| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 19, September 2022, pages 136-142
Synthetic pathways of sinensetin and derivatives as an alternate source in biological activity study
Xiaoqi Wanga, b, Jianfeng Zhana, Chi-Tang Hob, Shiming Lia, c, *
aCollege of Life Science, Huanggang Normal University, Huanggang 438000, Hubei, China
bDepartment of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
cMisabel Group, Shijiazhuang 051134, Hebei, China
*Corresponding author: Shiming Li, College of Life Science, Huanggang Normal University, Huanggang 438000, Hubei, China. E-mail: shiming@rutgers.edu
DOI: 10.31665/JFB.2022.18319
Received: September 16, 2022
Revised received & accepted: September 29, 2022
| Abstract | ▴Top |
Sinensetin is one of the polymethoxyflavones existed in citrus peels. The effective antioxidant, inhibitory effect of inflammation, anti-diabetic and anti-cancer activity of sinensetin has been revealed recently. However, the amount of sinensetin isolated from the nature source is extremely low relating to the needs for its in vivo and efficacy study. Herein we summarized the alternative routes, i.e. the organic synthesis of sinensetin and its associated derivatives to provide a complete but concise reference in the preparation of this class of compounds as an effective source for in-depth study of sinensetin and related molecules.
Keywords: Sinensetin; Sinensetin derivatives; Synthesis; Polymethoxyflavones; Metabolites
| 1. Introduction | ▴Top |
Polymethoxyflavones (PMFs) exist almost exclusively in citrus peels. The number and location of methoxyl groups on the skeleton of C6-C3-C6 benzo-γ-pyrone render poly-structures of PMFs, among which nobiletin and tangeretin are often found as the two major ones (Wang et al., 2018). With high structural similarity to nobiletin and tangeretin, sinensetin (5,6,7,3′,4′-pentamethoxyflavone or 2-(3,4-dimethoxyphenyl)-5,6,7-trimethoxy-4H-1-benzopyran-4-one) (Figure 1a) is another PMF that has drew researchers’ increased attention recently. As illustrated in Figure 1b, the number of publications has been steadfastly increasing for the past four decades starting from 1985.
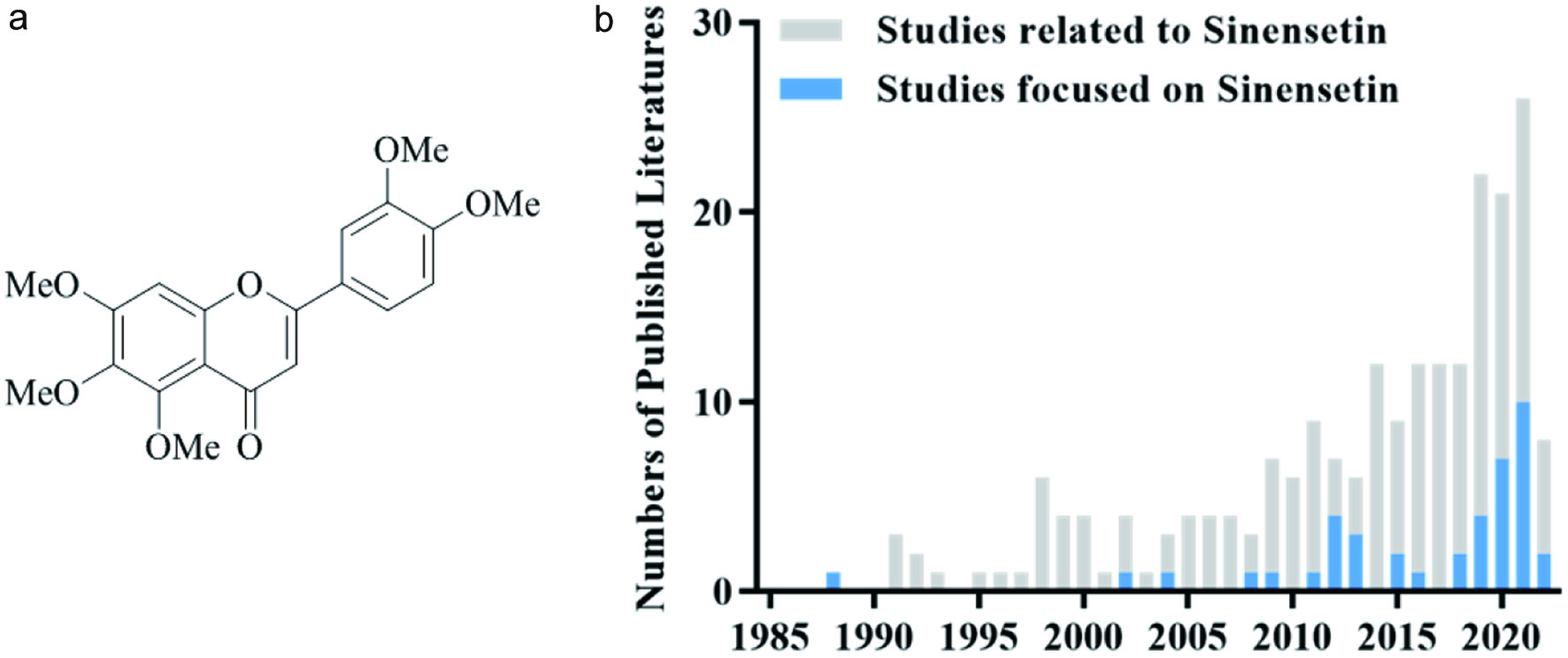 Click for large image | Figure 1. (a) Chemical structure of sinensetin and (b) number of annually published papers related to or focused on sinensetin since 1985 from the Web of Science (last search in June 2022). |
The content of sinensetin in the peels of some citrus cultivars is listed in Table 1, from which we can see that there is a large variation of the content among citrus peels, from 13 ppm in lemon to over 4,200 ppm in tangerine and sweet orange peels (Table 1). The differentiation of sinensetin amount in different citrus peels is attributed mostly to cultivars and next to climate and conditions such as soil and water system among other environmental factors. For instance, the concentration of sinensetin in the peels of Citrus aurantium L. grew in diverse regions could have a big difference (Green et al., 2007; Park et al., 2014). It seems a general trend that higher temperature could stimulate the accumulation of sinensetin in citrus peels, postulated to defend flourishing insects in tropical climate. Plethora of evidence has revealed that the content of secondary metabolites will increase dramatically when a plant is under