| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 18, June 2022, pages 98-108
Fate of polyphenols in black goji berry (Lycium ruthenicum Murr.) upon in vitro and in vivo digestion
Yue Gaoa, #, Maninder Meenua, #, Wai San Cheangb, Baojun Xua, *
aFood Science and Technology Program, BNU-HKBU United International College, Zhuhai, China
bInstitute of Chinese Medical Sciences, State Key Laboratory of Quality Research in Chinese Medicine, University of Macau, Macao SAR, China
*Corresponding author: Baojun Xu, 2000, Jintong Road, Tangjiawan, Zhuhai, Guangdong 519087, China. Tel: +86-756-3620-636; E-mail: baojunxu@uic.edu.cn
DOI: 10.31665/JFB.2022.18313
Received: June 14, 2022
Revised received & accepted: June 29, 2022
| Abstract | ▴Top |
The low stability of polyphenols during gastrointestinal digestion affects their biotransformation and bioavailability in the human body. That in turn affects the therapeutic potential of black goji berry. Thus, the present study was conducted to explore the impact of digestion on the fate of polyphenols present in black goji berries. Black goji berries exhibit high levels of phenolics which were reduced during gastrointestinal digestion. The crude extract of goji berry exhibited potent antioxidant activity as accessed by DPPH (151.34 mmol TE/g), ABTS (202.89 mmol TE/g) and FRAP assay (64.43 mmol TE/g), which were reduced after in vitro gastrointestinal digestion. However, the pronounced reduction was observed after in vivo intestinal digestion as determined by DPPH (23.23 mmol TE/g), ABTS (141.29 mmol TE/g) and FRAP assay (25.53 mmol TE/g). Furthermore, UPLC-Q-TOF-MS chromatograms revealed that the chemical structures of anthocyanins were stable during gastric digestion and significant alterations were observed during intestinal digestion.
Keywords: Black goji berry; In Vitro Digestion; In Vivo Digestion; Colonic Fermentation; Polyphenols
| 1. Introduction | ▴Top |
Lycium ruthenicum Murr. commonly known as black goji berry belongs to the genus Lycium L. (Solanaceae) and family anthophytes. It is widely distributed in Xinjiang and Qinghai Provinces of China (Gong et al., 2015; Levin and Miller, 2005). It is acknowledged as a traditional herbal medicine in China with potent anti-cancer, antioxidant and anti-senile activities. It is frequently employed for the treatment of multiple life-threatening diseases such as hyperlipidemia, immunosuppressive diseases, and diabetes (Islam et al., 2017; Peng et al., 2020; Zheng et al., 2011). These biological activities and health benefits of black goji berry are attributed to its phytochemicals such as phenolic acids, carotenoids, flavonoids, polysaccharides and anthocyanins (Potterat, 2010; Zheng et al., 2018). Among these phytochemicals, anthocyanins are the major polyphenols responsible for the health benefits of black goji berry (Aura et al., 2005; Braga et al., 2018; Islam et al., 2017; Zheng et al., 2018).
The variation in the physiochemical and chemical composition among polyphenolics are the important determinants in human digestion and their health benefits followed by consumption. Whereas the inability of certain polyphenolics to survive in physiological digestive conditions raises significant concerns about their ability to be absorbed or further metabolized and reach tissues and organs (Cipriano et al., 2022). Among the polyphenols, anthocyanins are generally perceived to have low bioavailability, with only 1% absorbed into plasma and the rest is eliminated in urine. The human pharmacokinetic studies found that anthocyanins have bioavailability ranging from <0.005 to 1.2 % based on the unmetabolized parent component and regardless of the amount given in a dose. Following absorption, these polyphenolics including anthocyanins reported to prevent and treat a wide range of diseases depending on their bioavailability (Braga et al., 2018; Cipriano et al., 2022).
During gastrointestinal digestion, the anthocyanins are exposed to different pH conditions which leads to the generation of various molecular structures of anthocyanins that, in turn, affects the therapeutic properties of anthocyanins (McGhie and Walton, 2007). Therefore, extensive knowledge of polyphenols composition, including anthocyanins from black goji berry during the digestion is required to further determine the dose and delivery method to deliver a particular quantity of polyphenols at the target site to exhibit therapeutic effects. Till now, researchers have extensively explored the impact of in vitro and in vivo digestion on the fate of polyphenols and anthocyanins from various plant materials, such as purple sweet potatoes, chestnut skin, blueberry, Myrciaria jaboticaba peel, berry pomace, mulberry, chokeberry, jambolan, pili and thai berries (Xiong et al., 2020; Tarone et al., 2021; Zang et al., 2022; Tu et al., 2021; Cipriano et al., 2022; Kim et al., 2020; Chamnansilpa et al., 2020). Whereas, in case of black goji berry, several studies have reported a significant amount of phenolics, flavonoids, anthocyanins, and fatty acids exhibit a potent antioxidant and antimicrobial potential. In addition, a study has also explored the impact of different drying process, storage temperatures, and color protector glucose on the retention of anthocyanins in black goji berries. The conservation of anthocyanins in situ by freeze-drying and storing the fruits at low temperatures with glucose was reported as a far more cost-effective method (Yan et al., 2018; Ilić et al., 2020; Wang et al., 2021; Nzeuwa et al., 2020; Wu et al., 2016; Islam et al., 2017). But, to the best of our knowledge, no study has been conducted regarding the biotransformation, bioaccessibility and antioxidant activity of black goji berry extracts during in vitro and in vivo digestion. Owing to the instability of polyphenols during digestion, understanding the biotransformation and bioavailability of polyphenols is vital to depict the health effects of black goji berry. Thus, this work was conducted to determine the impact of digestion on biotransformation, total polyphenols, bioaccessibility and antioxidant activity profiles of polyphenols present in black goji berry. For the same, in vitro gastrointestinal digestion model was established to simulate the digestive conditions of the stomach and intestine and followed by the comparison of in vitro data with the data generated by employing in vivo digestion models.
| 2. Materials and methods | ▴Top |
2.1. L. ruthenium Murr. sample
The dried fruits of goji berry (L. ruthenium Murr.) was purchased from Nuomuhong Farm, Qinghai Province, China. The longitude, latitude and altitude (m) of the sample collection site is 96.4°, 36.4° and 2,792, respectively.
