia et al., 2022). However, amino acids and peptides coexist in real food system and will compete for reducing sugars for ARP formation. Therefore, for the first time, the relative reactivity of glycine, diglycine and triglycine on ARP formation was investigated in this study by combining glycine and small peptides in the same Maillard model system to mimick the real food system.
| 2. Materials and methods | ▴Top |
2.1. Chemicals
Glucose, glycine, 15N-glycine, diglycine, triglycine, sodium hydroxide, hydrochloric acid, ammonium hydroxide solution, and 2,3,5-triphenyltetrazolium chloride were purchased from Sigma-Aldrich Chemical Co. (Shanghai, China). HPLC-grade acetonitrile, and formic acid were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Ultrapure water was made in house by purifying deionized water through Milli-Q equipment (Millipore, Bedford, MA, USA).
2.2. Preparation of three ARPs as standards (gly-, digly-, trigly-glucose)
Glycine, diglycine or triglycine was mixed well with glucose in deionized water at a molar ratio of 1:2 (0.1 M: 0.2 M), respectively. The pH value of solutions was adjusted to 7.0 by 6 M sodium hydroxide. The formed solutions were lyophilized to powder and then heated up at 70 °C for 40 min. Water was added back to each of the heated samples and followed by purification stated as the following section.
2.3. Purification of the three ARP standards
Initial purification was performed according to a previously published procedure with minor modifications (Xia et al., 2022). It was accomplished by using an open column filled with cation exchange resin (Dowex 50WX4, 200-400 mesh, H+-form) (Sigma, St. Louis, MO, USA). Prior to each separation, the resin was thoroughly rinsed sequentially with 1 M NaOH solution, deionized water, 1 M HCl solution, deionized water and 1M NaOH solution then finally deionized water. The reason that deionized water was applied in between acid-base solution change was to adjust pH to 7.0 and thus reduce salt formation. After loading appropriate amount of ARP reaction solution prepared above, the column was first flushed with deionized water to remove uncharged compounds such as glucose. The flow rate of deionized water was 2 mL/min and each 50 mL of eluted solution was collected as one fraction and detected with 2,3,5-triphenyltetrazolium chloride (TTC) test. A red color from the TTC test indicated the existence of glucose. Upon the depletion of glucose, ammonium hydroxide solution (0.22 mol/L) was then applied to the column at a flow rate of 1 mL/min for desorption of charged compounds including target ARPs. LC/MS/MS was used to analyze the content of ARPs for collected fractions. The fractions that contained ARPs were then combined, concentrated in vacuo and lyophilized to dryness for further purification.
The second purification was performed on a preparative high-performance liquid chromatography (HPLC, Yilite, Suzhou, China), equipped with an Atlantis Silica HILIC OBD Prep Column (19 mm × 150 mm, 5 μm, Waters Corporation (Milford, MA, USA), which was maintained at 35 °C. Isocratic mobile phase was composed of acetonitrile and water (v/v, 1:1). Flow rate was 1 mL/min and injection volume 100 μL. The eluted fractions were collected by an autosampler (QT-100FC-LCD, Qite Corporation, Shanghai, China). In the separation, the crude samples from last separation stated above were dissolved in Milli-Q water (50 mg/mL) and then filtered with a 0.22 μm PTFE syringe filter prior to loading onto the column. Fractions were double-checked by LC/MS/MS analysis for targeted ARPs, of which containing ARPs were combined, concentrated in vacuo to remove organic solvents and finally lyophilized to dryness using a freeze dryer (FreeZone 1, Labconco Corporation, Kansas City, MO, USA). The dried ARPs were stored at −20 °C for further usage.