stress, such as diseases and harsh weather conditions. In citrus genus, the content of citrus flavonoids would be dramatically elevated when citrus plant encounters Phytophthora citrophthora (Ortuno et al., 2002). As a specific example, the level of sinensetin in Citrus sinensis could increase by 26% when disease infection occurs. In addition, as shown in Table 1, three cultivars of Citrus sinensis, ‘Valencia’, ‘Parson Brown’ and ‘Navel’ all showed consistently greater extracted concentrations of sinensetin (Green et al., 2007), indicating the Citrus sinensis may generate more abundant sinensetin than other citrus species.
 Click to view | Table 1. Sinensetin content in the peels of different citrus cultivars |
Sinensetin possesses multiple biological activities and health promoting properties. It has been demonstrated that sinensetin has efficient inhibitory activity against inflammation on LPS-induced murine J774 macrophage cells (Laavola et al., 2012), LPS-stimulated RAW 264.7 cells (Shin et al., 2012) and virus-triggered human type ? alveolar epithelial cells (A549 cells) (Li et al., 2020) via regulating various cytokines as well as other inflammatory and proinflammatory mediators. Sinensetin at a dose of 50 mg/kg also exhibited significant ameliorating effect on the carrageenan-induced paw inflammation in mice (Laavola et al., 2012), which identified the in vivo impact of sinensetin on acute inflammation at the early stage. Additionally, the alleviating role of sinensetin has been evaluated on colitis (Xiong et al., 2019) and results indicated that in both DSS- and TNBS-induced colitis models, sinensetin (20−120 mg/kg.bw dosage) could dramatically restore the impaired epithelial TJ barrier functions via modulating the apoptosis and autophagy crosstalk, and inhibiting claudin-2 expression. The anti-obesity effect of sinensetin has been revealed that it could activate fatty acid β-oxidation, facilitate lipolysis, reduce insulin-stimulated glucose uptake and suppress lipogenesis (Kang et al., 2013; Kang et al., 2015). One of the mechanisms of sinensetin on the preventive effect of type 2 diabetes has been revealed as the potent inhibition of α-glucosidase (32.0−89.0% inhibition at concentration of 0.31−2.50 mg/mL) and α-amylase (85.8% inhibition at concentration of 2.5 mg/mL) (Mohamed et al., 2012), probably through spontaneous binding driven by hydrophobic interactions and the subsequent changes of enzyme conformation (Liu et al., 2021). In addition, sinensetin demonstrated anti-glycation effects by suppressing the formation of advanced glycation end products and oxidation levels (∼50% inhibition at concentration at 0.15 mg/mL) (Liu et al., 2021). Sinensetin also demonstrated effective anti-cancer activity via different mechanisms such as cell apoptosis and autophagy in human T-cell lymphoma Jurkat cells (Tan et al., 2019) and HepG2 cells (Samidurai et al., 2020; Kim et al., 2021). Other biological effects of sinensetin include antiangiogenesis (Lam et al., 2012; Li et al., 2022), chemosensitizing (Choi et al., 2002), anti-pulmonary fibrosis (Wan et al., 2022), anti-osteoarthritis (Liu et al., 2022) and age-related sarcopenia-preventing potencies (Kim et al., 2019) among others. However, further efficacy studies of sinensetin are warranted to comprehend the natural bioactive profiles of sinensetin.
