| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Article
Volume 2, June 2018, pages 112-118
Antioxidant activity of faba bean extract and fractions thereof
Ryszard Amarowicza, *, Fereidoon Shahidib
aInstitute of Animal Reproduction and Food Research, Polish Academy of Sciences, Tuwima Street 10, 10-748 Olsztyn, Poland
bDepartment of Biochemistry, Memorial University of Newfoundland, St. John’s NL, Canada A1B 3X9
*Corresponding author: Ryszard Amarowicz, Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, Tuwima Street 10, 10-748 Olsztyn, Poland. E-mail:
DOI: 10.31665/JFB.2018.2146
Received: April 18, 2018
Revised received & accepted: May 18, 2018
| Abstract | ▴Top |
Phenolic compounds were extracted into 80% (v/v) acetone. The crude extract so obtained was separated into fraction of low-molecular-weight compounds (Fraction I) and a fraction of condensed tannins (Fraction II) using Sephadex LH-20 column chromatography with ethanol and acetone/water (1:1, v/v) as respective mobile phases. The antioxidants activities of the crude extract and fractions thereof was investigated in an emulsion system and using ABTS, DPPH, and reducing power assays. Fraction II exhibited a higher antioxidant efficacy than those of the crude extract and Fraction I. In the crude extract, 21 compounds, namely phenolic acids (gallic, p-hydroxybenzoic, protocatechuic , p-coumaric, ferulic), flavonoids (catechin, epigallo catechin, kaempferol, apigenin, and luteolin derivatives), gallate procyanidins, vanillin, protocatechuic aldehyde, and tryptophan were identified by HPLC-DAD-MS. Gallate procyanidin dimer, gallate procyanidins, and acetylated kaempferol hexose were the major phenolics present in the extract.
Keywords: Broad bean; Antioxidant activity; Phenolic compounds
| 1. Introduction | ▴Top |
Phenolic compounds of plant origin play important role in food technology as well in human nutrition. As the natural antioxidants they can inhibit or delay the oxidation of nutrients present in food products. In the human organism phenolics can act as protectors of lipids, proteins and DNA against reactive oxygen and nitrogen species (RONS) (Halliwell and Gutteridge, 1992; Willett, 1994). The protective effect of consumption of phenolic-rich grains, legumes, berries, nuts, and oilseeds against several chronic diseases was demonstrated by several researcher groups (Zgang et al., 2018; Chang et al., 2016; Shahidi and Ambigaipalan, 2015; Alshikh et al., 2015; Shahidi and Chandrasekara, 2013).
Legumes are an important source of proteins, starch, oligosaccharides (prebiotics), dietary fibers, vitamins, and minerals in the human diet (Vaz Patto et al., 2015; Flight and Clifton, 2006). The can also serve as a rich source of natural antioxidants and thus can play an important role in caridio- and cancer-protection (Shahidi and Ambigaipalan, 2015; Bouchenak and Lamri-Senhadji, 2013; Aparicio-Fernandez et al., 2008; Madhujith and Shahidi, 2005Peeters et al., 2003;; Kolonel et al., 2000; Kushi et al., 1999).
Faba bean (Vicia faba) is a species of Fabaceace family. It is native to North Africa, South America, southwest and south Asia, and is extensively cultivated elsewhere. Like other legumes, faba bean seeds contain phenolic compounds (Turco et al., 2016). The presence of condensed tannins in faba bean was confirmed in the research of Zdunczyk et al. (2018), Jin et al. (2012), Makkar et al. (1997), and Khalil et al. (1995). Antioxidant potential of faba bean was also reported using several assays, including the DPPH (Khan et al., 2015), FRAP (Zdunczyk et al., 2018), as well as DPPH assay and ORAC (Siah et al., 2014). Very high antioxidant capacity of faba bean sprouts was reported by Okumura et al. (2016).
The present study was aimed to determine the polyphenolic profiles and antioxidant properties of faba bean extract and its low-molecular-weight as well as tannin fractions.
| 2. Materials and methods | ▴Top |
2.1. Materials
Faba bean (Vicia faba) seeds of the Polis cultivar were bought from the Plant Breeding Station in Olsztyn (Poland).