2.4. Identification of ARPs by LC/MS/MS
The isolated glycine-glucose-ARP, diglycine-glucose-ARP and triglycine-glucose-ARP were dissolved separately in ultrapure water at a concentration of 0.01 mg/mL and then filtered by a 0.22 μm PTFE syringe filter. As for the reaction models, samples were further diluted accordingly for subsequent LC/MS/MS analysis. The HPLC/MS/MS spectra of the compounds were acquired on an MS/MS (Agilent, Santa Clara, CA, USA) using a HILIC column (5 μm, 4.6 mm × 250 mm, Waters, USA). Ultrapure water (A) and acetonitrile (B) containing 0.1% formic acid were used as the mobile phase. The column temperature was set at 35 °C and the total flow rate of mobile phase was 0.5 mL/min. Then the samples were eluted with the following linear gradient: 0–15 min, 70% B; 15–17 min, 95% B; 17–20 min, 90% B. Injection volume was 0.5 μL. The ionization conditions of the MS/MS were as followed: collision energy, 6.0 eV; capillary voltage, 3.5 kV; cone voltage, 20 V; and detector voltage, 1.8 kV. Temperatures of source block and desolvation were 100 °C and 400 °C, respectively. The desolvation gas flow was 700 L/h and cone gas flow was 50 L/h. MS detection was in full scan mode over the range of m/z 50–1,000 with a scanning time of 1 s and an inter scanning delay time of 0.1 s. Specific MS detection of the three ARPs was in multiple reaction monitoring (MRM) mode: m/z 238.1→220.1 and 238.1→202.0 for glycine-glucose-ARP; m/z 295.2→277.1 and 295.2→211.1 for diglycine-glucose-ARP; m/z 352.1→334.1 and 352.1→268.0 for triglycine-glucose-ARP. Mass Lynx software (version 4.1, Waters, Milford, MA, U.S.A.) was used to analyze the datum of ARPs.
2.5. Preparation of reaction model of ARP formation reactivity
Basically, two batches of mixed solution were prepared using Milli-Q water: one as glucose/glycine/diglycine and glucose/glycine/triglycine as the normal glycine batch (NGB), and the other one as glucose/15N-glycine/diglycine and glucose/15N-glycine/triglycine (15NGB) in a concentration of 0.02 mol/L for each ingredient. The pH values of solutions were adjusted to four categories as 3.0, 5.0, 7.0, and 9.0, respectively, to illustrate the effect of initial pH value on the ARP formation. The various pH-adjusted solutions were then added to high-temperature high-pressure resistant reaction vessels containing small-sized magnetic stirring bars. The vessels were then sealed and heated in an oil bath for 60 and 120 min at four different reaction temperatures (80, 100, 130, 160 °C) with constant magnetic mixing at 600 rpm. Upon completion of the reaction, the vessels were cooled in an ice bath immediately to room temperature for subsequent LC/MS/MS, pH and UV-Vis analysis.
2.6. UV-Vis analysis
One hundred micro litter of each post-heating solutions was placed in 96-well plate for later ultraviolet-visible (UV-Vis) spectrometer (FLUOstar Omega, BMG Labtech, Ortenberg, Germany) analysis. The absorbance at 420 nm (A420) were presented as Maillard reaction intermediate and browning intensity, respectively (Ajandouz et al., 2001; Hong and Betti, 2016). The reaction solutions were diluted accordingly to the range of values between 0.2 and 0.8 to ensure accuracy. The final results were the measured absorbance values multiplied by the dilution factors to reflect the real corresponding values.
| 3. Results and discussion | ▴Top |
3.1. Preparation of ARPs
Lyophilization followed by thermal treatment was chosen to prepare the three target ARPs since reducing water content can facilitate ARP formation (Luo et al., 2021). The purity after two rounds of purification was double checked by LC/MS/MS and nuclear magnetic resonance (NMR) and achieved above 95%. The purified ARPs derived from glycine/glucose, diglycine/glucose, and triglycine/glucose were identified as N-(1-deoxy-D-fructos-1-yl)-glycine (GA), N-(1-deoxy-D-fructos-1-yl)-diglycine (DA) and N-(1-deoxy-D-fructos-1-yl)-triglycine (TA) by NMR, respectively (data were not shown here).
The LC/MS/MS spectra with proposed fragmentation pathways of the GA, DA and TA were clearly shown in Figures 1–3.