Owing to the ultra low sinensetin availability from natural sources such as citrus peels or other plants, large amount of sinensetin is required to the in-depth in vivo study and human clinical trials. In addition, metabolites of sinensetin need to be identified and characterized, but these metabolites are not commercially available. Isolation and identification from in vivo metabolism study often yields minute amount of metabolites and is impossible to acquire accurate structural information of sinensetin metabolites. Therefore, the synthesis of sinensetin and its metabolites becomes an important tool to perform in-depth in vivo biological study of sinensetin. In this review, we have summarized the recent progress on the synthesis of sinensetin and its potential metabolites and other derivatives.
| 2. Synthetic pathways of sinensetin and its derivatives | ▴Top |
Sinensetin can be isolated from citrus peels with limited quantity that is usually insufficient to conduct in vivo study. Moreover, certain amount of metabolites and other derivatives of sinensetin are also needed for pharmarcokinetic/pharmacodynamic study and further effective evaluation of their biological property. Hence, the alternative route to acquire sinensetin and its derivatives is through organic synthesis.
2.1. Synthesis of sinensetin
According to previous literatures, the synthesis of sinensetin could majorly refer to two studies with different synthetic pathways as illustrated in Figure 2 (Hossain and Ismail, 2004; Wei et al., 2013). In the synthesis, the key reaction is the aldol condensation between an acetophenone and a benzaldehyde. The major differences are the building blocks to start the synthetic scheme. As illustrated in Figure 2a, Hossain and Ismail (2004) started the synthesis with 2,3,4,6-tetrahydroxyacetophenone (1), protected the hydroxyl groups with methoxymethyl (MOM) ether to yield 2, then followed by aldol reaction with MOM-protected benzaldehyde (4) to form a fully MOM protected chalcone (5). Upon removal of