2.2. Chemicals
Mucin, α-amylase, pepsin, pancreatin, lipase and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich (St. Louis, U.S.A.). 6-Hydroxy-2,5,7,8-tetramethlchroman-2-carboxylic acid (Trolox), acetonitrile (HPLC grade), and formic acid (HPLC grade) were procured from Sigma-Aldrich Co. (Shanghai, China). Bile salts, 2,2′-azino- bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2-diphenyl-1-picryhy-drazyl (DPPH), potassium persulphate (K2S2O8), sodium nitrite (NaNO2), phosphate buffer saline, vanillin, (+)-catechin, gallic acid, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), ferrous sulfate heptahydrate (FeSO4·7H2O) were purchased from Shanghai Yuanye Bio-Technology Co. (Shanghai, China). The standards of cyanidin-3-O-glucoside, delphindin-3-O-glucoside chloride, malvidin-3-O-glucoside chloride, pelargonidin-3-O-glucoside chloride and peonidin-3-O-glucoside chloride were also procured from Shanghai Yuanye Bio-Technology Co. (Shanghai, China). The absolute ethanol was purchased from Tianjin Fuyu Fine Chemical Co., Ltd (Tianjin, China). Urea, potassium phosphate monobasic (KH2PO4), magnesium chloride (MgCl2), sodium phosphate monobasic dihydrate (NaH2PO4·2H2O), calcium chloride dihydrate (CaCl2·2H2O), ammonium chloride (NH4Cl), aluminum chloride hexahydrate (AlCl3·6H2O), sodium acetate (CH3COONa) and ferric chloride hexahydrate (FeCl3·6H2O) were purchased from Tianjin Damao Chemical Reagent Co., Ltd (Tianjin, China). All chemicals used in this study were of analytical grade unless mentioned specially.
2.3. Extraction of L. ruthenium Murr. sample
The extraction of L. ruthenium Murr. was carried out according to a previously described method with slight modification (Xu and Chang, 2007). Briefly, 100 g of ground sample was extracted twice with 1,000 mL 75% ethanol (v/v) for 3 h at 300 rpm using an orbital shaker. Then the sample extract was stored overnight in the dark followed by centrifugation of the extracted sample at 3,000 rpm for 10 min. The collected supernatants were stored at 4 °C in dark. The residues were extracted one more time and supernatants were mixed and evaporated at 40 °C using a rotatory evaporator followed by freeze-drying and storing at −80 °C until further analysis.
2.4. In vitro simulated gastrointestinal digestion
The in vitro simulated digestion was performed by employing the previously described model with slight modification (Flores et al., 2014) as shown in Figure 1. Briefly, 7.0 g crude extract was mixed with salivary solution (NaCl (117 mg/L), KCl (149 mg/L), NaHCO3 (2.10 g/L), urea (0.4 g/L), mucin (1.0 g/L), α-amylase (2.0 g/L) and distilled water) and stirred for 3 min. In addition, gastric solution (NaCl (5.504 g/L), KCl (1.648 g/L), NaH2PO4·2H2O (0.6916 g/L), CaCl2·2H2O (0.798 g/L), NH4Cl (0.612 g/L), urea (0.170 g/L), mucin (6.0 g/L), pepsin (5.0 g/L), concentrated HCl (13 mol/L) and distilled water) was added to the mixture and incubated at 37 °C for 2 h while maintaining its pH value at 1.9 ± 0.1 with HCl. After gastric digestion, intestinal juice (NaCl (14.024 g/L), KCl (1.128 g/L), NaHCO3 (6.776 g/L), KH2PO4 (0.16 g/L), MgCl2 (0.1 g/L), urea (0.2 g/L), pancreatin (18.0 g/L), lipase (3.0 g/L), concentrated HCl (0.36 ml) and distilled water) and bile salt solution (NaCl (10.518 g/L), KCl (0.752 g/L), NaHCO3 (11.57 g/L), urea (0.5 g/L), bile salts (60.0 g/L), concentrated HCl (0.30 mL) and distilled water) were added to the mixture followed by incubation at 37 °C for 2 h while maintaining the pH of solution at 7.9 ± 0.1. The aliquots collected at the end of each phase were centrifuged at 3,500 rpm for 10 min followed by freeze-drying and stored at −80 °C until further analysis.
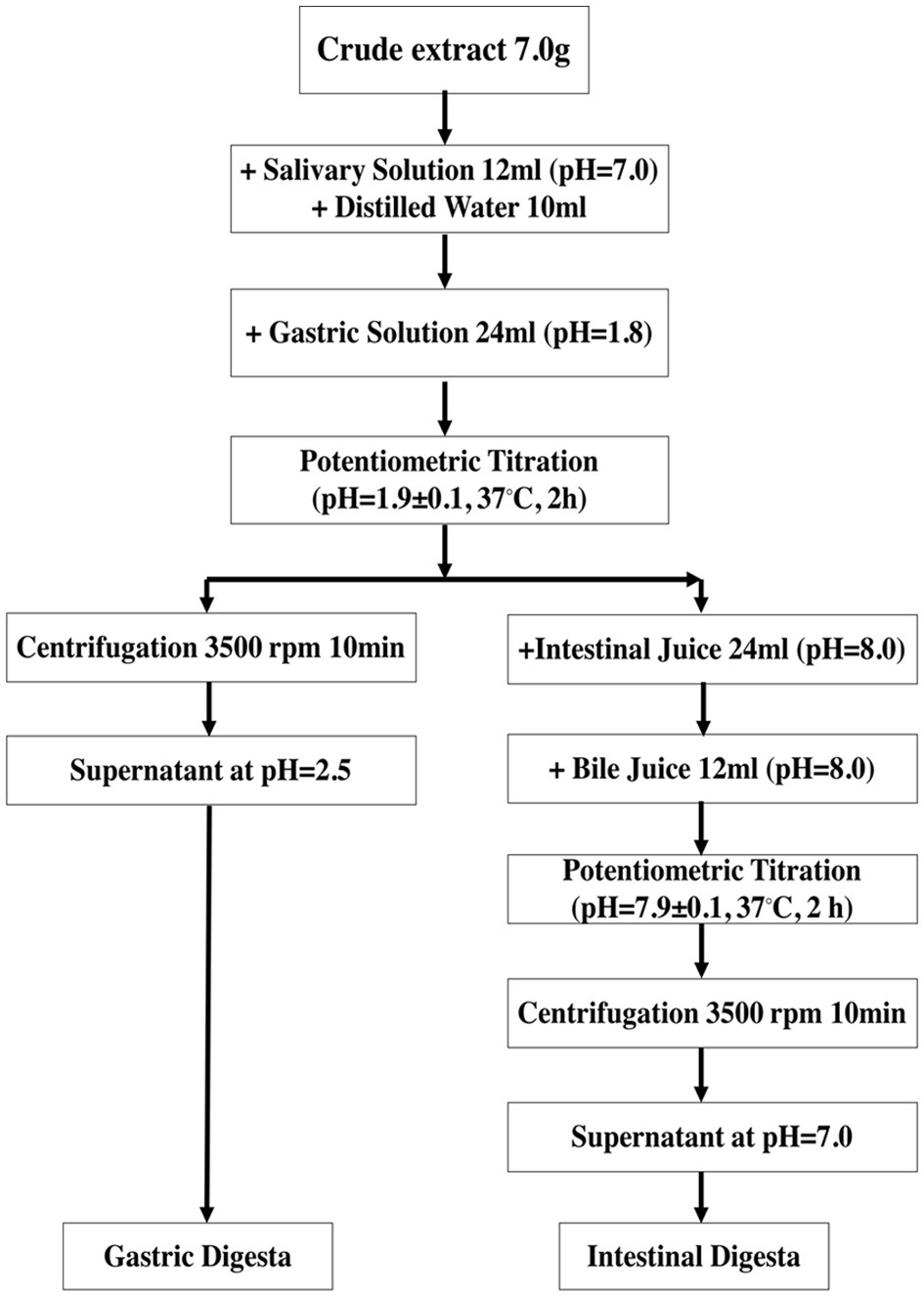 Click for large image | Figure 1. Simulated in vitro digestion process. |
2.5. In vivo digestion of L. ruthenium Murr.