2.2. Chemicals and standards
Organic solvents, trichloroacetic acid, potassium ferricyanide, and ferric chloride were purchased from the POCh Company (Gliwice, Poland). Linoleic acid, β-carotene, polyoxyethylenesorbitan monopalmitate (Tween 40), polyoxyethylenesorbitan monopalmitate (Tween 40), butylated hydroxyanisole (BHA), 6-hydroxy-2,5,7,8-tetram-ethylchroman-2-carboxylic acid (Trolox), Folin-Ciocalteu’s phenol reagent, Sephadex LH-20, 2,2′-diphenyl-1-picrylhydrazyl (DPPH), vanillin, gallic acid, p-hydroxybenzoic acid, trans-p-coumaric acid, (+)-catechin, (−)-epicatechin, and tryptophan were obtained from Sigma (Poznań, Poland). Luteolin, apigenin, kaempferol, epigallocatechin gallate, catechin gallate, and epicatechin gallate were purchased from Extrasynthese (Genay Cedex, France).
2.3. Extraction
Phenolic compounds were extracted from defatted seed samples with hexanes into 80% acetone (v/v). Condition of extractions were as follows: 3 × 30 min, 50 °C, a solid-to-solvent ratio of 1:10 (w/v) (Amarowicz and Shahidi, 2018). Extraction was carried out at 50 °C for 30 min in flasks placed in a shaking water bath (Elpan 357, Wrocław, Poland). Acetone from the combined extract was evaporated using a Büchi rotary evaporator. The sample was then freeze-dried.
2.4. Column chromatography
The separation of the low-molecular-weight phenolic and condensed tannin fractions from the crude acetonic extract was carried out using a Sephadex LH-20 column chromatography (Strumeyer and Malin, 1975). A portion of 800 mg of the extract was loaded onto the column (5 × 50 cm) packed with gel suspended in 95% (v/v) ethanol. Fraction I (low-molecular weight phenolic compounds) was eluted from the gel by using 1 L of ethanol. For elution of the Fraction II (condensed tannins) 600 mL of 50% (v/v) acetone was used. The organic solvents were then evaporated using a rotary evaporator and any remaining water from Fraction II was removed by lyophylisation.
2.5. Total phenolic compounds
The content of total phenolics in the crude extract and Fractions I and II was determined using Folin-Ciocalteou’s phenol reagent (Naczk and Shahidi, 1989). The results were expressed as (+)-catechin equivalents per g of the extract or fractions thereof.
2.6. Condensed tannins
Condensed tannins were determined using a vamillin/HCl colorimetric method (Price et al., 1978). The results obtained were reported as absorbance units at 500 nm per 1 g extract or its fractions (A500/g).
2.7. UV spectra
UV spectra of the extract and its fractions dissolved in 80% (v/v) methanol was recorded using A Beckman DU 7500 diode array spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA).
2.8. Determination of total antioxidant activity (TAA)
The total antioxidant activity was assayed using a Randox kit (Randox Laboratories Ltd., Crumlin, UK) and the analytical procedure outlined by the supplier. Results were expressed as mmol Trolox equivalents (TE)/g extract or fractions thereof.
2.9. Antioxidant activity in β-carotene-linoleate model system
The method of Miller (1970) was carried out for measuring the inhibition of the linoleic acid autoxidation in emulsion system. In this assay, 2 mg of crude extract or Fraction I, or 1 mg of Fraction II dissolved in 0.2 mL of methanol were added to 5 mL of a prepared emulsion of linoleic acid and β-carotene stabilized with Tween 40. The samples were incubated for 2 h at 50 °C and their absorbance was read at 15 min intervals.
2.10. Reducing power
Reducing power of the extracts and fraction thereof, was investigated using the method of Oyaizu (1978). The results were expressed by the increase of the sample absorbance read at 700 nm.
2.11. Antiradical activity against DPPH•
The method described by Yen and Chen (1995) was used for determination of the antiradical activity of the crude extracts and its fractions against DPPH radical. The sample was composed of 2 mL of methanol, 0.25 mL of 1 mM solution of DPPH radical, and 0.1 mL of methanolic solution containing between 0.05 and 2.5 mg of the crude extract or low-molecular-weight fraction—and between 0.1 and 0.5 mg of fraction tannin fraction. After 20 min of scavenging reaction at room temperature, the absorbance of the sample was read at 517 nm.