five MOM groups, the polyhydroxychalcone (6) underwent simultaneous cyclization and oxidation to yield 7, followed by methylation to finish the sinensetin (8) synthesis. Wei et al. (2013) employed the conventional route as demonstrated in Figure 2b to prepare 6-hydroxy-2,3,4-trimethoxyacetophenone (10) from the acetylation of 3,4,5-trimethoxyphenol (9) followed by an aldol condensation with 3,4-dimethoxybenzaldehyde (11). The latter route is more straightforward assuming the commercial availability of 3,4-dimethoxybenzaldehyde and 3,4,5-trimethoxyphenol. The synthetic route in Figure 2a has more flexibility for the starting agents and for obtaining intermediate 5,6,7,3′,4′-pentahydroxylated flavanone and flavones. In summary, these two synthetic routes could be readily adapted in the preparation of other PMFs or perhydroxylated flavonoid intermediates.
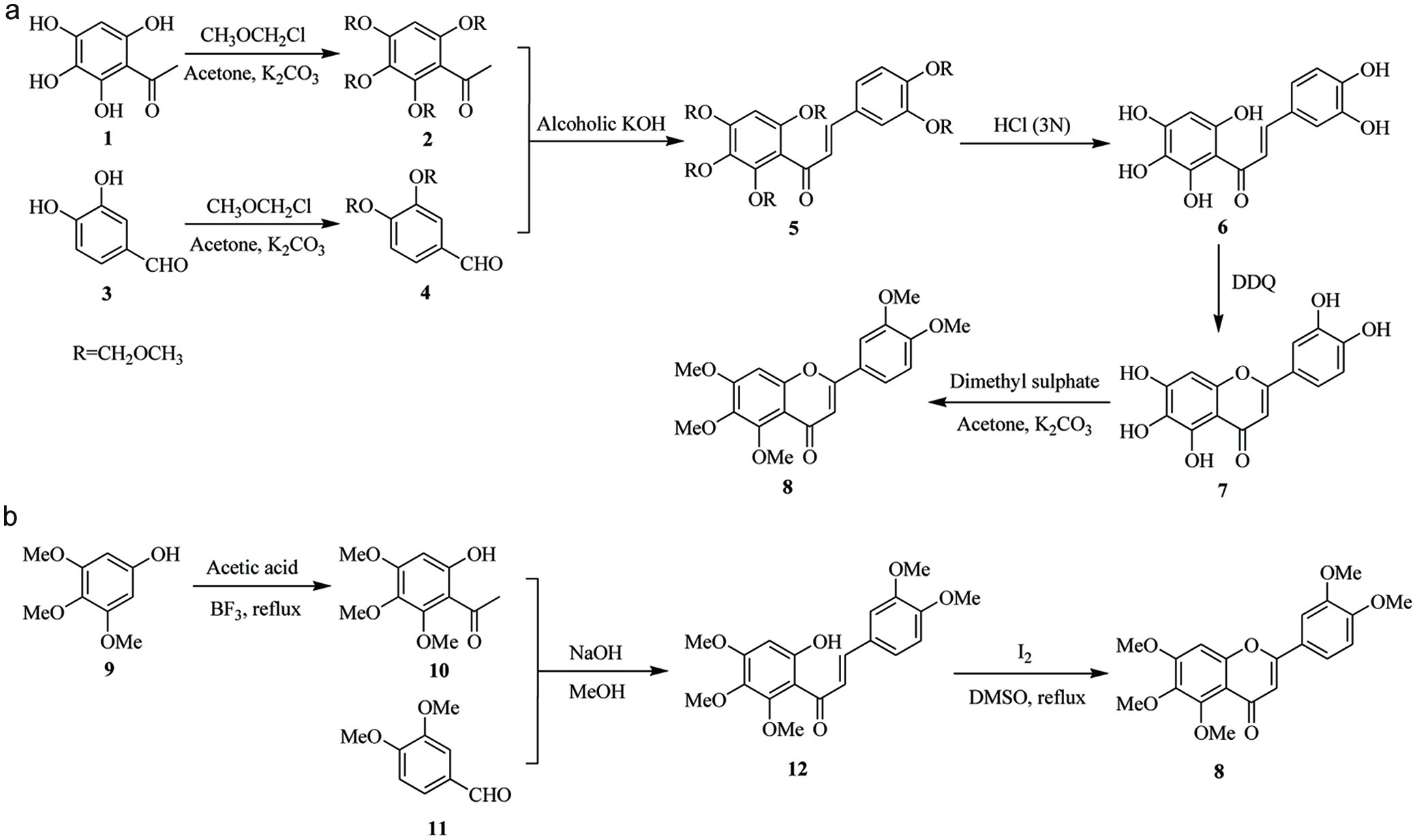 Click for large image | Figure 2. Synthetic pathways of sinensetin used by (a) Hossain and Ismail (2004) and (b) Wei et al. (2013). |
2.2. Synthesis of 5-demethylsinensetin
Due to the relative labile nature of the 5-methyl groups on the PMFs, 5-demethylsinensetin is usually prepared in a one-step reaction. Treatment of sinensetin with a strong acid or Lewis acid, 5-demethylsinensetin (13) can be synthesized in high yield (Figure 3). This synthetic scheme applies to prepare most 5-demethylated PMFs (Li et al., 2019).
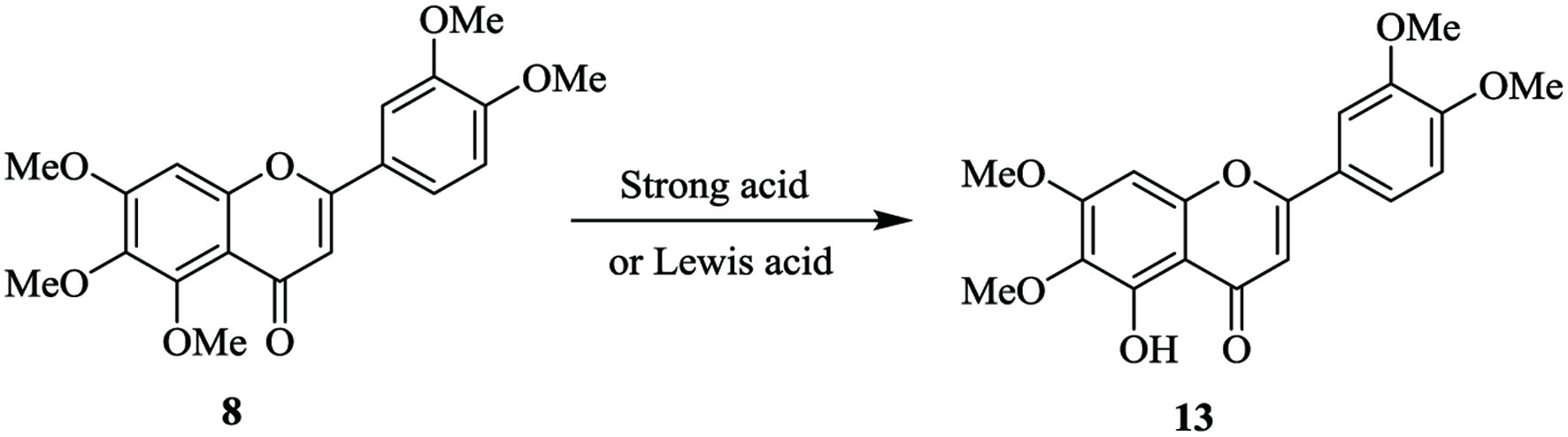 Click for large image | Figure 3. One-step synthesis of 5-demethylsinensetin. |
2.3. Synthesis of 3′-demethylsinensetin, 4′-demethylsinensetin and 3′,4′-didemethylsinensetin
As illustrated in Figure 4, the synthetic pathways of B-ring demethylated sinensetin, i.e. 3′-demethylsinensetin (16a), 4′-demethylsinensetin (16b) and potential 3′,4′-didemethylsinensetin (16c) was nearly the same except using different benzaldehydes without (Wang et al., 2021) or with a benzyl protected group at 3- or 4-postion, or 3,4-dibenzyl protection (Li et al., 2006; Li et al., 2007).