Eight adult male rats were purchased from Southern Medical University, Guangzhou, China. The body weight of each rat was 170 - 180 g. Based on the dose (400 mg anthocyanin/kg·b.w. of rat) 1.6 mL of crude extract was dissolved in sterilized deionized water and fed to rat per day for three continuous days. After three days, animals were sacrificed using chloroform. The small intestinal and large intestinal metabolites were obtained after dissecting followed by extraction with 50% ethanol and centrifugation at 3,000 rpm for 10 min. The resultant supernatant was filtered using a 0.22 μm sterilized polyethersulfone (PES) membrane, freeze-dried and then stored at −80 °C till further analysis.
2.6. Determination of total phenolic content (TPC)
The TPC value of samples was determined by employing the previously described Folin-Ciocalteu assay (Xu and Chang, 2007) using gallic acid as a standard. The absorbance of the resultant solution was measured at 765 nm using a UV-visible spectrophotometer. The TPC values were expressed as milligram gallic acid equivalents per gram of the sample on a dry weight basis (mg GAE/g). The standard calibration curve of gallic acid was found to be linear from 25 to 1,000 μg/mL (R2 = 0.9991).
2.7. Determination of total flavonoid content (TFC)
The TFC value of samples was determined by employing aluminum chloride colorimetric method as described previously (Xu and Chang, 2007). The absorbance of the resultant reaction mixture was measured at 510 nm using a UV-visible spectrophotometer. Different concentrations of catechin (10 to 500 μg/mL) were used to establish a linear standard curve with R2 = 0.9990. The TFC values of samples were expressed as milligram (+)-catechin equivalents per gram of the sample (mg CAE/g) on a dry weight basis.
2.8. Determination of condensed tannin content (CTC)
A previously described vanillin-HCl colorimetric method was employed to access the CTC values of samples under investigation (Xu and Chang, 2007). The absorbance of the reaction mixture was measured at 500 nm using a UV-visible spectrophotometer. A standard linear curve of Catechin was used established in the range of 10 to 1,000 μg/mL (R2 = 0.9995). The CTC values of samples were reported as milligram (+)-catechin equivalents per gram of the sample on a dry weight basis (mg CAE/g).
2.9. Determination of monomeric anthocyanin content (MAC)
The MAC values of samples were determined by employing a pH differential colorimetric method as mentioned in the literature (Xu and Chang, 2008). Briefly, the sample was diluted with KCl buffer (pH = 1.0) and CH3CO2·3H2O buffer (pH = 4.5). The absorbance was measured at 700 nm and 520 nm using a UV-visible spectrophotometer. The MAC values of samples were expressed as cyanidin-3-glucoside equivalents (CE) in mg/g using the molecular weight of 449.2 g/mol and an extinction coefficient of 26,900 L cm/mol.
2.10. ABTS free radical scavenging assay (ABTS)
The ABTS value of samples was determined by employing colorimetric method according to a previously described method (Miller et al., 1993; Re et al., 1999). The absorbance of reaction mixture was measured at wavelength 734 nm using a UV-visible spectrophotometer. A linear standard curve of Trolox was developed with a concentration of 50 to 800 μg/mL (R2 = 0.9990). The ABTS values were expressed as micromole of Trolox equivalents per gram of the sample on a dry weight basis (μmol TE/g).
2.11. DPPH free radical scavenging capacity assay (DPPH)
The DPPH value of crude extract and digested samples was determined according to a previously mentioned colorimetric method (Xu and Chang, 2007). The absorbance of the reaction mixture was recorded at 517 nm using a UV-visible spectrophotometer and different concentrations of Trolox (100 to 750 μg/mL) were used to establish a linear standard curve (R2 = 1.0). The DPPH values of samples were reported as micromole of Trolox equivalents per gram of the truffle extract on a dry weight basis (μmol TE/g).
2.12. Ferric radical scavenging power assay (FRAP)
A previously reported colorimetric method was used for the determination of FRAP values of samples (Xu and Chang, 2007). The absorbance of the resultant reaction mixture was measured at 593 nm using a UV-visible spectrophotometer. A linear standard curve of different concentrations of ferrous sulfate (100 to 1,000 μg/mL) was established (R2 = 0.9998) and the FRAP values were reported as millimoles of Fe2+ equivalent (FE) per 100 g samples on a dry weight basis (mmol FE/100g).