2.12. HPLC-DAD
For the HPLC analysis, a Water 2001 system (a quaternary pump, an autoinjector, a diode-array detector, Millenium software; Waters Corp., Milford, MA, USA) was used. Phenolic compounds were separated on a Nova-Pak C18 column (300 × 3.9 mm, 4 μm; Waters, Warsaw, Poland), using a gradient of acetonitrile in water according to Dueñas et al. (2004). All analyses of the samples were triplicated. The results were reported as mean value standard deviation.
2.13. HPLC-ESI-MS
Mass spectra were recorded using a Hewlett Packard 1100 mass selective detector (MSD, Palo Alto, CA, USA) equipped with an atmospheric pressure chemical ionization (APCI) source and an electrospray ionization (ESI) interface. Mass spectra were recorded in the negative-ion mode using a variable fragmentation voltage of 100 and 250 V for m/z 100–1,000 and 1,000–2,500, respectively (Dueñas et al., 2004).
2.14. Identification and quantification of phenolic compounds
The UV spectra and MS data of the compounds were compared with standards. Some un-known compounds with UV spectra similar, to those of flavonoids and gallates, were identified as their derivatives. Quantitative analysis was made by using an external standard method based on calibration curves for each compound; apigenin, luteolin, and kaempferol were used as standards for flavonoids. The contents of procyanidins were expressed as (+)-catechin equivalents.
| 3. Results and discussion | ▴Top |
3.1. Content of total phenolics and condensed tannins
Fraction II obtained using a Sephadex LH-20 column was characterized by the content of total phenolics compounds (285 mg/g and 116 A500/g) condensed tannins (116 A500/g ) was much higher than that was determined in the crude extract and in Fraction I (Table 1). The reason for the relatively low content of total phenolic in fraction I is the presence of sugars in this fraction. The solvent system used in this research was able to also extract sugars/oligosaccharides from faba bean seeds.
 Click to view | Table 1. Total phenolics, tannins content, total antioxidant activity in faba bean extract and its fractions and UV spectral data |
The results obtained in this research show that faba bean is a rich source of phenolic compounds as well as condensed tannins. A lower content of total phenolic compounds was previously reported for extracts of pea (Amarowicz and Troszyńska, 2003), white bean (Orak et al., 2016), red bean (Orak et al., 2015), broad bean (Amarowicz and Shahidi, 2017; Boudjou et. al., 2013).
The high content of condensed tannins in the fractions eluted with the mobile phase of acetone-water (1:1; v/v) from the column packed with Sephadex LH-20 gel was reported before for adzuki bean, broad bean, pea, lentil (Amarowicz et al., 2010, 2009, 2008; Amarowicz and Troszyńska, 2003). The presence of condensed tannins in faba bean seeds determined by vanillin method was reported by Amarowicz et al. (2004) and Baginsky et al. (2013). According to Luo et al. (2015), content of condensed tannins in faba bean extracts ranged from 0.9 to 1.9 g of gallic acid equivalents/100 g extract. In this research the authors precipitated tannins by using polypyrrolidone (PVPP) and used Folin-Ciocalteu phenol reagent for determination of phenolic compounds.
Low values of absorbance obtained for fraction after colour reaction with vanillin (Table 1) were caused by the presence catechins (flavan-3-ols) in this fraction which agrees with the results of HPLC-DAD-MS analysis (Table 2).
 Click to view | Table 2. Phenolic compounds identified by HPLC-DAD and HPLC-ESI-MS in a faba bean acetonic extract |
3.2. UV spectra
The UV spectra recorded for the crude extract of faba bean, and its low-molecular-weight and tannin fractions exhibited maxima at the same wavelength of 276 nm (Figure 1). The same wavelengths was obtained for the UV spectra of extract of broad bean crude extract and its two fractions (Amarowicz and Shahidi, 2017). The UV spectra depicted in Fig. 1 are agreement with the results from HPLC-DAD analysis reported in Table 2 where several separated phenolic compounds showed UV spectra with a maximum close to 276 nm.