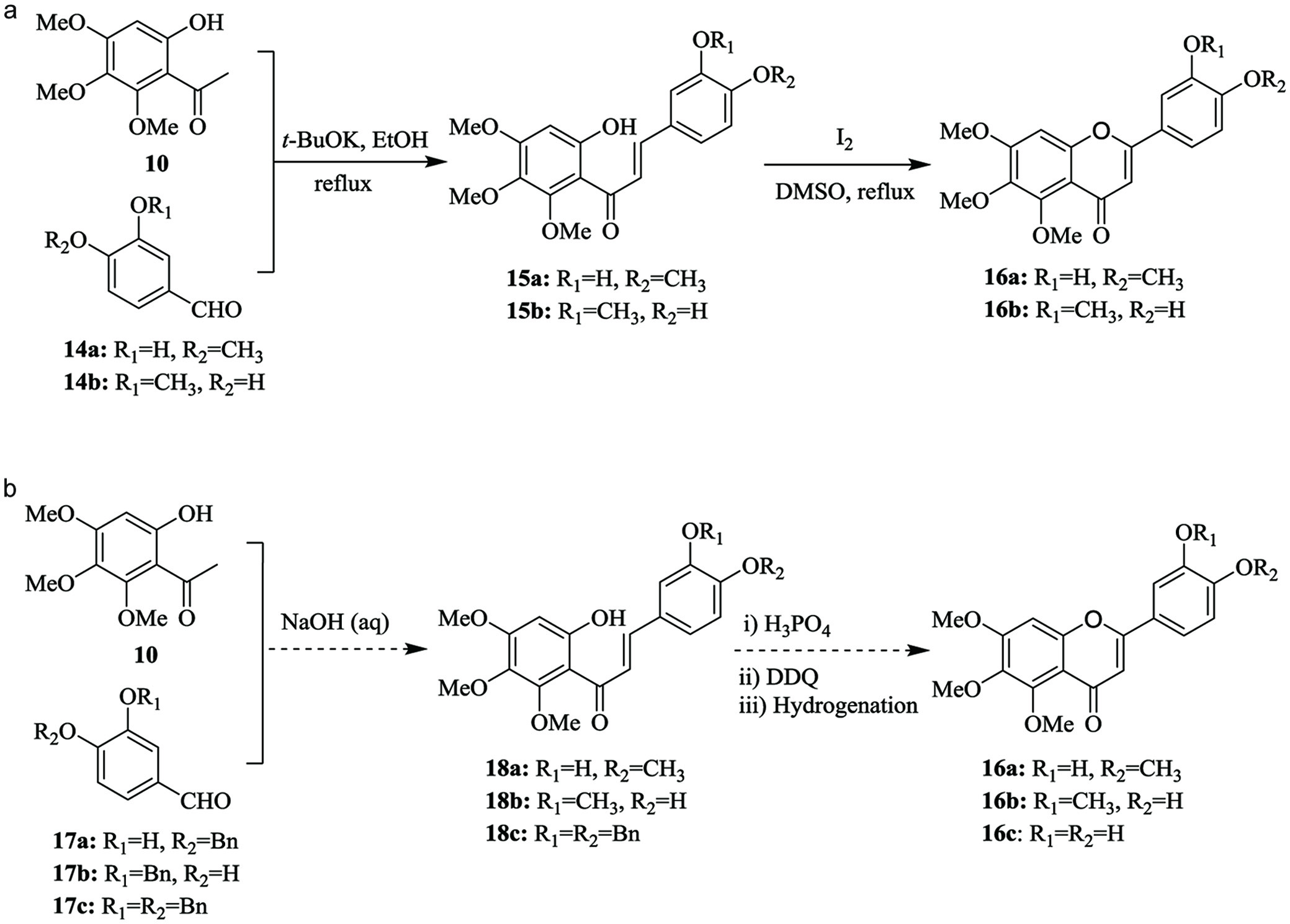 Click for large image | Figure 4. (a) Synthetic pathways of 3′-demethylsinensetin and 4′-demethylsinensetin by Wang et al. (2021) and (b) potential alternate routes to 16a, 16b and 3′,4′-didemethylsinensetin 16c (Li et al., 2006; Li et al., 2007). |
In the 2-step synthetic scheme (Figure 4a) (Wang et al., 2021), a direct condensation between 6-hydroxy-2,3,4-trimethoxyacetophenone (10) and 3-hydroxy-4-methoxybenzylaldehyde (14a) or 4-hydroxy-3-methoxybenzaldehyde (14b) generated a chalcone (15a or 15b), followed by simultaneous cyclization and oxidation to yield 3′-demethylsinensetin (16a) or 4′-demethylsinensetin (16b), which is straightforward and convenient.