2.13. Determination of anthocyanins by ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS)
The anthocyanins present in various samples were determined based on a previously reported method (Sánchez-Patán et al., 2012) with slight modification. An Acquity UPLC I-Class and Xevo G2-XS TOF mass spectrometer equipped with an Acquity UPLC BEH C18 (1.7 μM, 2.1 × 100 mm) analytical column (Waters, Milford, MA, U.S.A.) at 40 °C was employed for the separation of anthocyanins. The mobile phases consisted of 0.1% formic acid (v/v) and acetonitrile and the flow rate was 0.3 mL/min. The injection volume was set at 20 μL and the run time for each sample was 12.17 min. The electrospray ionization (ESI) was operated in positive mode with a capillary voltage of 3 kV, source temperature of 130 °C, desolvation temperature of 400 °C, desolvation gas (N2) at a flow rate of 750 h/L and cone gas (N2) flow rate of 60 h/L. The full scan range of ions in MS1 and MS2 was from m/z 100 to m/z 1,000, and from m/z 50 to m/z 1,000, respectively.
2.14. Statistical analysis
The in vitro digestion treatments were carried out in duplicate and all colorimetric experiments were performed in triplicate. The data were expressed as mean ± standard deviation. The significant differences among the mean values were analyzed by employing one-way ANOVA. The Duncan test was used to determine the significant difference (p < 0.05) among mean values of a group using IBM SPSS Statistics version 25 (IBM Corporation, New York, U.S.A.).
| 3. Results | ▴Top |
3.1. Mass balance calculation
After freeze-drying, the crude extract obtained from 100 g dried L. ruthenium Murr. was 51.2 g with 51.2% yield. Regarding the mass balance during in vivo digestion, 46 mL of solution were added for gastric digestion of 7.0 g crude extract and after freeze-drying, the gastric digesta was observed to be 6.25 g in weight. For the intestinal digestion, 7.0 g crude extract was digested with 82 mL of solution and 5.37 g intestinal digesta was obtained after freeze-drying.
3.2. Changes in polyphenols profiles before and after in vitro and in vivo digestion
The TPC values of all samples, including crude extract, in vitro gastric digesta, in vitro intestinal digesta and in vivo intestinal digesta are presented in Table 1. Crude extract presented a significantly higher (p < 0.05) amount of TPC (49.47 mg GAE/g) that was 17.10 % higher than the TPC value of gastric digesta (41.01 mg GAE/g). Besides, from gastric digestion to intestinal digestion, TPC was further decreased to 25.84 mg GAE/g. However, there was no significant difference (p > 0.05) was observed in the TPC value of in vitro intestinal digesta (25.84 mg GAE/g) and in vivo intestinal digesta (25.04 mg GAE/g).
 Click to view | Table 1. Polyphenol profiles of L. ruthenicum Murr. before and after in vitro and in vivo digestion |
As shown in Table 1, crude extract exhibits the highest value for TFC (13.86 mg CE/g) among samples. During in vitro gastric digestion, TFC value was decreased (34.7%) to 9.05 mg GE/g dramatically. Moreover, from gastric digestion to intestinal digestion, an insignificant decrease (p > 0.05) in the TFC values was observed between the two phases. However, in terms of in vivo intestinal digesta, the TFC value was relatively lower compared to the in vitro intestinal digesta.
The CTC values of, crude extract, in vitro gastric digesta, in vitro intestinal digesta and in vivo intestinal digesta are depicted in Table 1. Among the CTC values of all samples, crude extract presented the highest value (25.96 mg CE/g). In the context of recovery, CTC was decreased by 35.47%, indicating the significant effect (p < 0.05) of digestion on CTC value. Moreover, a significant difference in the CTC values was observed after in vitro intestinal digestion (11.65 mg CE/g) and in vivo digestion (7.00 mg CE/g).
The MAC values of crude extract and different digesta are mentioned in Table 1. Among all samples, the crude extract exhibited the highest value for MAC (154.7 mg/g). During intestinal digestion, the MAC value was observed to be decreased dramatically. The MAC value of in vitro gastric and intestinal digesta was 114.18 mg/g and 35.09 mg/g, respectively. Furthermore, it was observed that the in vivo intestinal digesta exhibited the lowest value for the MAC (7.06 mg/g) compared to other samples under investigation.
3.3. Changes in antioxidant activity before and after in vitro and in vivo digestion
As shown in Table 1, the ABTS value of in vitro gastric digesta (154.92 mmol TE/g) was relatively lower compared to the in vitro intestinal digesta (164.45 mmol TE/g). However, among all samples, crude extract exhibits the highest ABTS radical scavenging capacity, 202.89 mmol TE/g. Moreover, a significant difference (p < 0.05) was also observed in the ABTS values of in vitro (164.45 mmol TE/g) and in vivo intestinal digesta (141.29 mmol TE/g).
As shown in Table 1, DPPH value of crude extract presented significantly the highest value for DPPH assay (151.34 mmol TE/g). DPPH value was decreased significantly (p < 0.05) during in vitro gastric digestion (71.05 mmol TE/g). The DPPH values of in vitro intestinal digesta (64.51 mmol TE/g) presented only a slight decrease compared to the DPPH value of in vitro gastric digesta. However, in vivo intestinal digesta presented the lowest value for DPPH assay (23.23 mmol TE/g) that was 63.99% lower compared to the DPPH value of in vitro intestinal digesta.
As shown in Table 1, crude extract exhibited the highest value for FRAP assay (64.43 mmol TE/g). It was interesting to note that gastric digestion and intestinal digestion presents a significant effect (p > 0.05) on ferric reducing antioxidant capacity as depicted from the small difference among the FRAP value of crude extract, in vitro gastric and intestinal digesta. Moreover, a significant difference (p > 0.05) was observed between the FRAP values of in vitro (34.06 mmol TE/g) and in vivo intestinal digesta (25.52 mmol TE/g).