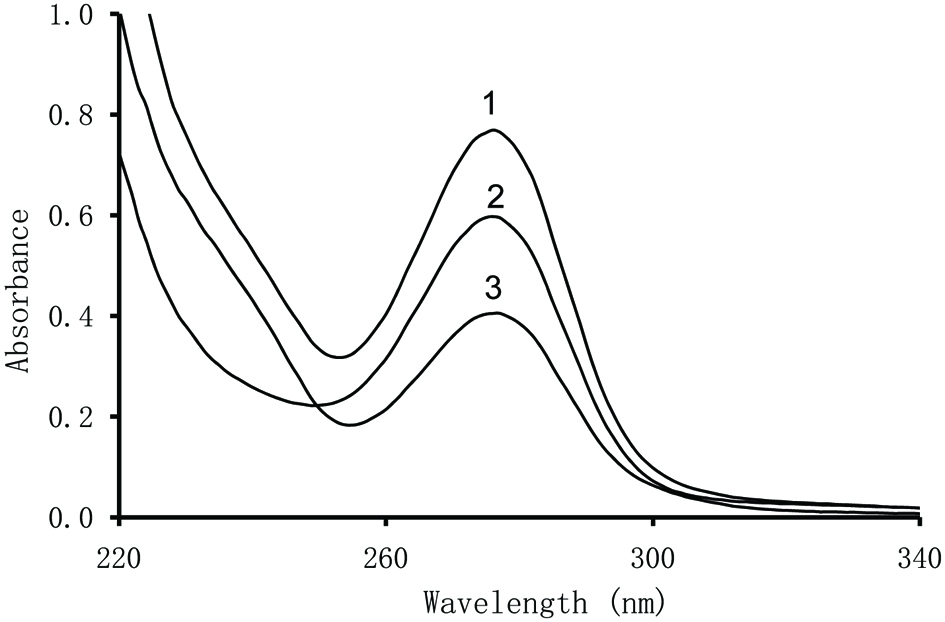 Click for large image | Figure 1. UV spectra of a faba bean acetonic extract and its Fractions. 1, crude extract; 2, Fraction I; and 3, Fraction II). |
3.3. Antioxidant activity
The total antioxidant activity (TAA) of the Fractions II (4.21 mmol TE/g) ) was several times higher than that of crude extract (0.88 mmol TE/g) and Fraction I (0.66 mmol TE/g). The same relation between TAA of the crude extracts and its two fractions were previously reported for adzuki bean (Amarowicz et al., 2008), red lentil (Amarowicz et al., 2009), red lentil (Amarowicz et al., 2010), and broad bean (Amarowicz and Shahidi, 2017). The ability to scavenge ABTS radical cation by phenolic compounds of legume seeds was reported before by several authors (Orak et al., 2015, 2016; Alshikh et al., 2015; Ramírez-Jiménez et al.,2014; Huber et al., 2014). In relation to cited authors, the results of the faba bean obtained in this study are considered to be high.
The crude extract of phenolic compounds of faba beam and its two fractions exhibited antioxidant activity in a β-carotene-linoleate model system (Figure 2). The effect of extract on the coupled oxidation of linoleic acid and β-carotene was the highest. However, the amounts of tannins fraction added to the emulsion system was a half of that of the crude extract and Fraction I. The results obtained are important because they prove the fact that condensed tannins also exhibited their antioxidant activity in the emulsion system. Our previous studies in a β-carotene-linoleate model system found lower antioxidant activity for extracts of leguminous seeds such as broad bean, lentil, adzuki bean, and effectively (Amarowicz and Shahidi, 2017; Amarowicz et al., 2010, 2009, 2008).