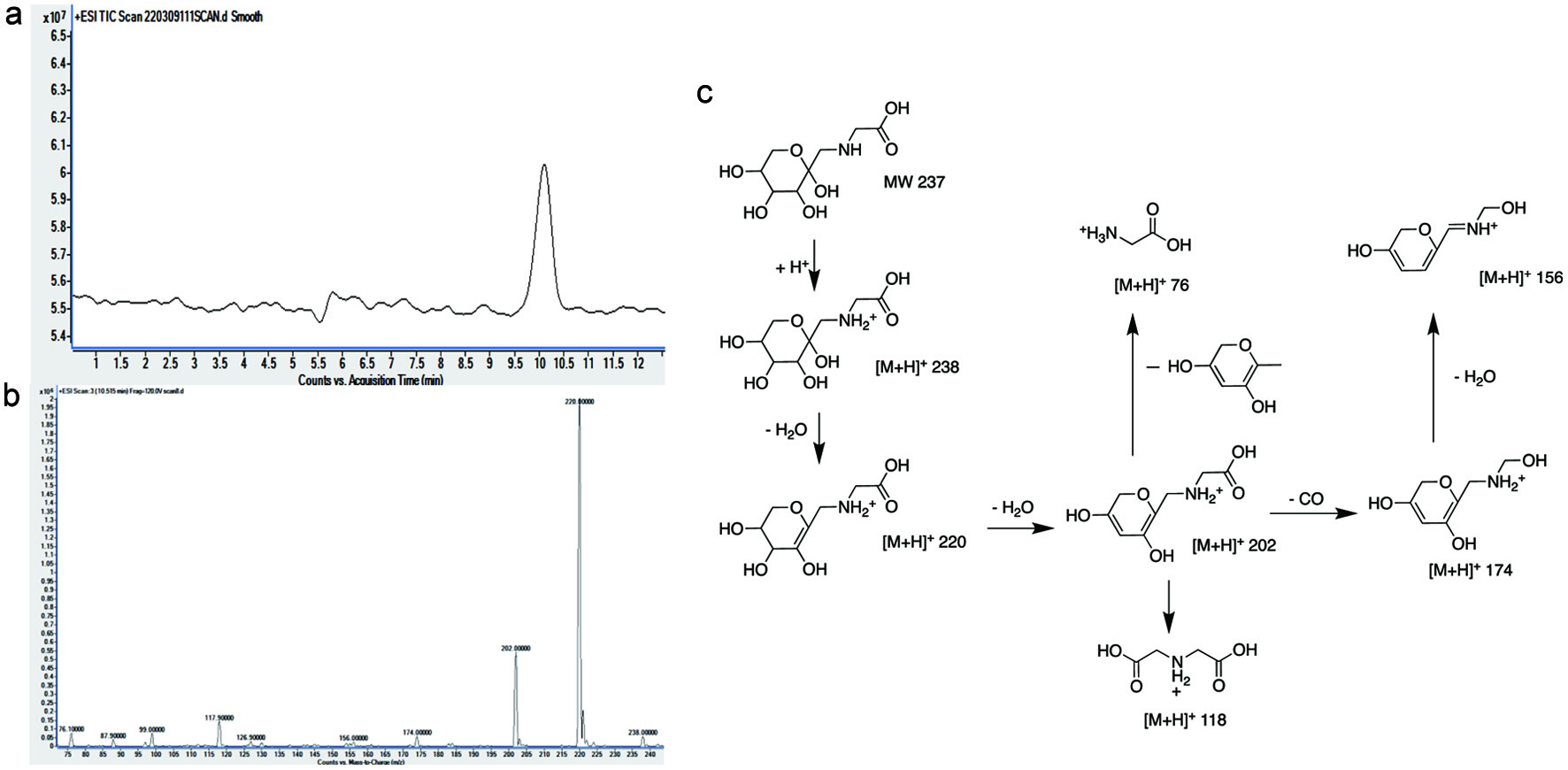 Click for large image | Figure 1. LC/MS/MS analysis of glycine-ARP: full scan spectrum (a); secondary spectrum (b); proposed fragmentation pathway (c). (120.0 v–m/z: 76.1, 118.0, 156.1, 174.0, 202.0, 220.0, 238.0) |
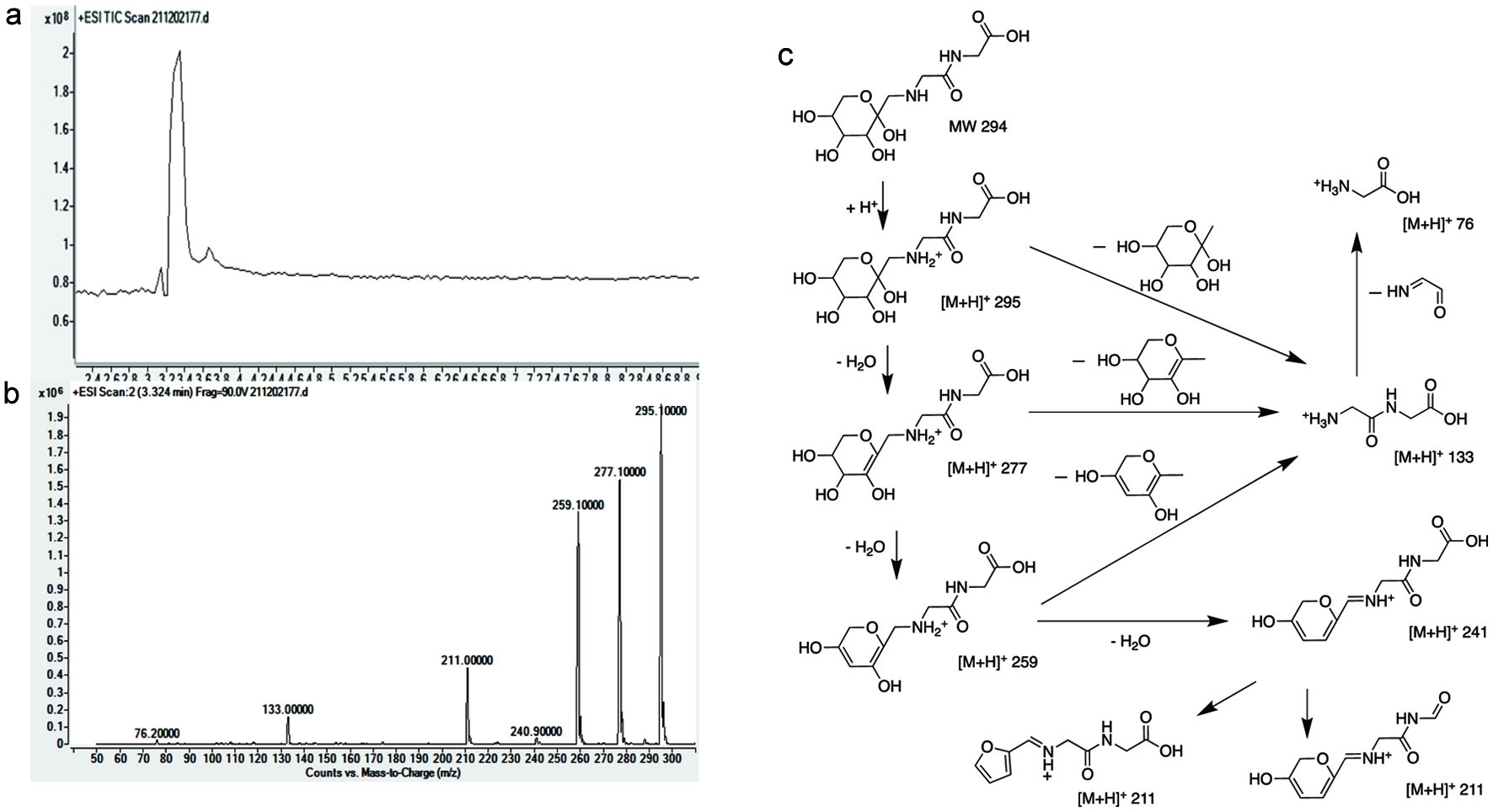 Click for large image | Figure 2. LC/MS/MS analysis of diglycine-ARP: full scan spectrum (a); secondary