3.4. Quantitative analysis of changes in polyphenols in L. ruthenicum before and after in vitro digestion
As shown in Table 2, except for TFC (41.70%), the loss of other polyphenols during gastric digestion was about 30%. For the antioxidant activity, during gastric digestion, 58.08% loss was observed in the DPPH value, while 20.40% loss was found in the FRAP value. While proceeding from gastric digestion to intestinal digestion, the loss in TPC, CTC and FRAP value was about 34%. In addition, slight loss in TFC, ABTS and DPPH values were also observed while analyzing digesta after gastric digestion to intestinal digestion. However, a significant loss was also observed in the case of MAC value (48.5%) during this process.
 Click to view | Table 2. Quantitative changes in polyphenols in L. ruthenicum Murr. before and after in vitro digestion |
3.5. Analysis of anthocyanins by UPLC-Q-TOF-MS
The TOF-MS profiles of all samples including, dried L. ruthenicum Murr. crude extract, in vitro gastric digesta, in vitro intestinal digesta and in vivo intestinal digesta are presented in Figure 2. Based on the m/z of precursor ions of crude extract and in vitro gastric digesta and their daughter ions, it was elucidated that the chemical structures of anthocyanins from L. ruthenicum Murr. remain stable during in vitro gastric digestion. However, after in vitro intestinal digestion, the chemical structures of anthocyanins were altered as exemplified due to significant variations in retention time, m/z of precursor ion and its daughter ions. For example, when comparing the peaks observed in chromatograms of in vitro gastric digesta and in vitro intestinal digesta, several peaks have been disappeared, such as the peaks at retention time 7.59 min and 9.19 min in case of in vitro gastric digesta. Moreover, multiple new peaks have been evolved followed by the intestinal digestion process, such as the peaks at retention time 6.85 min and 9.54 min in case of in vitro intestinal digesta. Similarly, after in vivo digestion, it was depicted that the chemical structures of anthocyanins have been changed significantly. In addition, several new peaks were also evolved as observed at retention time 6.42 min and 7.74 min in the case of chromatogram presenting the profile of in vivo intestinal digesta (Figure 2d).
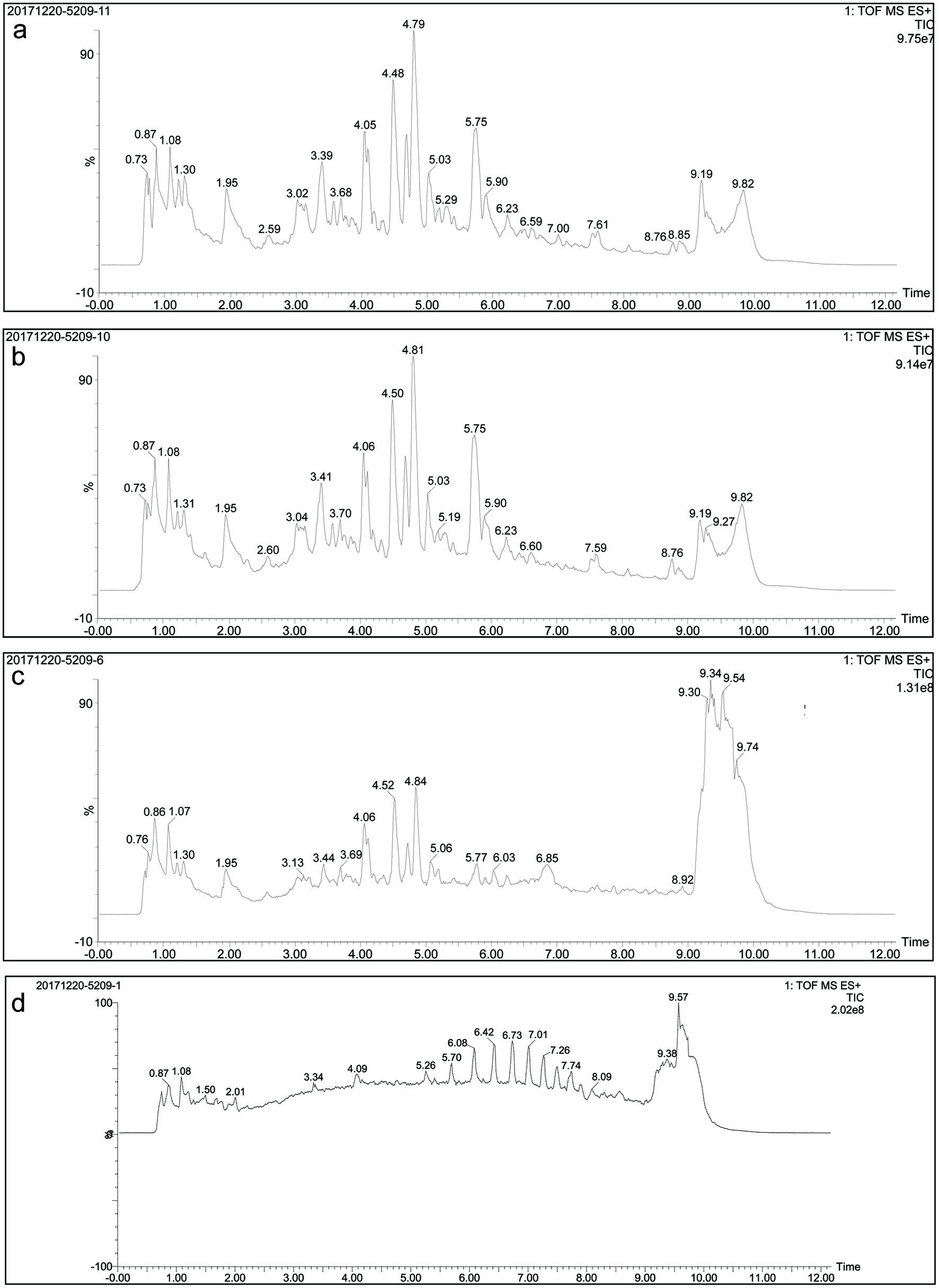 Click for large image | Figure 2. TOF-MS chromatograms of (a) crude extract, (b) digesta of in vitro gastric digestion, (c) digesta of in vitro intestinal digestion, and (d) digesta of in vivo intestinal digestion. |
| 4. Discussion | ▴Top |
4.1. Qualitative analysis of changes in polyphenols during in vitro digestion
In case of simulated in vitro digestion, all polyphenols namely, total phenolics, total flavonoids, condensed tannins and anthocyanins were presented a slight variation in response to gastric digestion. The possible reason may be the marginal absorption of polyphenols in the stomach (Marín et al., 2015; Pereira-Caro et al., 2021). During intestinal digestion, the small intestine plays a crucial role since it is responsible for the partial absorption of polyphenols in the form of aglycones and glucosides. The unabsorbed polyphenols that escaped from the gastric digestion leave the stomach and moved to the intestine where the degradation of phenolic compounds occurs due to the cleavage of sugar moieties, which, in turn, induces the absorption of polyphenols (Marín et al., 2015; Cipriano et al., 2022). In addition, it was also reported that after ingestion, anthocyanin glycosides are quickly absorbed in the stomach, and the unabsorbed anthocyanins move to the small intestine. In the colon, colonic bacteria induce the cleavage of glycosidic bonds of anthocyanins that result in the formation of phenolic acids and aldehydes which are responsible for the therapeutic effects associated with the consumption of anthocyanin-rich foods (Keppler and Humpf, 2005; McGhie and Walton, 2007). It serves as a plausible explanation for low recovery values for TPC, CTC and MAC after in vitro intestinal digestion. A study focused on the stability and biological activity of wild blueberry during simulated in vitro GI digestion provides solid evidence regarding the recovery of polyphenols. Based on this study, recovery of total phenolics in crude extract, gastric digesta and intestinal digesta was 100%, 93.5% and 49.1%, respectively. However, in case of anthocyanins, the highest recovery was again observed in crude extract (100%) followed by gastric digesta (96.8%) and intestinal digesta (17%). Previously, in case of chokeberry, the total loss of anthocyanins was 42.6% and the total loss of flavonols was 26.5% during the whole process of digestion (Bermúdez-Soto et al., 2007; Correa-Betanzo et al., 2014). Therefore, intestinal digestion exhibits a significant impact on the reduced recovery of polyphenols from gastric to intestinal digesta. In case of TFC, the gastric digesta exhibit around 65.3% recovery, which was relatively lower compared to other polyphenols. The reason behind the low recovery of flavonoids might be their interaction with other compounds that in turn lead to early absorption of these compounds (Tu et al., 2021).