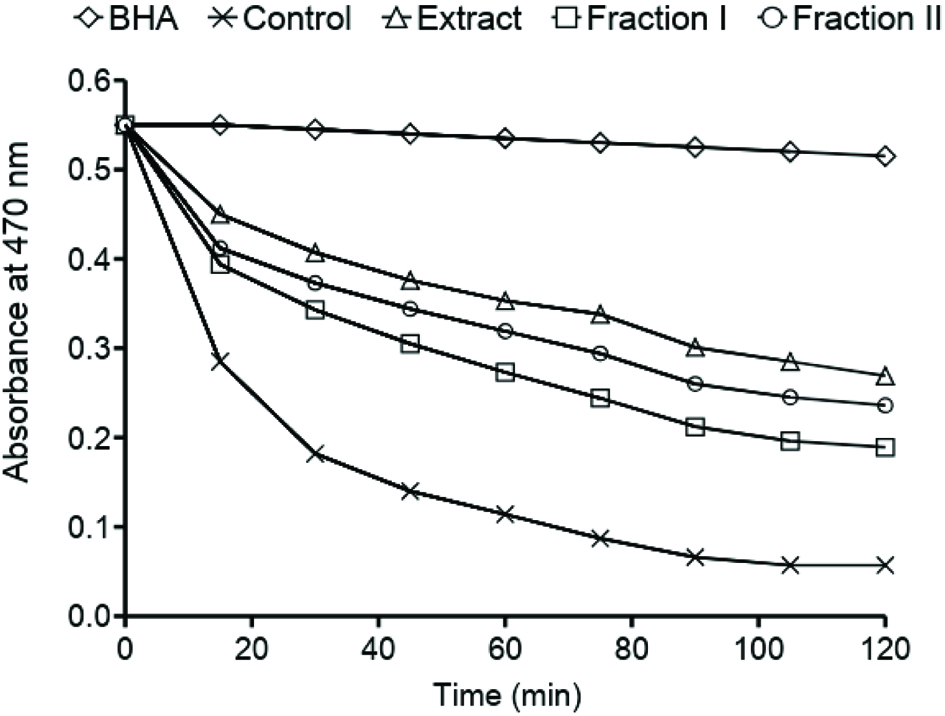 Click for large image | Figure 2. Antioxidant activity of a faba bean acetonic extract and its fractions in a β-carotene-linoleate model system. |
Figure 3 depicts the effect of phenolic compounds present in the extract of faba bean and its two fractions (Fraction I and II) on the formation of Fe2+ from Fe3+/ferricyanide. This effect was monitored by the measuring optical density at 700 nm. The tannin fraction exhibited much stronger reducing activity than those of the crude extract and Fraction I. The strong reducing power obtained for tannins was reported before for peas (Amarowicz and Troszyńska, 2003). However, tannin fractions isolated by Sephadex LH-20 from broad bean. as well as green and red lentils, and adzuki bean, reduced Fe3+ more effectively (Amarowicz and Shahidi, 2017; Amarowicz et al., 2010, 2009, 2008). The ability of phenolic compounds of lentil, common bean, chickpea, yellow pea, green pea, and broad bean for reducing Fe3+ was also assayed by other authors using the FRAP method (Alshikh et al., 2015; Xu and Chang, 2007; Heimler at al., 2005; Ou et al., 2002).
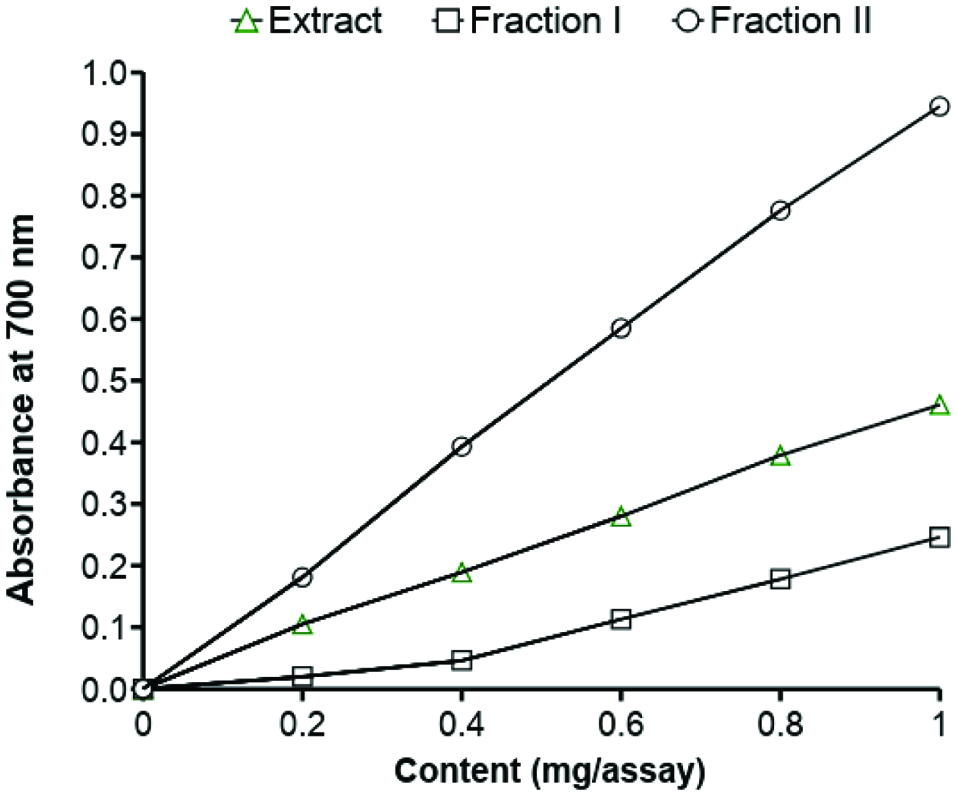 Click for large image | Figure 3. Reducing power of a faba bean acetonic extract and its fractions. |