spectrum (b); proposed fragmentation pathway (c). (90.0 v–m/z: 76.2, 133.0, 211.0, 240.9, 259.1, 277.1, 295.1) |
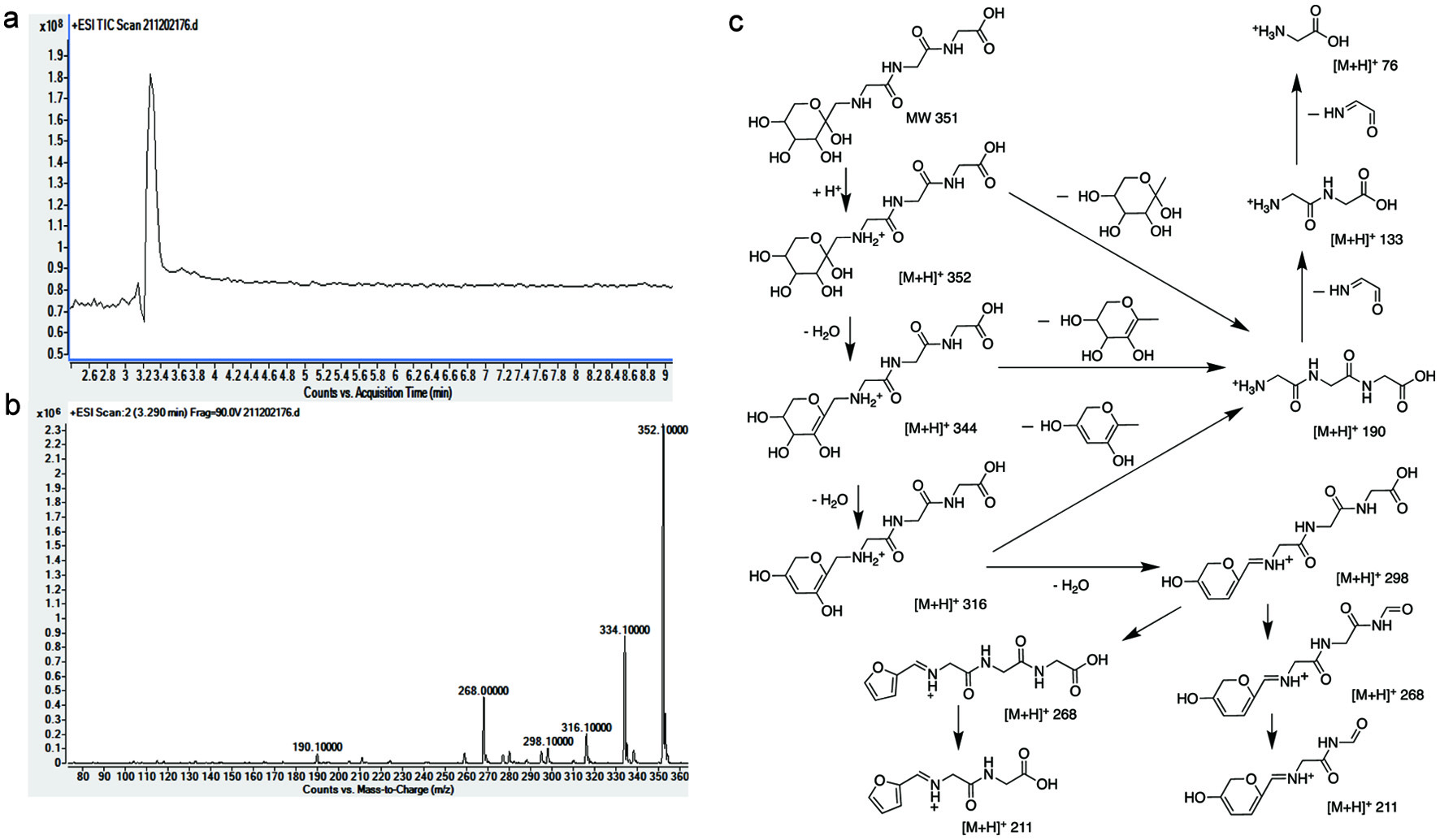 Click for large image | Figure 3. LC/MS/MS analysis of triglycine-ARP: full scan spectrum (a); secondary spectrum (b); proposed fragmentation pathway (c). 90.0v–m/z: 190.1, 211.1, 268.1, 298.1, 316.1, 334.1, 352. |
3.2. Actual GA formation of initial glycine reactant
In order to compare the relative reaction reactivity of glycine, diglycine and triglycine with glucose, and their corresponding ARP formation, the combinations of two amino compounds in one reaction system were prepared as (i) glycine and diglycine plus glucose, and (ii) glycine and triglycine plus with glucose. The comparison was achieved by comparing the contents of three ARPs.