4.2. Quantitative analysis of changes in polyphenols during in vitro digestion
During in vitro digestion process, the weight of substances exhibits significant variations. Initially, 7.0 g of crude extract was digested into the gastric digesta that weighed 6.25 g and the yield was found to be 89.3%. Subsequently, 6.25 g of gastric digesta was further digested into the intestinal digesta and the resultant weight was 5.37 g (Table 2). The yield of intestinal digestion was 76.7%. After multiplying the value of polyphenols (expressed as equivalents per gram), the quantitative change in polyphenols of crude extract during in vitro digestion was obtained. In case of in vitro digestion, the loss of crude extract in the stomach was analyzed quantitatively and the %loss for polyphenols was observed to vary from 25.9% to 34.1%. While in the intestine, the loss was ranged from 12.8% to 48.5%. In the intestine, 48.5% of MAC was lost, which might have been absorbed directly or degraded and the total loss of TFC was 54.5% in the case of in vitro digestion. A similar finding was also reported previously after in vitro digestion where researchers have employed (+)-catechin as standard. However, in the present study, in case of gastric digestion, the loss of TFC was higher compared to the loss of TFC in (+)-catechin reported previously (Bermúdez-Soto et al., 2007). This might be due to the interaction of flavonoids with other compounds or rapid absorption. The other possible reason behind this finding may be the influence of pH fluctuation on the stability of flavonoids during digestion. In addition, in vitro digestion model used previously was static that could not ensure the minimal pH fluctuation during digestion (Bermúdez-Soto et al., 2007). However, in the present study, minimal pH fluctuation during digestion was ensured by utilizing a potentiometric Titrando to build a dynamic model.
4.3. Analysis of the difference between in vitro and in vivo intestinal digesta
As shown in Table 1, no significant difference (p < 0.05) was observed in the TPC value of in vitro and in vivo intestinal digesta. However, other parameters namely, TFC, CTC and MAC exhibit significantly (p < 0.05) higher values in case of in vitro intestinal digesta compared to the in vivo intestinal digesta. The reason behind this finding may be the interference of oxygen with the simulated colonic fermentation during the decomposition of undigested foods via enzymatic activity (Rocchetti et al., 2017). To mimic the in vivo digestion, a sophisticated anaerobic workstation needs to be employed for simulated colonic fermentation. Theoretically, colonic microbiota in the large intestine can cause polyphenol catabolism and absorption, which was evident from the difference observed in the polyphenolic contents in the case of in vitro and in vivo intestinal digesta in this study. These results were in agreement with a previous study reported on wild blueberry. In that case, the recovery of total phenolic decreased from 49.1% (intestinal digesta) to 42.5% after colon fermentation. Whereas, the recovery of total anthocyanins was reduced dramatically from 15.0% (intestinal digesta) to 1.5% (Correa-Betanzo et al., 2014). The same trend was also observed in present study. However, decrease in the recovery was not as evident as mentioned in the previous study (Correa-Betanzo et al., 2014). This disparity in the recovery results of total phenolics and anthocyanins of present and the previous studies (Correa-Betanzo et al., 2014) may be attributed to the difference in species of berries, composition and content of anthocyanins and phenolics before and after digestion. In addition, this study has performed UPLC-Q-TOF-MS analysis of black goji berry for analysis of anthocyanins before and after digestion. During in vitro and in vivo digestion, TOF-MS chromatogram (Figure 2) presented the emergence of several new peaks, along with the disappearance of original peaks after digestion. These changes indicated the alteration in the chemical structures of anthocyanins present in black goji berry followed by the in vitro and in vivo digestion. This alteration in the chemical structure of anthocyanin was also supported by the emergence of new peaks in MS and MS-MS spectra of anthocyanins after in vitro and in vivo intestinal digestion (Figures 3 and 4).