The 4-step synthetic scheme (Figure 4b) of the preparation of other B-ring demethylated PMFs (Li et al., 2007) is similar to the 2-step preparation of sinensetin (Figure 2b) with an additional debenzylation step. This scheme can be also readily adapted and applied to prepare B-ring demethylated derivatives of sinensetin. The stepwise synthetic details are described as follows. The aldol condensation reaction between 6-hydroxy-2,3,4-trimethoxyacetophenone (10) and a benzyl protected benzaldehyde, 3-benzyloxy-4-methoxybenzaldehyde (17a), 4-benzyloxy-3-methoxybenzaldehyde (17b), or 3,4-dibenzyloxybenzaldehyde (17c) was conducted in a basic solution to yield a chalcone (18a, 18b, or 18c), which was underwent a cyclization and oxidation reaction to obtain a flavone. Removal of the benzyl group with palladium catalyzed hydrogenation yielded B-ring demethylated sinensetin (16a, 16b, or 16c) and finished the synthesis.
Starting reagent, 6-hydroxy-2,3,4-trimethoxyacetophenone (10) could be commercially available or made by the acetylation of 3,4,5-trimethoxyphenol (9) with an acetyl agent, such as acetic acid, acetyl chloride, acetic anhydride or similar ones. The condition to finish the acetylation is usually under the catalysis of a Lewis acid such as boron trifluoride diethyl etherate and under inert atmosphere. This step avoided using expensive and limited resources of PMFs as a starting material, unlike the previous preparation of nobiletin derivatives, in which isolated nobiletin and tangeretin were used to prepare 6-hydroxy-2,3,4,5-tetramethoxyacetophenone as the start chemical agent (Li et al., 2006; Li et al., 2007). The preparation of 6-hydroxy-2,3,4-trimethoxyacetophenone (10) has reconfirmed the need and necessity to synthesize 6-hydroxy-2,3,4,5-tetramethoxyacetophenone in replacing the ring fission of nobiletin and tangeretin to make the derivatives of nobiletin and tangeretin among others.
2.4. Synthesis of 6-D3-sinensetin
6-D3-Sinensetin (23) was also synthesized as a replacement of sinensetin in identifying sinensetin metabolites (Wei et al., 2013). In Figure 5, acetylation of 3,5-dimethoxy-4-hydroxyphenol (19) yielded 3,6-dihydroxy-2,4,-dimethoxyacetophenone (20), which was then methylated with deuterated methyl iodide to produce 3-D3-6-hydroxy-2,3,4,-trimethoxyacetophenone (21). Aldol condensation between 21 and 3,4-dimethoxybenzaldehyde (11) synthesized a deuterated chalcone (22), which further underwent cyclization and oxidation to yield 6-D3-sinensetin (Wei et al., 2013).