Since diglycine and triglycine would break down and form glycine at high temperature, the interference of newly-formed glycine from glycine di- and tri-peptides with original glycine monomer in forming glycine-ARP should be eliminated. This obstacle would be ideally overcome by using 15N-glycine at the beginning of ARP formation reactions and directly quantifying the 15N-glycine-ARP using self-made external 15N-glycine-ARP standard. However, the preparation of 15N -glycine-ARP is costly and cumbersome.
Therefore, an indirect method was applied as two batches of glycine including normal glycine (NGB) and 15N-glycine (15NGB) were used at the beginning, where 15N-glycine-ARP would form from 15N-glycine correspondingly and become distinct under MS detection condition of glycine-ARP. The 15N-glycine-ARP thus represented solely ARP formed from glycine monomer and was distinguished from glycine-ARP resulted from the newly-formed glycine of glycine di- and tri-peptides. Since MRM mode was used and specific paired ions were chosen to analyze the normal glycine-ARP, the 15N-glycine-ARP would not be detected for its different specific paired ions from that of the normal one.
While the glycine-ARP content in the NGB batch was the sum of that from glycine monomer, glycine di- and tri-peptides, the glycine-ARP content in the 15NGB batch was the sum of that from glycine di- and tri-peptides only. Therefore, the actual glycine-ARP content of initial glycine monomer was then equaled to the difference of glycine-ARP content between NGB and 15NGB batches, as shown in Tables 1 and 2.
 Click to view | Table 1. Actual contents of glycine-ARP and diglycine-ARP formed in combination reaction mode (R1, mmol/L) |
 Click to view | Table 2. Actual contents of glycine-ARP and triglycine-ARP formed in combination reaction mode (R2, mmol/L) |
3.3. pH and temperature effects on ARP formation
As shown in Table 3, the concentrations of each ARP at different temperature and pH values were listed. At relative lower temperature such as 80 and 100 °C, all ARPs formed more at higher pH values, i.e. weak basic condition than acidic lower pH values, especially for GA at pH 9. This is aligned with a previous study that the rate of phenylalanine-ARP formation remained very low in acidic pH value but sharply increased when the pH value is larger than the pKa value of amino group of the amino acid (Ge and Lee, 1997). The reason behind is that unprotonated amino groups and acyclic sugar are the active forms in ARP formation, and the percentage of unprotonated amino groups is largely related with pH value. For instance, glycine, owing a pI of 5.97 and pKa of 9.60 for its amino group, has a rapid percentage increase of free amino groups right around its pI value (Martins et al., 2000). Glycine-ARP has a lower activation energy (Ea) of 64.8 kJ/mol at pH 10.0 than 84.76 kJ/mol at pH 7.5 (Yu et al., 2018). Therefore, ARPs of glycine, diglycine and triglycine tend to form more amount in alkaline condition if solely considering availability of free amino groups affected by pH values.
 Click to view | Table 3. The ratio of peptide-ARP over glycine-ARP and ratio between diglycine-ARP and triglycine-ARP under different initial pH and temperature in relative reactivity analysis |
The small peptides will theoretically form more ARPs than glycine at the same environment for their lower pKa values of amino groups, which increases percentage of the unprotonated amino groups. In addition, ARP formation rate of triglycine, diglycine and glycine was investigated in isolated Maillard system and it was found to be reversely related with peptide length since the Ea was 63.48, 72.84 and 84.76 kJ/mol for tri- and di-glycine, respectively (Xia et al., 2022). The longer the peptide length, the lower the activation energy of ARP formation, and the higher the relative reactivity of ARP formation. It is partially aligned with the result of this study in competing Maillard reaction systems. As shown in Table 3, small peptides especially diglycine either had higher or similar ARP formation reactivity as glycine at lower temperature. However, the situation changed as temperature increased, indicating that Ea was not the sole factor affecting ARP formation reactivity in competing Maillard system.