Very strong antiradical activity against DPPH• was observed for tannin fraction separated from faba bean crude extract (Figure 4). The low-molecular-weight phenolic fraction (Fraction I) was a weak scavengers of the DPPH radical. Antiradical activity of phenolic compounds present in legume seeds extracts was previously confirmed in several studies (Ombra et al., 2016; Veggi et al., 2014; Xu and Chung, 2007; Ranilla et al., 2007). The strong antiradical activity of the condensed tannins separated from plant materials such as canola hulls, beach pea, and evening primrose a were reported by Amarowicz et al. (2000). Radical scavenging activity of condensed tannins as determine by the DPPH assay was described by Muir (1996).
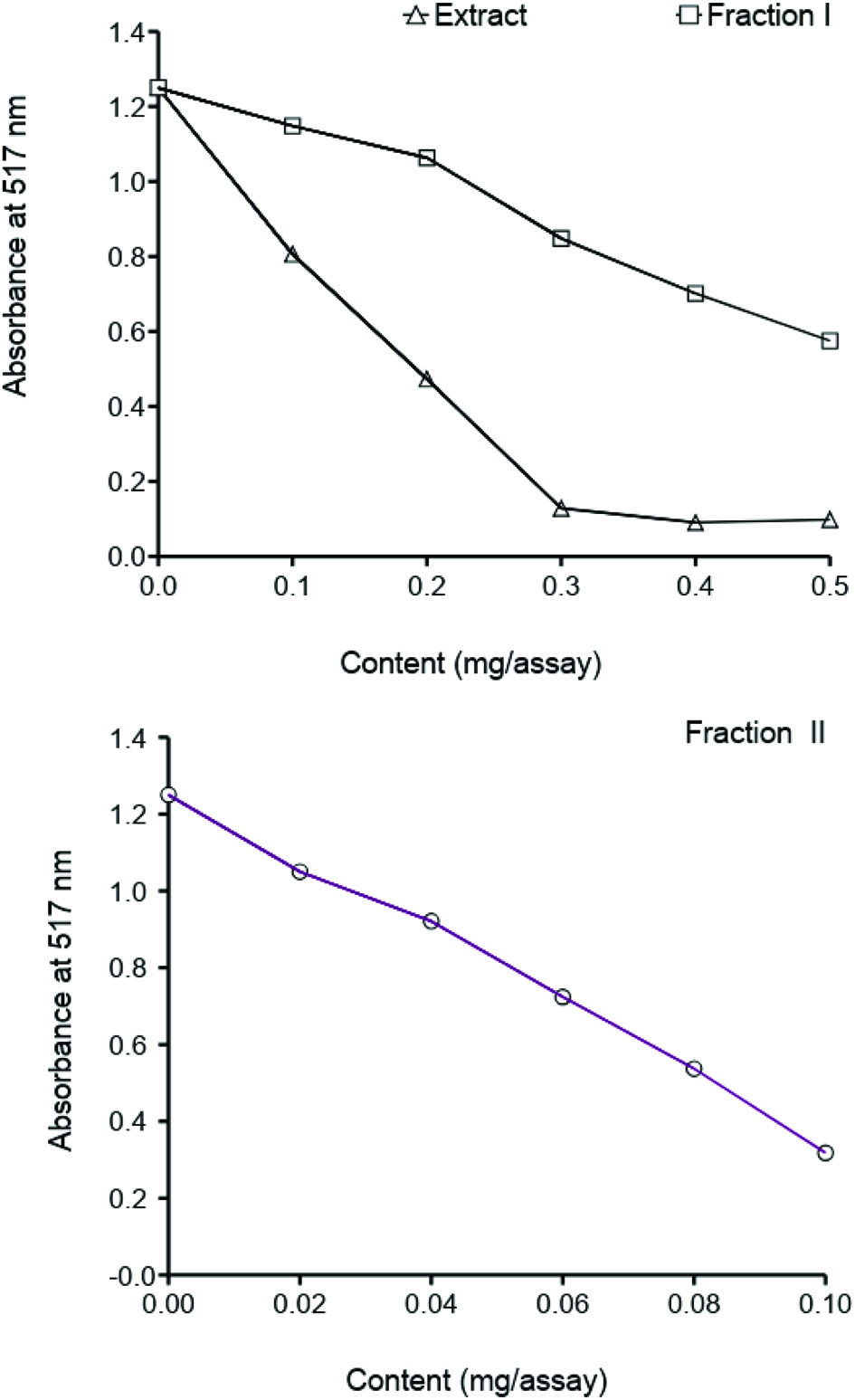 Click for large image | Figure 4. Scavenging effect of a faba bean acetonic extract and its fractions on DPPH radical. |
3.4. Content of individual phenolic compounds
Figure 5 shows an HPLC chromatogram of a faba bean acetonic extract. The results of HPLC-DAD and HPLC-ESI-MS are given in Table 2. Based on the retention times and UV spectra of commercial standards, the presence