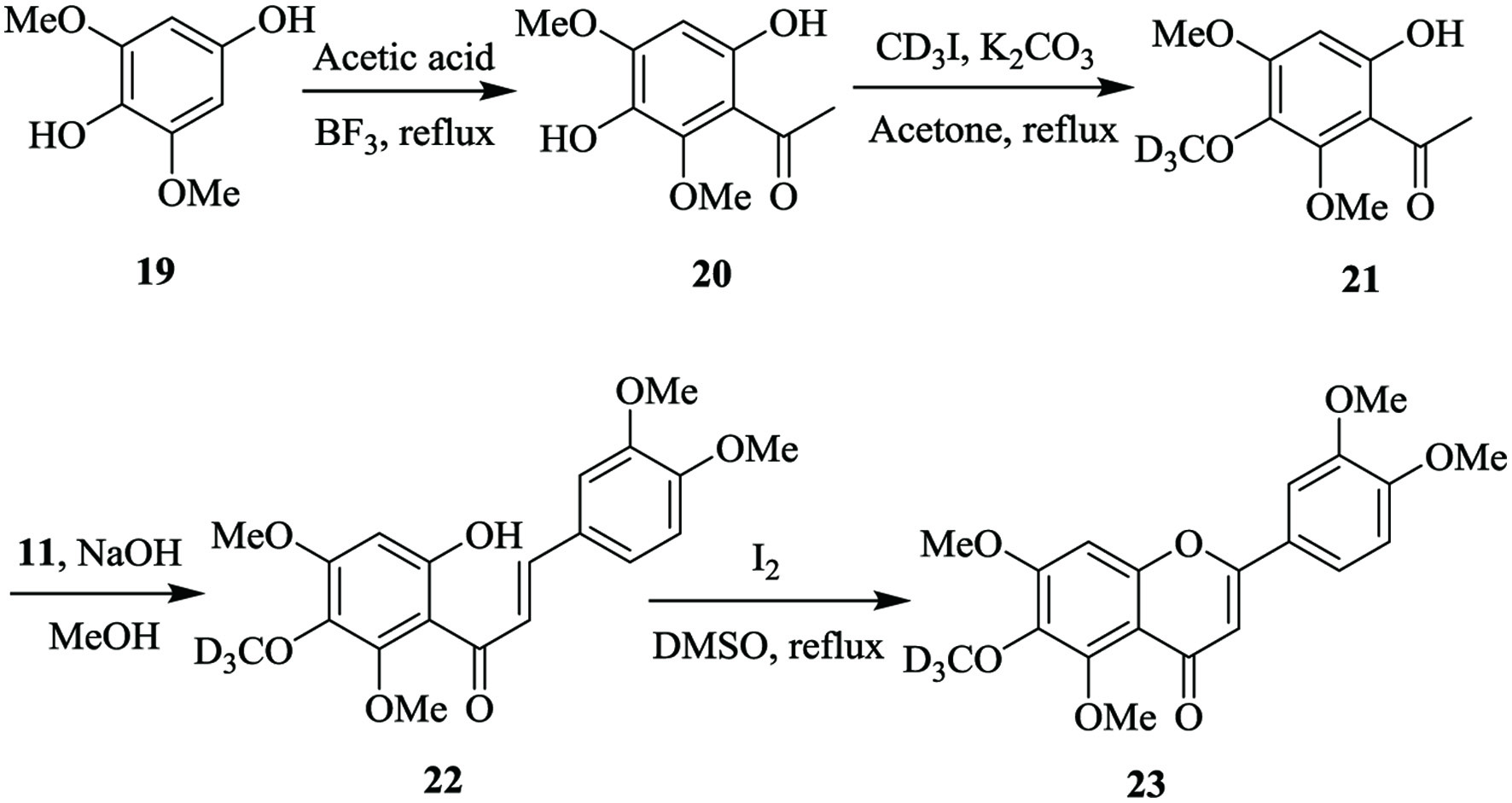 Click for large image | Figure 5. The synthetic pathway of 6-D3-sinensetin (Wei et al., 2013). |
2.5. Synthesis of 5-demethylsinensetin derivatives
5-Demethylated PMFs have been demonstrated to have more potent anti-cancer and anti-inflammatory activities than their corresponding PMFs (Li et al., 2007). Therefore, in the characterization of 5-demethylsinsensetin metabolites, the chemical synthesis of potential 5-demethylsinensetin metabolites, such as 5,3′-didemethylsinensetin (24a, eupatorin), 5,4′-didemethylsinensetin (24b, cirsilineol) and 5,3′,4′-tridemethylsinensetin (24c, cirsiliol) was the direct one-step 5-demethylation (Figure 6) from the prepared B-ring demethylated sinensetin (16a, 16b or 16c) illustrated in Figure 4. In detail, 5-demethylation of 3′-demethylsinensetin (16a), 4′-demethylsinensetin (16b) and 3′,4′-didemethylsinensetin (16c) yielded eupatorin (24a), cirsilineol (24b) (Wang et al., 2022) and cirsiliol (24c), respectively. Previously, benzyl ethers were employed for the protection of hydroxy group at the C-4′ position, but it complicated cirsilineol synthesis (Matsuura et al., 1973). In addition, the synthesis of eupatorin through Baker-Venkataraman rearrangement and alkali-ring isomerization was also suggested to be lack of universality (Farkas et al., 1969; Chen and Uong, 1972). Thus, 5-demethylsinensetin and its metabolites were synthesized through a single-step, i.e. hydrochloric acid hydrolysis reaction (Wang et al., 2022). Overall, the syntheses of B ring demethylated derivatives of 5-demethylsinensetin were achieved with one step reaction in high yields, which could be also introduced to the similar derivatization of 5-demethylated PMFs.