As the temperature arose, the formation of three ARPs was intensified thoroughly for one-hour thermal treatment in every pH value, indicating enhanced ARP formation reactivity with elevated temperature. However, the ARPs content of diglycine and triglycine were slightly decreased as heating time extended to two hours at 130 °C due to possible intrinsic degradation at high temperature. The reduction of small peptide ARPs was even more oblivious at 160 °C as diglycine-ARP reduced to around one half of that at one-hour timepoint and triglycine-ARP almost vanished. The instability of triglycine also exhibited in the formation of diglycine-ARP as a degradation product of triglycine-ARP. In contrast, glycine-ARP formation maintained a relative stable status and thus resulting in a better relative ARP formation reactivity as shown in Table 3. Therefore, small peptides only have relative better ARP formation reactivity than glycine at lower temperature where ARP stability could be guaranteed. The relative weaker stability of small peptide-ARPs may be resulted from more bond breakage points than amino acid-ARP. However, systematic stability study of small peptide-ARPs is almost scarce. Lack of actual degradation characterization of pure ARPs at various environment could be a big drawback in their potential usage as flavor additives, which is under investigation by our group.
Based on aforementioned results, reactivity of ARP formation is dynamically affected by various complex factors, which mostly fall into two categories: i) the intrinsic chemical characterization such as chemical structure, composition and stability of initial reactant; ii) matrix conditions including pH value, temperature, water activity, interference with salt, sugar and metal ions, process or storage time length and microorganism involvement and other factors. Peptide length and amino acid composition may influence ARP formation (Tang et al., 2019; Xia et al., 2022). In addition to amino compounds, reducing sugar also have large impact on the ARP formation. Pentose is more reactive than hexose in Maillard reaction. For instance, ribose has a reduced Ea of 84.76 comparing to that of 30.00 kJ/mol for glucose at pH 7.5 (Zhan et al., 2020). Free form of amino groups is largely influenced by pH value which further changes along with elevated temperature and prolonged time of reaction due to acid formation like acetic acid and formic acid (Martins et al., 2003). Temperature elevation results in severe ARP degradation, which is clearly displayed in glycine-ARP degradation curve at 90, 100 and 120 °C (Davidek et al., 2002; Martins et al., 2003). The ARP degradation is accompanied with the formation of dicarbonyl compounds and their subsequently formed browning compounds like melanoidin in advanced Maillard reaction. It was also found in this study as shown in Figure 4 that browning intensity is positively related to temperature. Water activity and initial concentration of reactants are the main factors controlling ARP formation rate at the early Maillard reaction phase, therefore, compounds influence water activity like salt and sugar will impact the ARP formation reactivity (Davidek et al., 2018).