of p-hydroxybenzoic aldehyde, gallic, protocatechuic, p-hydroxybenzoic, trans-p-coumaric acids, (−)-epicatechin, catechin gallate, epicatechin gallate, epigallocatechin gallate, vanillin, and tryptophan in the crude extract of broad bean was confirmed.
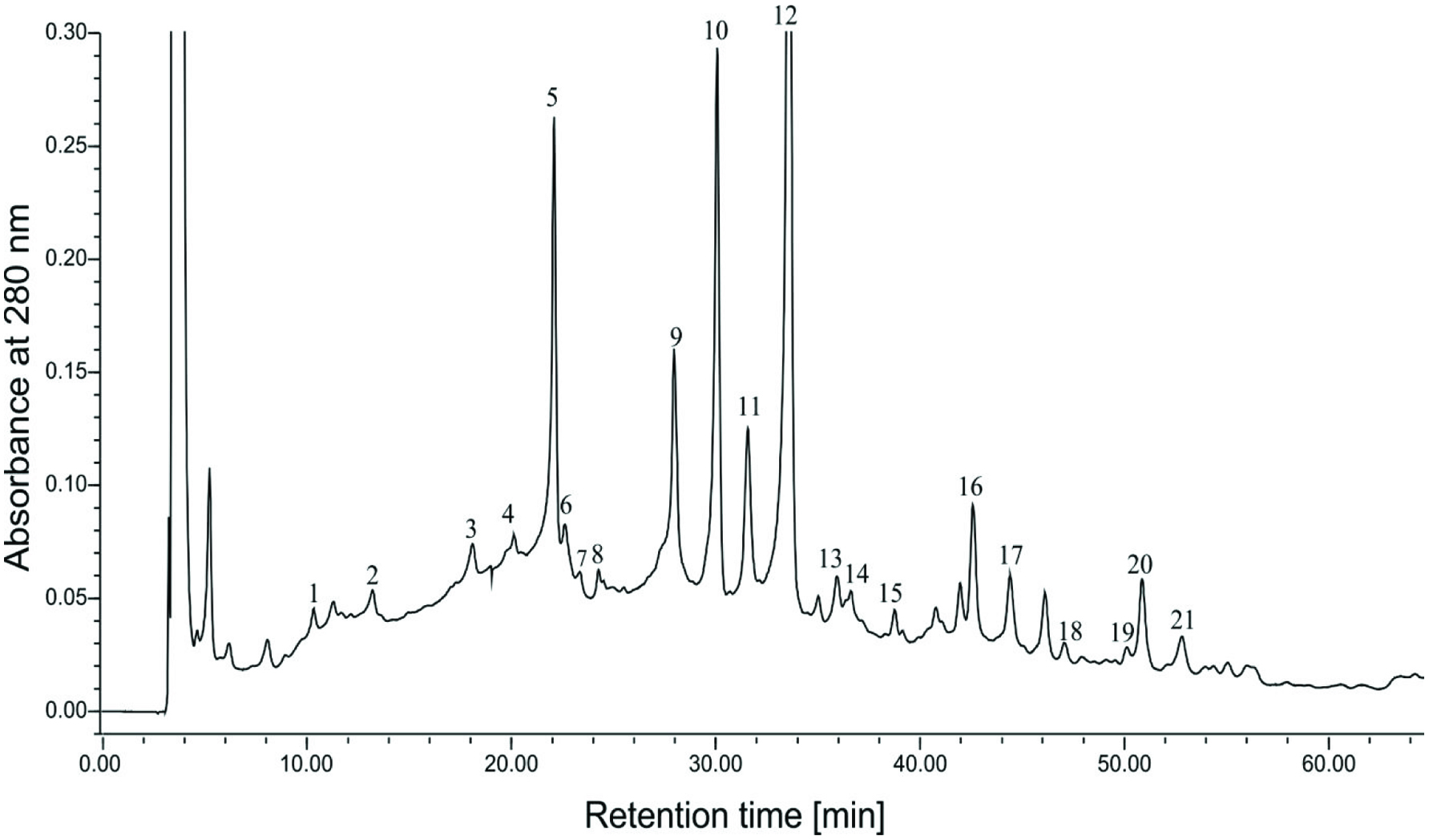 Click for large image | Figure 5. HPLC chromatogram of a faba bean acetonic extract. |
Compound 5 showed a negative molecular ion [M–H]− at m/z of 745 and two fragment ions [F–H]− at m/z of 577 and 169, corresponding to a procyanidin dimer and gallic acid, respectively. This compound was identified as gallate procyanidin dimer. Compounds 9, 10, 17 and 20 with a maximum at 278 nm according to fragment ions [F–H]− at m/z of 289 and 169 corresponding to epicatechin or catechin and gallic acid, respectively, were identified as procyanidin gallates. Compounds 8 and 19 with a negative molecular ion [M–H]− at m/z of 447 and compounds 18 with a negative molecular ion [M–H]− at m/z of 442 were identified as kaempferol and apigenin hexoses, respectively. Based on the UV and MS spectra compounds 16 and 21 were identified as acetylated luteoliv and acetylated kaempferol hexsoses.
Gallate procyanidin dimer, gallate procyanidins, and acetylated kaempferol hexose were the major phenolics present in the extract of faba bean (Table 2). The presence of catechins is very typical for legume seeds (Yeo, J., and Shahidi, 2017. 2015; Alshikhet et al., 2015; Limón et al. 2015; Dueñas et al., 2015). The presence of caffeic, p-coumric, ferulic, and sinapic acids was confirmed by Yao et al (2011) in such legumes as lima bean, broad bean, coman bean, pea, jack bean, adzuki bean, rise bean, mung bean. Gallate procyanidin dimer, gallate procyanidins were previously reported in the extracts of broad bean and lentils (Amarowicz and Shahidi, 2017; Amarowicz et al., 2010, 2009). Procyanidin in faba bean were also determined in faba bean by Abu-Reidah et al. (2014) using UHPLC-ESI-QTOF-MS method.
| 4. Conclusion | ▴Top |
Faba bean is a potentially valuable legume crops with high antioxidant potential. The tannin fraction separated from the crude extracts using a Sephadex LH-20 column chromatography displayed a stronger antioxidants activity than the low-molecular-weight phenolic fraction. Faba bean seeds may serve as an important can be a valuable component in the diet of the vegetarian and the general populations.
| References | ▴Top |