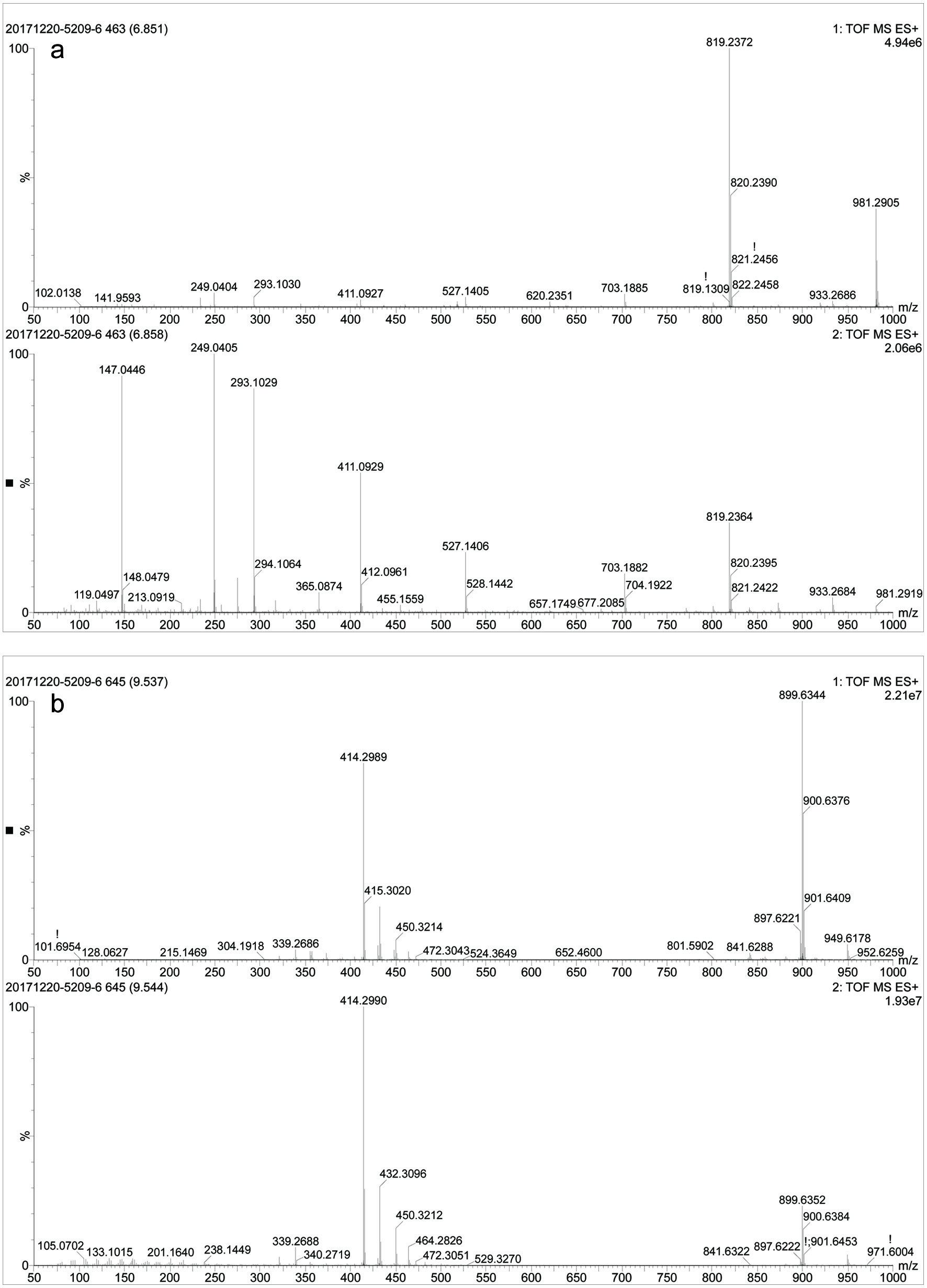 Click for large image | Figure 3. MS, MS-MS spectra of in vitro intestinal digesta (a) at retention time 6.85 min and (b) 9.54 min. |
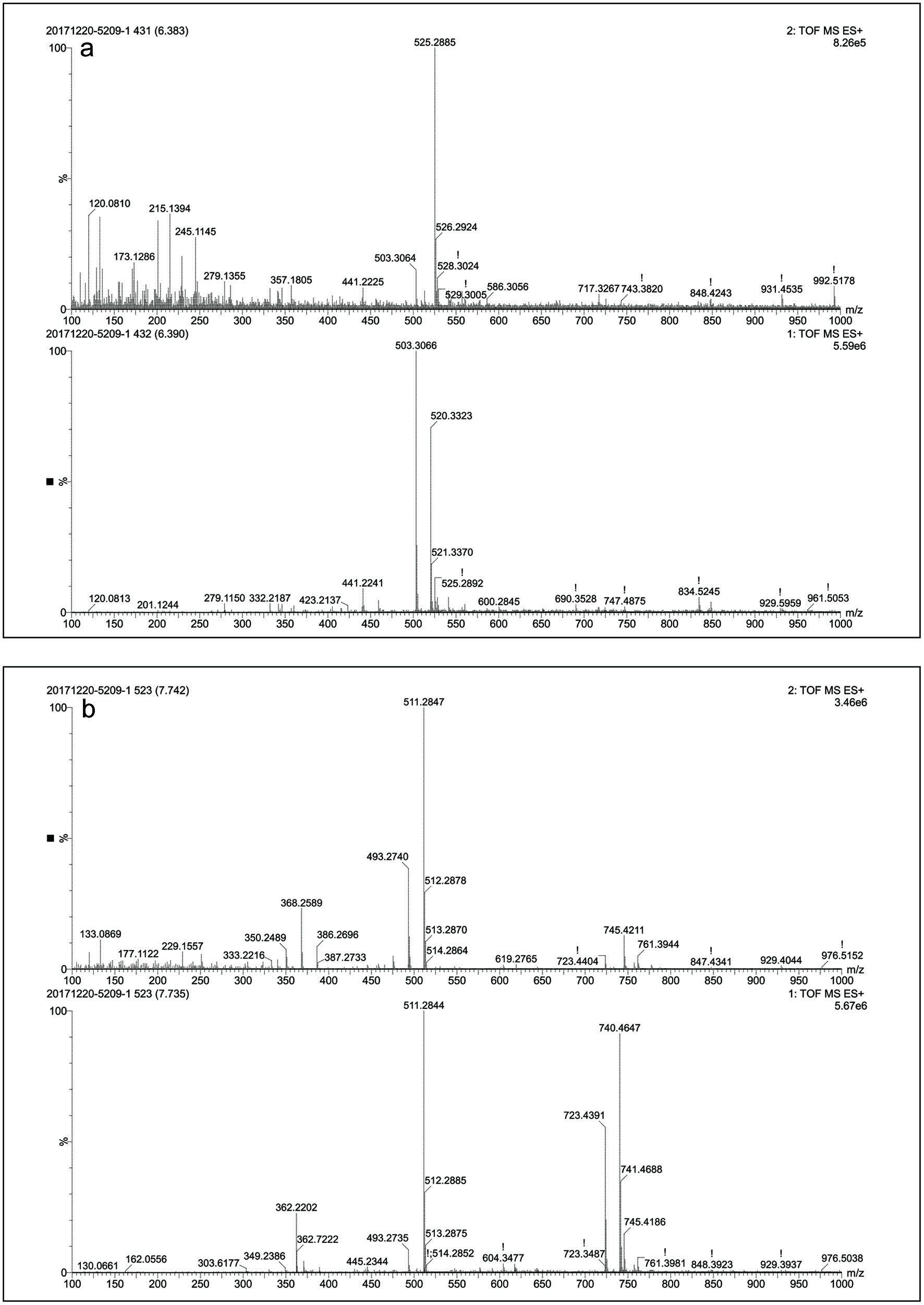 Click for large image | Figure 4. MS, MS-MS spectra of in vivo intestinal digesta (a) at retention time 6.42 min and (b) 7.74 min. |
Previous studies on wild blueberry (Correa-Betanzo et al., 2014) and red wine (Sánchez-Patán et al., 2012) have shown that the ring cleavage of anthocyanins by gut microflora at different degradation rates. Moreover, another study on mulberry (Liang et al., 2012), reported the emergence of certain phenolic derivatives during colonic fermentation that were not present in the gastric digesta, while some phenolic compounds were disappeared after colonic fermentation. Researchers have also demonstrated the loss of the majority of anthocyanins followed by the treatment of fecal slurry with active gut microflora. Once the partial deglycosylation of some anthocyanins occurred (e.g. cyanidin-3-rutinoside), the majority of anthocyanins were observed again (Aura et al., 2005). All these variations in anthocyanins are attributed to colonic fermentation. The enzymatic activity of colon microflora also contributes towards the significant absorption of polyphenols present in foods, which in turn significantly impact the composition and recovery of polyphenols before and after digestion (in vitro and in vivo).
4.4. Comparison of antioxidant activity
Polyphenols are capable of slowing or preventing the oxidation of other molecules, by acting as strong antioxidants. During GI digestion, the antioxidant capacity may alter owing to the occurrence of chemical transformations. To gain a comprehensive understanding, the change in antioxidant followed by digestion was evaluated by employing three different methods based on different principles namely, free radical scavenging assay (DPPH), radical scavenging assay (ABTS) and ferric reducing antioxidant power (FRAP) methods (Meenu et al., 2018; Xu et al., 2020; Yu et al., 2021; Zhang et al., 2020). The FRAP relies on the reduction of antioxidants, while the principles of DPPH and ABTS depend on the scavenging ability of the antioxidants.
As shown in Table 1, the crude extract of black goji berry exhibits potent antioxidant activity as accessed by ABTS (202.89 mmol TE/g), DPPH (151.34 mmol TE/g) and FRAP assays (64.43 mmol TE/g). The FRAP value of the black goji berry sample in this study was significantly less, whereas DPPH and ABTS values in this study was high compared to a previous study (Islam et al., 2017). After in vitro gastric digestion, a significant reduction in antioxidant activity was observed that is also presented a reduced % recovery in Table 1. Furthermore, a significant decrease in the % recovery and antioxidant activity in terms of DPPH and FRAP assay was observed after in vitro intestinal digesta. This decrease in the antioxidant activity attributed to the significant decrease in the TPC, TFC, CTC and MAC values of crude extract after in vitro intestinal digestion. Similar findings were also observed in previous studies on black carrots, chestnut outer-skin and inner-skin (Pereira-Caro et al., 2021; Tu et al., 2021).
It is interesting to note a pronounced reduction in the antioxidant activity of crude extract after in vivo intestinal digesta in terms of ABTS, DPPH and FRAP assays. Overall, the antioxidant activity of crude extract measured by different assays was found to be reduced at different levels after both in vivo and in vitro digestion. This significant decrease in the antioxidant activity was correlated with the significant decrease in the TPC, TFC, CTC and MAC content after in vitro and in vivo intestinal digestion. Similar results were also observed in case of simulated digestion and colonic fermentation of black carrot (Pereira-Caro et al., 2021). Antioxidant compounds are more reactive at acidic pH conditions during gastrointestinal digestion and less reactive at neutral pH during intestinal digestion. As a result, biotransformation of antioxidant compounds to others with lower antioxidant potential or the synthesis of new antioxidant metabolites can occur during digestion. That, in turn, results in lower values for antioxidant assay after gastric and intestinal assays (Pereira-Caro et al., 2021; Andrade et al., 2022).
| 5. Conclusions | ▴Top |
In conclusion, the black goji berry is a rich source of polyphenols, especially anthocyanins. The biological effects of black goji berry such as antioxidant activity are associated with its polyphenols. A significant alteration in the polyphenol contents and antioxidant activity of black goji berry was observed followed by in vitro and in vivo digestion, particularly after the intestinal digestion part. Moreover, the results of UPLC-Q-TOF-MS analysis revealed the alteration in the chemical structures, formation and degradation of anthocyanins in black goji berries after digestion. However, further identification of the specific chemical structure of anthocyanins in L. ruthenium Murr. during the whole digestion process is required along with an explanation of compounds presented in the MS/MS spectra. In future, a greater number of detailed studies are required to be conducted to completely understand the fate of polyphenols from black goji berry change during in vitro and in vivo digestion.
These authors contributed equally to this study.
This project is jointly supported by two grants (project code: UIC202016 and UIC202017) from BNU-HKBU United International College.
YG: Investigation, Calculation, Writing Original draft preparation. MM: Calculation, Writing, Reviewing and Editing. WSC: Writing, Reviewing and Editing. BX: Conceptualization, Methodology, Software, Supervision, Writing, Reviewing and Editing.
| References | ▴Top |