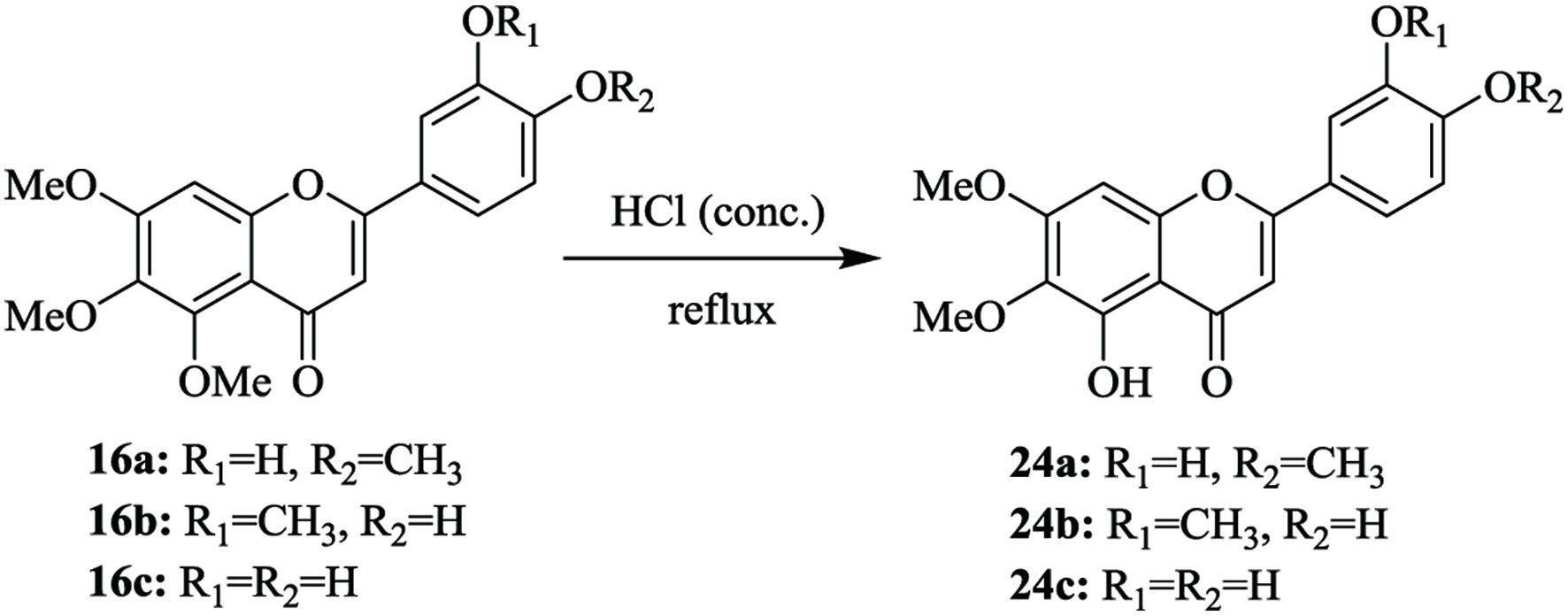 Click for large image | Figure 6. Synthetic pathways of 5,3′-didemethylsinensetin (24a), 5,4′-didemethylsinensetin (24b) and 5,3′,4′-tridemethylsinensetin (24c). |
| 3. Concluding remarks | ▴Top |
In summary, sinensetin is a bioactive component from citrus peels and other plants with several potent biological activities such as antioxidant, inhibitory effect of inflammation and anticancer among others. However, due to the limited availability of sinensetin and its potential metabolites, synthetic routes have been explored recently. This review provides a summary of the synthetic schemes and concepts of the preparation of sinensetin and its biofunctional derivatives.
Acknowledgments
Authors wish to thanks for the following funding source: 1) Advanced Education Programme from Huanggang Normal University, Hubei Province, China (Grant number 202108504) & The Department of Science & Technology, Hebei Province for the Grant of International Collaboration for Model Enterprises of Misabel Group, Shijiazhuang, Hebei (Grant number #24).
| References | ▴Top |