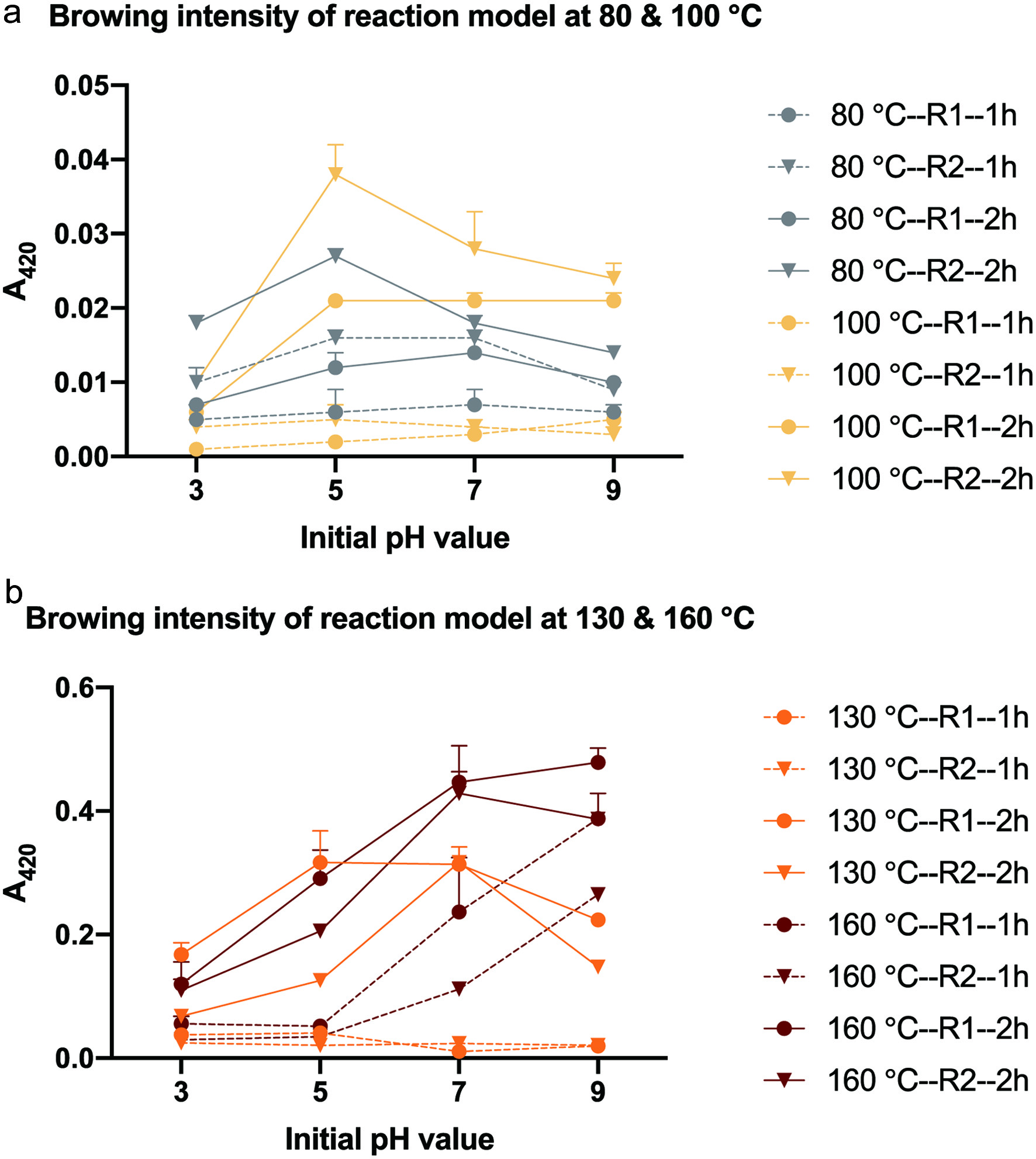 Click for large image | Figure 4. Browning intensity change under different initial pH and temperature in relative reactivity analysis. *1h and 2h means thermal treatment of 1 hour and 2 hours, receptivity. *R1 and R2 means the model system of glucose/glycine/diglycine = 1:1:1 and glucose/glycine/triglycine = 1:1:1 (molar ratio), respectively. |
In brief, the ARP content is the result of a competition between formation and degradation. Under lower temperature, the ARP formation reactivity was led by formation largely affected unprotonated amino group and accumulation of ARP with time was observed. While under higher temperature, degradation instead took a dominant role and therefore depletion of ARP was found, and medium temperature like 130 °C showed the best performance at ARP formation activity. The small peptides have better relative reactivity of ARP formation than amino acid at low temperature and acidic to neutral conditions, of which diglycine has relative better reactivity than triglycine even at high temperature possibly due to better stability of corresponding ARP.
| 4. Conclusion | ▴Top |
Amadori rearrangement products act as promising flavor precursors. As major components in real food system, peptides and formed peptide-ARPs deserve more attention in potential usage as food additives in the future. This study showed that small peptides have better relative reactivity in ARP formation than amino acid at low temperature and in a wide range of pH values from acidic to neutral, indicating strong ARP formation in real food systems during long-term fermentation and storage and implying a potential important role in flavor contribution. Since majority of peptides has not been studied for the formation of corresponding ARPs, further researches need to be conducted to get a big picture of peptide-ARPs based on comprehensive understanding. Whereas, preparation and model system study of each peptide-ARP would be onerous, data analysis method like machine learning should be introduced to relieve some laboriousness.
Acknowledgments
This study was supported by one NSFC matching grant (project code: UICR0300003) from BNU-HKBU United International College. Special thanks to Dr. Heping Cui of Jiangnan University, China for the helpful discussion on the preparation of Amadori compounds.
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |