| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 18, June 2022, pages 52-66
Effect of processing on the preservation of bioactive compounds in traditional and exotic fruits: a review
Fereidoon Shahidi*, Renan Danielski, Grasiela Rocha Barros da Silva
Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1C 5S7
*Corresponding author: Fereidoon Shahidi, Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1C 5S7. E-mail: fshahidi@mun.ca
DOI: 10.31665/JFB.2022.18308
Received: June 13, 2022
Revised received & accepted: June 24, 2022
| Abstract | ▴Top |
Bioactives are natural substances that may function as antioxidant, anti-inflammatory, antimicrobial, and anticarcinogenic agents. They include phenolic compounds, carotenoids, and vitamins that can exert health-promoting effects. Conventional fruit processing (e.g., heat treatment) can negatively affect the content and possibly the integrity of bioactives in the source material. Meanwhile, non-conventional techniques, such as high pressure processing and pulsed electric field, may increase the extractability of bioactives from the food matrix and enhance their availability for intestinal absorption. Although berries are usually perceived as outstanding sources of antioxidants, other conventional fruits also stand out, such as apple, banana, grape, mango, and orange. Nevertheless, exotic fruits, such as Buriti, mamey, açaí, pitanga, camapu, and tucumã are less frequently consumed, even though they can provide relevant bioactives. Additionally, fruit processing generates by-products containing high-value bioactives that can re-enter the industry cycle while minimizing the quantity of waste generated. Future studies should further examine the potential of exotic fruits using their discarded portions. Thus, identifying the best techniques for their use and maximum phytochemical extraction would be essential to reducing their environmental impact. Additionally, novel functional foods and nutraceuticals can be obtained by exploring the bioactive potential of these feedstocks and their processing discards.
Keywords: Traditional fruits; Exotic fruits; Biological activity; Phenolic compounds; Food processing
| 1. Introduction | ▴Top |
Fruits can be consumed as such or processed using different techniques such as drying, juicing, canning, freezing and preserving by pickling or jam preparation (FAO, 1994). Due to their high susceptibility to microbiological deterioration, processing is often used to extend their shelf-life. According to the Food and Agriculture Organization (FAO, 2020), traditional fruits are widely consumed and produced, including bananas (120 million tonnes), apples (87 million tonnes), mangos (55 million tonnes), oranges (76 million tonnes), and grapes (78 million tonnes). All fruit production annually generates around 28% waste produced by the food industry (Parfitt et al., 2010).
Besides traditional fruits, some exotic fruits have demonstrated excellent market potential due to their high levels of natural antioxidants, which has given them the status of superfruits (Chang et al., 2019). Nevertheless, few studies have investigated their bioactive composition and health effects, as it can be seen for Amazonian fruits such as pitanga (Eugenia uniflora) (Dorman and Deans, 2000), buriti (Mauritia flexuosa), mamey (Mammea americana), camapu (Physalis angulate) (Lima et al., 2009), tucumã (Astrocaryum aculeatum), and açaí (Euterpe oleracea) (Cabral et al., 2020).
Fruit bioactives can trigger cellular responses in tissues, ameliorating their function in the body once consumed, which positively impacts the health (Guaadaoui et al., 2014). Many of these compounds also have antioxidant activity, which can function as such or as synergists to minimize the risk of chronic diseases (Pisoschi and Negulescu, 2012). According to multiple epidemiological studies, fruits’ intrinsic compounds are responsible for their health impact (Serna-Saldivar, 2010); these include ocular, and neurological conditions, strokes, some types of cancer, diabetes, hypertension, cardiovascular ailments, cerebrovascular issues, and multiple blood ailments (Barrett et al., 2005).
The primary bioactive groups found in fruits include some vitamins, phenolic compounds, and carotenoids which have been regarded as key factors in health promotion and disease risk reduction associated with the consumption of fruits and vegetables (Sricharoen et al., 2016). The profile and content of bioactives in fruits depend on the cultivar, growing and climate conditions, storage, and transport, among others (Bennett et al., 2011).
World Health Organization/Food Agricultural Organization (WHO/FAO) recommends 400–500 g per day of fruits and vegetables to prevent micronutrient deficiencies and related health conditions, as insufficient supply of these foods may result in an array of non-communicable diseases (NCDs) (WHO, 2005). On the contrary, a diet high in fruits and vegetables reduces the occurrence of non-contagious diseases and improves overall health (Shahidi and Ambigaipalan, 2015).
Processing may provide a means for adding value to fruits by transforming them into a variety of products, such as juices, jams, and pie fillings, among others. In terms of bioactive compounds, processing can impact by affecting (decreasing or increasing) their content and bioacessibility, as well as by causing isomerization, polymerization, and other transformations (van Breda and de Kok, 2018).
The present review provides up-to-date information on the impact of processing techniques on major bioactives present in selected common and exotic fruits , with a special emphasis on phenolic compounds, vitamin C, and carotenoids. The chemical transformations and bioaccessibility aspects will be addressed in order to evaluate the extent of modifications caused by processing, and whether conventional and non-conventional techniques work to increase or decrease the health-promoting effects of the fruits discussed in the present review.
| 2. Bioactive compounds from fruits and their health-promoting effects | ▴Top |
Food bioactive compounds provide important fundamental ingredients that allow active response in living tissues. They can improve health by modifying normal physiological functions or ameliorating the organism’s biological activities. The benefits of bioactives may be related to their antioxidant, antimicrobial or anti-inflammatory function and these may be dictated by their specific bioactivity, chemical structure, and dose (Guaadaoui et al., 2014). Regarding fruits and vegetables, not only their edible portions are sources of bioactives but their processing discards also serve as rich sources of phenolic compounds, carotenoids, phytosterols, omega-3 fatty acids, and fat-soluble vitamins, among others (Saini et al., 2019).
Several bioactive classes of compounds can be encountered in fruits, with vitamins, especially vitamin C, phenolics, and carotenoids being especially prominent. Figure 1 shows the structure of commonly found bioactives in traditional and exotic fruits.
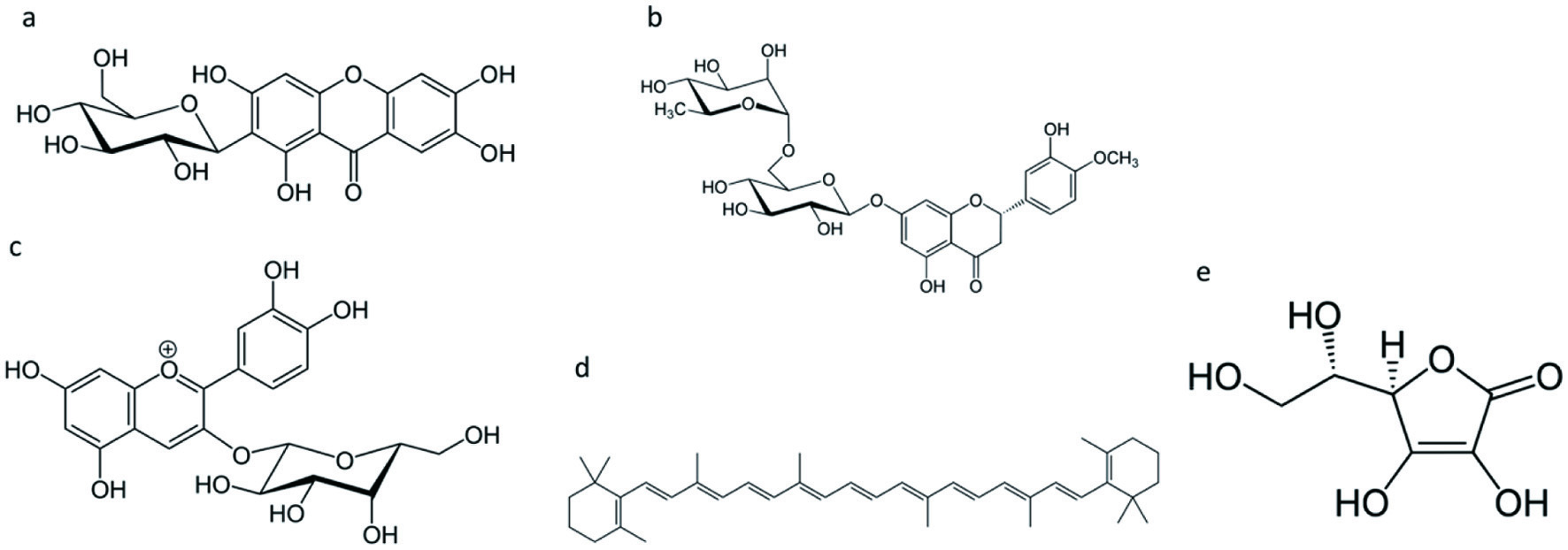 Click for large image | Figure 1. Commonly found bioactives in traditional and exotic fruits. (a) mangiferin; (b) hesperidin; (c) cyanidin-3-O-galactoside; (d) β-carotene; and (e) ascorbic acid. |
Vitamin C (ascorbic acid) is a hydrophilic molecule that can be found in the body in the reduced (ascorbic acid or ascorbate) or oxidized forms (dehydroascorbic acid) (Kocot et al., 2017). The physiological and metabolic functions promoted by this vitamin include common cold prevention, wound healing properties, antioxidant activity, and maintenance of skin, blood vessels, bones, and cartilage health. Ascorbic acid can also restore the antioxidant function of α-tocopherol after free radical neutralization. Even though these functions are essential, humans cannot synthesize vitamin C due to the lack of the enzyme L-gulono-1,4-lactone oxidase. Therefore, consuming foods rich in vitamin C is necessary to obtain this substance (Sunil Kumar et al., 2017). Table 1 shows the vitamin C content found in traditional and exotic fruits.
 Click to view | Table 1. Vitamin C content of traditional and exotic fruits |
Carotenoids are present in several fruits, vegetables, and marine species. They are fat-soluble pigments with over 700 identified compounds responsible for providing yellow, red, and orange colors to their natural sources. This group can be broken down into carotenes, strictly hydrocarbon structures, and xanthophylls which are oxygenated derivatives of carotenes. In terms of their biological activity, carotenoids bearing one or more ionone rings in their structures may serve as vitamin A precursors. Some examples are β-carotene, β-cryptoxanthin and α-carotene (Kris-Etherton et al., 2002). Moreover, these substances also exhibit antioxidant activity and are especially efficient in quenching singlet oxygen, thus preventing photooxidation. Total carotenoid levels and identified carotenoids from selected traditional and exotic fruits are shown in Table 2.
 Click to view | Table 2. Carotenoid content and profile in traditional and exotic fruits |
Among the bioactive classes of compounds present in fruits, phenolic compounds stand out for their diversity and bioactivity, which include antioxidant, anti-inflammatory, and anticancer activities, as well as lowering the risk of type 2 diabetes, cardiovascular diseases, and neurodegenerative disorders. Phenolic compounds are secondary plant metabolites and their basic structures consist of a benzene ring to which one or more hydroxyl groups are attached. Depending on their structural features, phenolics can be broken down into several subgroups, including phenolic acids, flavonoids, tannins (hydrolysable and condensed), stilbenoids, lignans, coumarins, and tyrosol (Shahidi et al., 2021).
In food matrices, phenolics may exist in the soluble (free or esterified/etherified to fatty acids, carbohydrates, and soluble proteins) and insoluble-bound forms (covalently bound to cell wall molecules, such as fiber, cellulose, and structural protein) (Shahidi et al., 2021; Schefer et al., 2021). Flavonoids, the main phenolic class, are present in high amounts in fruits (Pan et al., 2009); their seeds may possess flavonoid glycosides (attached to a sugar molecule) or aglycones (Fang et al., 2005). Based on their hydroxylation and methylation pattern, this class may further be categorized into flavones, flavonones, flavonols, flavan-3-ols, flavanonols, isoflavones, and anthocyanins. The latter is predominantly found in dark-colored fruits, such as blueberries, grapes, strawberries, blackberries, and blackcurrants, among others. Anthocyanins are water-soluble pigments, and structural changes caused by pH variations alter their color, ranging from red to purple and blue (Shahidi et al., 2021).
The antioxidant activity of phenolic compounds are exerted through neutralization of free radicals, which mainly occurs by the donation of a hydrogen atom from the phenolic’s hydroxyl group. Phenolics bearing catechol and galloyl moieties also have the ability to chelate transition metals, which are prooxidant factors. The mitigation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) by antioxidants prevents damage to DNA, lipids, and proteins, thus reducing the risk of chronic diseases, such as cancer, type 2 diabetes, Alzheimer’s, and atherosclerosis, among others. This can be achieved with consumption of a balanced diet, low in refined sugar and saturated fat and rich in fruits and vegetables, as these foods are rich sources of phenolics and other natural antioxidants (Shahidi et al., 2019).
2.1. Traditional fruits
Although many traditional foods are outstanding sources of bioactive compounds, the following reports on only a selected set of examples, which are used to demonstrate the importance of these substances and how they can be affected by processing.
2.1.1. Apple
Apple is the most cultivated fruit worldwide due to its seasonal availability and high nutritional content. In 2020, apple production reached around 87 million tonnes, which were destined to be consumed fresh or serve as raw material for other products, such as juice, chips, cider, and vinegar. In terms of phenolic compounds, apples are rich in a variety of phenolic classes, including dihydrochalcones, flavan-3-ols, flavonols, anthocyanins, hydroxycinnamic acids, and proanthocyanidins. A summary of the bioactive profile of apples and other traditional fruits is given in Table 3. Apple peels, seeds, and flesh predominantly contain (on dry weight, DW basis) phenolics such as chlorogenic acid (3.9–32.90 mg/100g), (+)-catechin (0.3–9.41 mg/100g), (−)-epicatechin (0.9–7.43 mg/100g), procyanidins B1 (0.0–6.8 mg/100g ) and B2 (0.9–9.72 mg/100g DW), rutin (0.0–24.3 mg/100g DW), and phloridzin (0.7–864.42 mg/100g), with the seeds also containing protocatechuic acid (2.43–9.41 mg/100g) and quercetin (2.73–5.51 mg/100g, Feng et al., 2021).
 Click to view | Table 3. Phytochemicals from selected traditional fruits |
The color of apple is mainly due to the accumulation of cyanidin-3-O-galactoside, an anthocyanin, on the peel (Dar et al., 2019). Anthocyanins act as antioxidants when the body’s endogenous antioxidants cannot deplete ROS/RNS for inefficiency or excessive amounts. They facilitate the body’s intrinsic antioxidant function, mitigating the attack of ROS/RNS on lipids, protein, and DNA (Mickle et al., 2019).
The phenolic composition of apples, similar to other fruits, is highly influenced by the variety, degree of ripening, environmental factors, and storage conditions. For instance, procyanidins B1, B2, and C1 have been found in significant amounts in Idared apple, while the sample compounds were not detected in the McIntosh, red delicious, and gala varieties. The same is true for quercetin-3-galactoside, which has a concentration of 1.52–4.31 mg/g DW in Idared apple, but it is not found in the McIntosh variety. The content of chlorogenic acid can also be discrepant among varieties, with Red Rome apple yielding 2.31 mg/g DW, while Idared and Granny Smith apples present 1.77 and 0.67 mg/g DW, respectively (Kalinowska et al., 2014). Moreover, differences in phenolic extraction methods, solvent system, and assay protocols can also cause discrepancies when analyzing the phenolic content and profile of apple and other fruits across multiple studies (Ambigaipalan et al., 2016).
Apple pomace, the waste fraction of apple juice extraction, represents 30% of the original fruit and is composed of peels, core, seeds, calyx, stems, and soft tissue. The phenolics reported in this fraction include phloridzin, phloretin, quercetin, and quercitrin (Rana et al., 2021). This portion also contains high levels of protocatechuic, 4-hydroxybenzoic, caffeic, p-coumaric, ferulic, and isoferulic acids in the insoluble-bound form (Li et al., 2020).
A phenolic-rich extract obtained from apple pomace was found to have anticancer activity by decreasing the viability of oral carcinoma cell line after 24 and 30 h of the extract application. The same extract also displayed anti-inflammatory effect by inhibiting nitric oxide production by 88.88–89.54% at 100 μg/mL (Rana et al., 2021).
Apples (Malus domestica) and grapes (Vitis vinifera) contain quercetin, a flavonol with anti-inflammatory properties. This molecule interrupts the expression of pro-inflammatory cytokines in RBL-2H3 and HMC-1 mast cell lines (Park et al., 2008), inhibits tumour necrosis factor (TNF)-induced NF-κ B and p300-CBP (transcription factor) rescue to the pro-inflammatory gene promoter in murine small intestinal epithelial cells (Ruiz et al., 2007). Besides, quercetin can reduce the levels of total cholesterol, triacylglycerols, and low-density lipoprotein; it also de-escalates hyperglycemia and high-density lipoprotein levels in animal models (Vinholes et al., 2016).
2.1.2. Banana
Similar to apple, banana is also an extremely popular fruit, being one of the primary food crops of the world, behind rice, wheat, and maize. Banana peel, the fruit’s main by-product, makes up around 40% of banana’s total weight. Although regarded as waste, this portion is rich in bioactive compounds, such as α- and β-carotenes, flavonoids, and non-flavonoid phenolics, depending on the cultivar (Bashmil et al., 2021). In terms of total phenolic content, banana peel is a superior source of phenolic compounds than its edible counterpart, ranging from 4.95 to 47 mg gallic acid equivalents (GAE)/g DW, which is 1.5–3 times higher than the levels encountered in banana flesh (Vu et al., 2018). Their phenolic profiles also differ, with the peels presenting a variety of phenolic acids, such as 3-hydroxyphenylpropionic acid and p-coumaroyl glycolic acid, as well as glycosylated flavonols, including isorhamnetin-3-O-glycoside, myricetin-3-O-rutinoside, and quercetin-3-O-xylosyl-glucuronide. The pulp contains ferulic and caffeic acids and the isoflavonoid 2′-hydroxyformononetin. Meanwhile, the anthocyanin delphinidin, as well as the coumarins scopoletin and umbelliferone have been found in both edible and no-edible fractions (Bashmil et al., 2021). Ferulic acid and rutin have also been reported as major components in the phenolic fraction of banana’s peel and flesh.
The antioxidant compounds present in banana peel lend themselves for use in food formulation. For example, Zaini et al. (2022) have compiled results from several studies where banana peel powder and extract were used to protect meat products against oxidation. These included chicken nuggets (55% greater lipid oxidation protection than the control), raw poultry meat (38.3% less thiobarbituric acid reactive substances than the control), and fish ball (0.5–1.5% peroxide value reduction regarding the control) (Zaini et al., 2020; Devatkal et al., 2014; Ali et al., 2019). It should be noted that enhanced oxidation protection of bioactives extracted from natural sources, such as banana peel, can be achieved by encapsulation of the natural extracts in order to protect their antioxidant efficacy.
As a health-promotion ingredient, flavonoid-rich banana peel extract has been assessed for its antitumor activity against MCF-7 breast cancer cell line (Durgadevi et al., 2019). The findings showed that cell viability was drastically reduced from 91.14 to 24.7% when increasing the concentration of the extract from 20 to 200 μg/mL. The extracts also exhibited typical apoptotic and necrotic morphological characteristics, including condensed nuclei, membrane blebbing, and formation of apoptotic bodies.
2.1.3. Mango
The recovery of bioactive compounds from fruits and their discarded fractions may open several possibilities for their use as novel functional ingredients. Besides, giving a proper destination to residues helps decrease the burden of agri-food waste on the environment. The processing of mango, one of the most popular tropical fruits, generates 15–25 million tonnes of by-products every year worldwide (Marçal and Pintado, 2021). As a source of fiber, vitamins C and E, phenolics, and carotenoids, these waste fractions are currently being studied as functional ingredients and natural antioxidants systems. According to Sanchez-Camargo et al. (2019), a carotenoid-rich extract obtained from mango peel through supercritical CO2 extraction displayed a higher oxidation protection factor for sunflower oil than pure all-trans-β-carotene. In the work of Siacor et al. (2020), phenolics from mango seed kernel were encapsulated with maltodextrin by spray drying. The newly formed capsules presented total phenolic contents ranging from 50.62 to 96.14 mg GAE/g and DPPH radical scavenging activity of 114.14–129.46 mmol Trolox equivalents (TE)/100 g.
Mango presents a rich and diverse phenolic profile. Salinas-Roca et al. (2018) identified gallic (the predominant one), dihydroxybenzoic, and chlorogenic acids, as well as mangiferin and quercetin in freshly-cut mangos. However, after 14 days of storage at 4 °C, dihydroxybenzoic and chlorogenic acids were degraded and could no longer be found in the samples. Meanwhile, Lee et al. (2021) investigated the phenolic composition of discarded mango flesh and found that they were not appealing to consumers. A total of 86 phenolics were identified in five different mango varieties, including hydroxybenzoic and hydroxycinnamic acids, anthocyanins, flavan-3-ols, flavones, flavanones, flavonols, isoflavonoids, coumarins, lignans, and stilbenes. Significant concentrations of quercetin, kaempferol, chlorogenic, caffeic, and gallic acids were also found.
In order to optimize the bioactive profile and biological activity of fruit by-products, an appropriate treatment should be conducted, as demonstrated by de Ancos et al. (2018). The effects of freeze-drying and hot-air drying on the phenolics and carotenoids of mango peels and paste have been compared. The samples presented a wide range of phenolics, including xanthones, benzophenones, gallates, ellagic acid derivatives, and flavonoids, with mangiferin detected as the main phenolic compound in all samples. As for carotenoids, all-trans-β-carotene, 9-cis-β-carotene, all-trans-lutein, and 13-cis-β-cryptoxanthin were identified in all samples. The carotenoids showed higher concentrations in the freeze-dried samples compared to the hot-air-dried ones, while most phenolics were unaffected by both drying treatments.
Phenolics isolated from mango seeds, namely 6-O-galloyl-5′-hydroxymangiferin, mangiferin, and a mixture of 5-hydroxymangiferin and methyl gallate demonstrated hypoglycemic effects in diabetic rats to a greater extent than glimepiride, an anti-diabetic medication (Amran et al., 2013). In addition, (+)-catechin and chlorogenic acid have previously shown a selective and noncompetitive inhibition of the maltase-glucoamylase and sucrase-isomaltase subunits of alpha-glucosidase (Simsek et al., 2015).
2.1.4. Orange
Oranges are popular sources of vitamin C. Some varieties, such as Sanguinelli and Tarocco have been reported to have an ascorbic acid content of 66 mg/100g of edible portion, which represents around 68% of the required daily requirement for this nutrient. This fruit is also a source of flavonoids, such as naringenin, hesperidin, quercetin, and rutin, in both the pulp and peels, and phenolic acids, including gallic, vanillic, caffeic, ferulic, p-coumaric, and chlorogenic acids (Farag et al., 2020).
Color is one of the main attributes sought by the consumers when buying sweet oranges. The carotenoid composition is responsible for providing the characteristic color of this fruit. According to Zacarías-García et al. (2021), xanthophylls make up a large proportion of Valencia Late and Valencia Ruby oranges, with β-cryptoxanthin, zeaxanthin, antheraxanthin, luteoxanthin, and violaxanthin detected. Phytoene and lycopene are the major carotenoids in Valencia Ruby, with 13.31 and 0.86 mg/100 g FW, respectively, while carotenoids in Valencia Late are predominantly composed of violaxanthin (0.38 mg/100 g FW).
2.1.5. Grapes
Grapes and grape-derived products are excellent sources of anthocyanins and proanthocyanidins. This fruit has a massive global annual production of around 75 million tonnes, being classified as one of the major fruit crops of the world. From the total volume of grapes produced, approximately 50% is destined to wine production, from where many by-products are generated (e.g., seeds, pomace, skins, stems, leaves). Grapes are outstanding sources of proanthocyanidins, ranging from 0.5 to 6.4 mg of catechin equivalent/g of fresh fruit, with the seeds contributing about 30% to the total content (Unusan, 2020).
The proanthocyanidin profile of grape seeds include the monomers (+)-catechin and (−)-epicatechin, as well as procyanidins B1, B2, B3, B4, B1-3-O-gallate, B2-3-O-gallate, B2-3′-O-gallate, and C1. According to Kuhnert et al. (2015), procyanidins with a degree of polymerization higher than five make up 47–81% of grape seed procyanidins, while 7–14% correspond to monomers and dimers, and 0 to 5.3% to trimers and tetramers. The administration of a proanthocyanidin-rich extract obtained from grape seeds to mice fed a high-fat diet mitigated the formation of pro-inflammatory cytokines, namely tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1), while enhancing insulin sensitivity. The extract also modulated gut microbiota by stimulating the growth of Protobacteria phylum, which is related to leanness and metabolic health (Liu et al., 2017).
On the other hand, red grape skins have high levels of anthocyanins, responsible for their color. Some of the anthocyanins identified in grape skins include cyanidin-3-O-rutinoside, peonidin-3-O-glucoside, malvidin-3-O-glucoside, and delphinidin-3-,5-O-diglucoside (Pereira et al., 2020; Tan et al., 2020). Anthocyanins from grape skin have demonstrated antitumor effects on MCF-7 breast cancer cell lines. The application of the anthocyanin-rich extract led to cell apoptosis by increasing ROS generation. This effect was more prominent for the anthocyanin extract when compared to purified anthocyanins, which suggests a synergistic effect between the different anthocyanins present in the extract.
2.2. Exotic fruits
The importance of traditional fruits in the diet is noteworthy, if consumed on a large scale. Nevertheless, other lesser-known and underutilized fruits also show a wide variety of bioactive compounds with health-promoting effects. The study and popularization of these unconventional fruits help diversity of the available sources of bioactives and opens new possibilities for the development of functional ingredients and nutraceuticals. Table 4 summarizes the bioactive compounds encountered in selected exotic fruits.
 Click to view | Table 4. Phytochemicals form selected exotic fruits |
2.2.1. Buriti
Buriti (Mauritia flexuosa) is classified as a neotropical dioecious palm species usually distributed in swamp areas across South America. Its fruits possess a bittersweet taste and vibrant orange color due to the high concentration of β-carotene. Figure 2 shows the physical appearance of buriti and other exotic fruits. The pulp and peel of buriti contain 5.82–26.7 and 1.86–2.86 mg/g of β-carotene, respectively. These fractions also present polyphenols, such as gallic acid, catechin, ellagic acid, and quercetin (Milanez et al., 2018). According to Hamacek et al. (2018), buriti is also a good source of vitamin C at 59.93 mg/100 g. The presence of a great variety of antioxidant substances explains the high antioxidant capacity observed in several studies for extracts obtained from this fruit.
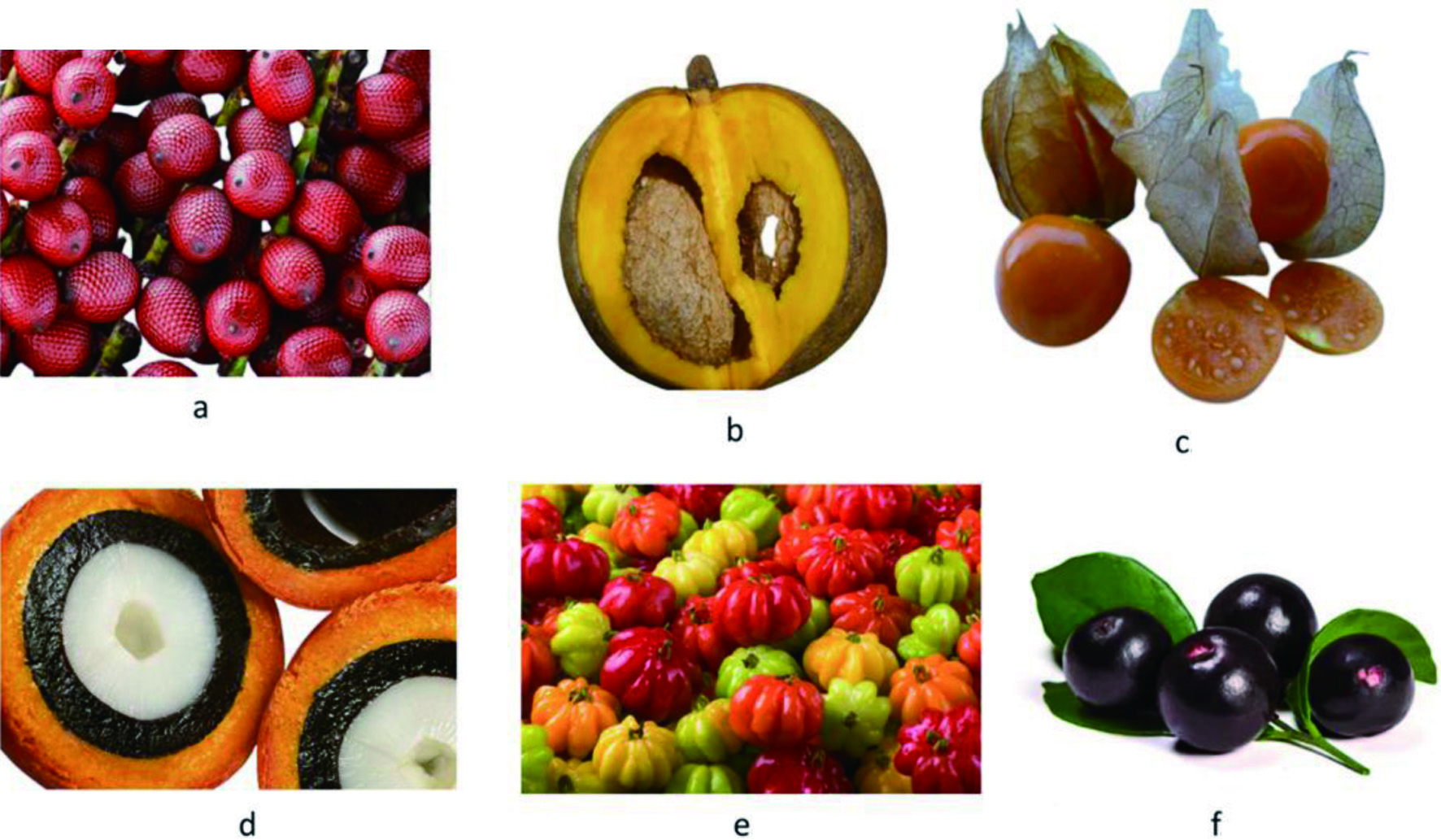 Click for large image | Figure 2. Exotic fruits found in the Amazon region. (a) buriti, (b) mamey apple, (c) camapu, (d) tucumã, (e) pitanga, (f) açaí. |
Buriti seeds and shells make up to 40 and 22% of the fruit’s total mass, respectively, and are usually used for animal feed. However, Rudke et al. (2021) reported their phenolic profile, with 28 phenolics quantified in buriti seeds and 33 in the shells, predominantly flavonoids, phenolic acids, coumarins, and aldehydes. Protocatechuic acid was the main phenolic present in both samples, ranging from 107 to 445.59 μg/g of the seed extract and 114.77–688.49 μg/g of the shell extract, depending on the method of extraction. Buriti shell extract obtained by pressurized liquid extraction also showed a high content of quercetin (885.38 μg/g).
As a source of antioxidants, buriti by-products (shells, endocarp, and bran) have been used to produce flour for further use as a functional ingredient (Resende et al., 2019). The blanched shells flour retained the highest amount of total soluble phenolics (934.6 mg GAE/100 g) while their unblanched counterparts had the highest concentration of carotenoids (1,186.7 μg/100 g) among flours of all by-products. The technological properties of the flours (water and oil retention capacities, water solubility index, and swelling capacity) were comparable to by-product flours obtained from other natural sources, such as rice bran flour, orange peels, pequi peels, and coconut fiber.
The bioaccessibility and bioavailability aspects of some bioactive compounds is a major concern as previous studies have shown their low absorption rate and further metabolism in the gastrointestinal system. Berni et al. (2020) have used microemulsion encapsulation of buriti carotenoids in order to enhance their bioefficiency. The fruit pulp was used to create oil-in-water microemulsions with corn oil, being further subjected to dynamic gastrointestinal system to assess the bioaccessibility of the carotenoid fraction. The results showed a low β-carotene recovery after the gastric phase (3.1% for the non-encapsulated pulp and 5.4% for the microemulsions). Although the overall bioacessibility was low, microemulsion encapsulation was able to slightly increase it from around 4% (non-encapsulated pulp) to approximately 6% (microemulsion form).
2.2.2. Mamey apple
The Amazon region is home to a wide range of native fruits. Some of them are unheard of in many parts of world, but the study of their bioactive profile could allow for further investigation of their benefits. Some examples of underexplored Amazonian fruits are mamey apple (Mammea americana) and camapu (Physalis Angulata). A comparison between the two fruits has shown a higher total phenolic content for mamey than camapu. Regardless, a UPLC-MS analysis has revealed a wide variety of phenolic compounds, with a predominance of terpenoids (61 compounds), followed by phenolic acids (58 compounds), and flavonoids (53 compounds) in both fruits (Lima et al., 2020).
Mamey apple is described as a climacteric fruit, protected by a thick skin (20–27% of the whole fruit) and bearing one to four bitter seeds (7–21% of fruit’s mass). The fruit’s pulp is a source of carotenoids and phenolic compounds, as described by Armelle et al. (2022). The authors demonstrated the presence, on a fresh weight basis in mg/100g, of (−)-epicatechin (1.96–2.47), sinapic acid hexoside (0.42–1.12), isoquercitrin (0.06–0.19), and procyanidins B1 (0.43–0.78), B2 (0.04–0.07), B5 (0.05–0.12), and C1 (0.50–3.48). Procyanidins are oligomers and polymers of catechin and epicatechin that impart astringency, bitterness, and sourness to products. They present numerous health benefits, such as suppression of blood glucose increase after starch intake, improvement of blood circulation by strengthening capillaries, arteries, and veins, protection against sun damage, and enhanced joint flexibility, demonstrated in vitro and in animal studies (Rauf et al., 2019). Therefore, consumption of procyanidin sources, such as mamey apple, is highly desirable from a health-promoting standpoint.
The carotenoid content of mamey apple (4.42 mg β-carotene equivalents/100 g) is comparable to that of carrot (5.47 mg β-carotene equivalents/100 g), a popular source of this bioactive category. A carotenoid-rich extract from mamey apple could supress oxidative damage in Caenorhabditis elegans by 20–30%, presenting a better outcome when compared to pure β-carotene, arising from synergistic effect between different carotenoids in the extract (González-Peña et al., 2021).
2.2.3. Camapu
Camapu is a small fruit (1–1.5 cm in diameter), containing 100–300 seeds in its core (de Oliveira et al., 2020). The fruit’s bioactive profile includes significant concentrations of vitamin C (26.70 mg/100 g), total phenolic compounds (59.9 mg GAE/100 g), and carotenoids (5.95 μg/100 g) (Guiné et al., 2020).
The pulp of the green camapu is rich in protocatechuic (11.0 mg/100 g DW), p-hydroxybenzoic (34.0 mg/100 g DW), caffeic (10.0 mg/100 g DW), and sinapic (9.0 mg/100 g DW) acids in the soluble form. However, as the fruit ripens, their contents decline sharply (0.8, 2.0, 1.0, and 2.0 mg/100 g DW, respectively). Ferulic acid is mainly present in the insoluble-bound form, and present at 17.0 mg/100 g DW in the green and and 13.0 mg/100 g DW in the ripe fruits (de Oliveira et al., 2020). A simulated in vitro gastrointestinal digestion showed that only around 40–50% of camapu’s phenolic compounds remain available after the small intestine phase, with the remaining not being detected after the oral and gastric phases (Guiné et al., 2020). Therefore, strategies to increase their bioefficiency are necessary.
2.2.4. Tucumã
Tucumã (Astrocaryum aculeatum), another Amazonian fruit, has an oval shape with a yellowish epicarp and a fleshy orange mesocarp. The endocarp is hard and black, while the pulp presents an oily texture and a sweet taste. Tucumã’s color is due to the presence of carotenoids, mainly all-trans-β-carotene. As a lipophilic pigment, this carotenoid is primarily encountered in the pulp oil (21.269–74.77 mg/100 g), followed by the fresh peel (32.608 mg/100 g), and fresh pulp (10.7 mg/100 g) (da Fonseca Machado et al., 2021). Overall, all-trans-β-carotene corresponds to 75% of all carotenoids present in this fruit, followed by significantly lower concentrations of 13-cis-β-carotene (2%), all-trans-α-carotene (2%), and all-trans-β-cryptoxanthin (2.8%) (Sagrillo et al., 2015). The bioactive profile of tucumã also comprises a variety of flavonoids (in mg/100g), namely rutin (1,276–3,054), quercetin (103–1,272), kaempferol (513), and catechin (107), as well as phenolic acids, including gallic (375–831), caffeic (833–984), ellagic (845), and chlorogenic (304) acids (da Fonseca Machado et al., 2021).
The rich bioactive composition of tucumã is a key factor for positive outcomes related to health promotion. Sagrillo et al. (2015) observed cytoprotective effects of ethanolic extracts from tucumã’s peel and pulp on lymphocyte cultures subjected to oxidation by hydrogen peroxide, a major ROS. The extracts were able to increase cell viability in the dose range of 300–900 μg/mL, with optimum resultsbetween 300 and 600 μg/mL. Extract concentrations greater than 100 μg/mL were sufficient to protect DNA from oxidative damage. In addition, the activity of caspases 1, 3, and 8, which play a role on orchestrating apoptosis, was significantly reduced by the introduction of bioactive-rich extracts.
In a study by Jantsch et al. (2021), the effect of tucumã extracts (concentration of 250 mg/kg) on memory loss and brain cortex redox balance was assessed in hyperlipidemia rats. The administration of the extract significantly reduced protein and lipid oxidation, with thiobarbituric reactive substances (TBARS) in the latter reaching the level in the control animals (no hyperlipidemia). Reactive oxygen species were also sharply declined by the application of tucumã extract, and memory loss was improved as demonstrated by an enhanced object recognition index.
2.2.5. Pitanga
Pitanga (Eugenia uniflora L.), also known as Brazilian berry, is composed of 77% pulp and 23% of seeds. The fruit has a sweet and acidic taste accompanied by an intense aroma. Depending on the genotype, ripe pitanga can be yellow, red, or purple in color. Its popularity as an antioxidant-rich fruit has been growing in South American countries, such as Brazil, Argentina, Uruguay, and Paraguay, where pitanga is the main ingredient of several products (e.g., jams, frozen pulp, ice creams, juices, liquors) (Fidelis et al., 2022).
From all pitanga genotypes, the purple one contains the highest total phenolics at 463 mg GAE/100 g, followed by the red variety at 210 mg GAE/100 g. Besides, purple pitanga is a source of anthocyanins, where delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, pelargonidin-3-O-glucoside, cyanidin-3-O-pentoside, and cyanidin derivative are present with the peels presenting a higher concentration of these compounds compared to the pulp (Fidelis et al., 2022). Red pitanga is one of the major sources of lycopene in nature. Filho et al. (2008) found 33.22 μg/g (3.322 mg/100g) of all-trans-lycopene in pitanga pulp. Besides, lutein, zeaxanthin, and β-cryptoxanthin was also identified.
An anthocyanin-rich extract (predominantly composed of cyanidin-3-O-glucoside and delphinidin-3-O-glucoside) obtained from pitanga fruit (seedless) was able to increase the lifespan and the reproduction rate of Caenorhabditis elegans worms. The effect was significantly higher than that in worms not subjected to the extract treatment. Furthermore, upon exposure to hydrogen peroxide, oxidative stress was sharply decreased in nematodes treated with pitanga extract (Tambara et al., 2018). Moreover, pitanga juice was shown to inhibit enzymatic activity of α-glucosidase (57.91–69.47%), a target for type 2 diabetes treatment (Siebert et al., 2020).
2.2.6. Açaí
Throughout the last two decades, açaí (Euterpe oleracea) has experienced an exponential popularity, going from an exotic fruit appreciated by the local population of Northern Brazil to a highly demanded superfruit in other parts of Brazil as well as internationally. This growth is associated with a general perception that açaí and its many derived products are healthy, nutritious, and have a pleasant flavor. In 2019, Brazil produced 1,398,328 tonnes of processed açaí, the raw material for a wide range of products, including beverages, energy drinks, and ice creams, among others (Barbosa and de Carvalho Junior, 2022).
As observed for other fruits, the processing of açaí also generates by-products, with the seeds being the main one for this particular fruit. Açaí seeds make up around 80–85% of the fruit’s total mass. With that being said, it is estimated that for every 100 tonnes of açaí processed, 80 tonnes of waste are produced. In most cases, these by-products are inappropriately discarded in landfills, even though they could be re-incorporated into the food chain, following a circular economy concept (Barbosa and de Carvalho Junior, 2022).
Açaí seeds are a rich source of oligomeric proanthocyanidins, with levels ranging from 1.5 to 6.1 mg of procyanidin B1 equivalents/g. They are predominantly composed of procyanidins type A and B, with medium degree of polymerization (>3,000 Da). Oligomeric proanthocyanidins have been related to anticancer activity by promoting apoptosis of malignant cells (Del Pozo-Insfran et al., 2006; Matta et al., 2020).
Additionally, açaí pulp is a well-known source of anthocyanins, with a predominance of cyanidin-3-glucoside and cyanidin-3-rutinoside, making up a large proportion of the fruit’s total phenolic content. For comparison, white açaí pulp contains 8.2–11.0 mg GAE/g of total phenolics, anthocyanin-rich purple açaí pulp has 4.3–44.7 mg GAE/g (Matta et al. 2020). Phenolic acids can also be found in açaí, including vanillic, caffeic, syringic, ferulic, p-coumaric, 3,4-hydrozybenzoic, and p-hydroxybenzoic acids (Carvalho et al., 2017).
Alqurashi et al. (2017) investigated the fate of açaí polyphenols after digestion, using an in vitro model. According to the authors, 49.8% of the initially identified phenolics reached the colon. By using mixed-culture fermentations with fecal inoculate, it was observed that the presence of polyphenols in the colon reduced the growth of pathogenic Bacteroides prevotella ssp. and Clostridium histolyticum besides stimulating the production of short-chain fatty acids.
| 3. Effect of food processing on bioactive compounds from traditional and exotic fruits | ▴Top |
The bioactives from dietary sources become available to the human body in the gastrointestinal tract by going through different metabolic processes and reaching the target tissue in sufficient amounts to render their intended effect. Processing can affect the bioavailability of bioactive compounds by decreasing/increasing the compound’s release from the matrix, as well as degrading or modifying the original compound’s structure, which can impact its bioactivities (Skrede et al., 2000). Additionally, different types of bioactives and/or food matrices are affected in distinct ways by the same processing. Therefore, a case-by-case evaluation is necessary to understand how processing transformations can either enhance or compromise the nutritional value and bioactivity of food and its components.
Minimally processed food includes fresh/cut fruits and vegetables. The possible modifications include slight changes in the physical form of the raw material in order to make it more appealing to the consumers, which include trimming, peeling, washing and cutting (Cantwell, 2013). Processing can be classified into chemical, biological, and physical methods. The chemical processing applies preservatives or pH manipulation, biological processing usually involves fermentation, while physical processing can be either thermal or non-thermal. During thermal processing, the product is exposed to high temperature for a short time (pasteurization and sterilization) or low temperature for a long time (retorting and drying). On the other hand, non-thermal processing does not involve temperature fluctuations, such as irradiation, high pressure processing, and ultrasonication (Clark et al., 2009; Karel and Lund, 2003; Ohlsson and Bengtsson, 2002).
Regardless of the type of processing, exposure to oxygen and light may occur, thus degrading the bioactive substances (Bode et al., 1990). For instance, oxidized phenols cause browning as a response to polyphenol oxidase (Vámos-Vigyázó and Haard, 1981). In addition, damage to the fruit’s tissue produces diverse physiological disarrays, hence reducing quality (Soliva-Fortuny and Martín-Belloso, 2003).
On the other hand, higher bioactive yields can be obtained after processing. For instance, heat can release insoluble-bound phenolics by breaking the linkage between them and cell wall macromolecules, making them more available for intestinal absorption (Howard et al., 1999). However, excessive temperature and exposure to high temperature for long periods can degrade some phenolics, carotenoids, and other bioactive classes (Leong and Oey, 2012). Thus, optimum conditions must be employed to retain and possibly enhance the bioactives present in the source material.
3.1. Effect of food processing on vitamin C
During thermal processing, vitamin C can be exposed to high temperature, light, and oxygen, which may drastically reduce its content (Hung et al., 2007). Depending on the operational parameters and oxygen content, heat processing can decrease the content of vitamin C by 20–90% (Uddin et al., 2002).
Apart from processes involving the application of high temperature, even minimal processing can deplete ascorbic acid due to oxygen exposure (Cantwell, 2013), directly impacting the vitamin’s antioxidant activity (Leong and Oey, 2012). The quantity of vitamin C in selected fruits after processing is given in Table 1.
A study by Gil et al. (2006) reported that fresh-cut mangoes stored at 5 °C retained over 95% of their original ascorbic acid content after six days, significantly higher than that for pineapple, kiwifruit, and cantaloupe which lost 10, 12 and 25% of their vitamin C, respectively, over the same period. Low-temperature storage, such as refrigeration (4 °C), has been shown to preserve vitamin C to a greater extent than room temperature storage (Li et al., 2017). However, very low freezing temperatures (−16 °C) showed a 30% reduction in the vitamin C level of apples (Mieszczakowska-Frąc et al., 2021).
The use of non-conventional processing techniques can be considered as a strategy to preserve thermosensitive bioactive compounds such as vitamin C. Tiwari et al. (2009) demonstrated that ultrasound treatment of orange juice increased shelf-life (27 to 33 days at 10 °C) compared to the thermal treatment (90 °C/21 sec), which lasted for 19 days under the same storage conditions. Gomes et al. (2022) also observed a similar outcome for sonoprocessed freshly squeezed orange juice which kept its original vitamin C content, as opposed to thermally treated juice (7% ascorbic acid reduction).
High pressure processing (HPP) is another alternative to thermal treatment with the goal of inactivating enzymes and microorganisms. Usually, HPP does not negatively affect low-molecular-weight bioactive compounds. At the same time, the modifications caused on the microstructure of foods due to the high pressure can increase the extractability and bioaccessibility of bioactives. According to de Ancos et al. (2020), Naval and red-fleshed Cara Cara orange juices treated by HPP at 200 and 400 MPa showed only a slight degradation (<5%) of their vitamin C content, with the only exception being a treatment involving 400 MPa and 40 °C for 1 min, which decreased 30% in the ascorbic acid concentration; possible pressure-induced activation of peroxidase and ascorbate oxidase enzymes, might have been responsible for degradation of vitamin C.
Pulsed electric field (PEF) is another promising technology that prevents microbiological spoilage of food without the use of heat treatment. The samples are treated with high intensity electric field pulses for microseconds in a processing chamber. This method is generally used for liquid foods, such as juices, soups, and dairy beverages. PEF-treated apple juice maintained the same vitamin C content as untreated apple juice during refrigerated storage after 72 h (Dziadek et al., 2019). In another study (Wibowo et al., 2019), apple juice was processed by PEF, HPP, and pasteurization (low intensity −72 °C/15 sec and high intensity −85 °C/30 sec) and stored for 3 weeks at 4 °C. Over 90% vitamin C retention was observed for most treatments, except for high intensity pasteurization. However, after the storage period, there was a sharp decline for ascorbic acid content (<25% of retention) in all treated samples. Nevertheless, PEF- and HPP-treated apple juice still presented higher vitamin C concentrations than their pasteurized counterparts. Ascorbic acid oxidation during storage may occur due to increased oxygen diffusion into the juice and produces dehydroascorbic acid and later degrades to 2,3-diketogulonic acid. Formation of furfural and 3-hydroxy-2-pyrone can also be observed upon vitamin C degradation during storage.
Freeze-drying is a common dehydration technique which is particularly used in products with a high content of thermolabile compounds. Nevertheless, as highlighted by Silva-Espinoza et al. (2019) and Barbosa et al. (2015) when dehydrating orange puree and probiotic orange powder, respectively, optimum freeze-drying conditions should be established as long drying periods can diminish vitamin C levels to a greater extent than high-temperature processes conducted for a short time, such as spray drying.
3.2. Effect of food processing on phenolic compounds
The phenolic profile of fruits can significantly change according to the type of processing employed. Usually, processes involving high temperature over a long period of time cause loss and/or reduction in the quantity of thermo-sensitive phenolics. On the other hand, moderate heat can release insoluble-bound phenolics by breaking the bond between phenolic compounds and macromolecules from complex matrices, making them more available for intestinal absorption. A clinical study conducted by Quirós-Sauceda et al. (2017) detected higher maximum plasma concentrations of mango phenolics, including chlorogenic acid, upon consumption of mango juice when compared to mango flesh.
The manufacture of fruit-based products can be highly beneficial from a nutritional standpoint, but other portions of fruits can also be procured as functional ingredients for novel formulations. Sogi et al. (2013) produced mango powders from the fruit’s peel and kernel by four different drying technologies, namely hot-air, vacuum, infrared, and freeze-drying. The powders produced form freeze-drying presented the highest total phenolic content, as well as the highest antioxidant capacity as evaluated by ABTS, DPPH, ORAC, and FRAP assays.
Pasteurization has routinely been used in the production of fruits to ensure microbiological safety. However, non-conventional approaches have also been studied as alternatives to better preserve the bioactives of such products. Barrón-García et al. (2022) compared the effects of pasteurization (72 °C/2 min) with Ohmic heating (15–20 V/cm) on the phenolic composition of mango pulp. This technique relies on the Joule effect, where the application of an alternating current through the food matrix is dissipated as heat. Heat formation is proportional to the voltage and the material’s electrical resistance. For food samples, moderate electric fields (<100 V/cm) are usually applied. According to the authors, changing the treatment did not affect the phenolic profile and both were able to preserve the composition of the fresh pulp. In addition, mangiferin and gallic acid were detected as main contributors to the antioxidant activity, measured by the ABTS assay.
Novel technologies can increase the yield of phenolics recovered from their natural sources. For instance, high hydrostatic pressure and pulsed electric field increased the yield of anthocyanins by more than 50% from grape by-products (skins, stems, and seeds) as opposed to heating at 70 °C. While anthocyanin monoglucosides were better extracted with PEF, HPP was a more suitable method for obtaining acylated anthocyanins. Solvent pH decreases during HHP, thus enhancing the extraction of acylated anthocyanins since they are more stable at pH <4, where flavylium cations are prevalent (Corrales et al., 2008). PEF has also been applied to orange peel, promoting a significant increase in the total phenolic yield (20–159%), antioxidant capacity (51–192%), and naringin (from 1.0 to 3.1 mg/100 g FW) and hesperidin (from 1.3 to 4.6 mg/100 g FW) levels when compared to the untreated samples (Luengo et al., 2013).
Some processes are able to inactivate pathogenic/spoilage microorganisms and browning enzymes while preserving the quality parameters of the food. Cold plasma is another alternative to thermal processing where samples are subjected to ionized gases produced by microwave, radio-frequency, or electrical discharges. Farias et al. (2020) applied dielectric barrier discharge plasma (excitation frequencies ranging from 50 to 900 HZ) on apple cubes. Besides promoting a partial inactivation of polyphenol oxidase activity, cold plasma also preserved or even surpassed the total phenolic content of untreated samples. Phenolic concentration was proportional to polyphenol oxidase inactivation as this enzyme uses phenolics as a substrate. In another study (Ramazzina et al., 2016), atmospheric double-barrier discharge was used on fresh-cut apples. A slight decrease in the level of phenolics was observed as opposed to the untreated sample. However, phenolic extracts from cold plasma-treated apples were tested in Caco-2 cell lines, where no reduction in cell viability, caused by the accumulation of ROS, was observed. In addition, the extracts did not interfere with the physiological cell response to oxidative stress.
Cold plasma has also been used as a pre-treatment for drying tucumã pulp. After cold plasma application, the samples were hot-air dried at 60 °C for 3 h. Dehydration caused a significant loss of total phenolics in comparison with fresh tucumã pulp. Among dehydrated samples, the cold plasma-treated samples showed higher phenolic levels (approximately 45 mg GAE/g) than their untreated counterparts (approximately 20 mg GAE/g) (Loureiro et al., 2021).
The ability of non-conventional processing techniques in increasing the bioaccessibility of phenolic compounds has also been assessed. Apples subjected to high-pressure processing (400 MPa/35 °C/5 min) showed increased bioaccessibility for hydroxycinnamic acids and dihydrochalcones than their untreated counterparts, but this was not observed for flavonols. Regardless, phenolic bioaccessibility was reduced stepwise from the oral phase to the intestinal phase digestion (Fernández-Jalao et al., 2020).
Açaí pulp has also been subjected to atmospheric cold plasma (Dantas et al., 2021), where a medium excitation frequency of 500 Hz for 5 min increased the level of total phenolics by 38.8%, while maintaining the same concentration of anthocyanins observed in the untreated sample. Cold plasma also had a positive effect on the bioaccessibility of açaí pulp phenolics, enhancing the release of catechin, epicatechin, epigallocatechin gallate, procyanidin B1, rutin, caffeic acid, and chlorogenic acid by 194.70, 383.3, 68.84, 130.71, 16.84, 341.48, and 57.63%, respectively.
On the other hand, thermal treatment may not be a good option when it comes to anthocyanin preservation. Pasteurization at 85 °C for 1 min caused a reduction of 40% in anthocyanin content in açaí juice compared with the untreated sample. Meanwhile, high pressure processing (HPP; 400–600 MPa/5min/20 °C) was able to maintain the same anthocyanin level as the control. Moreover, the use of 500 MPa aided cell rupture and promoted a higher release of non-anthocyanin phenolics than the thermal treatment. This increased phenolic concentration reflected the juice’s antioxidant activity, which was also enhanced, as measured by the ORAC assay (da Silveira et al., 2019).
3.3. Effect of food processing on carotenoids
Innovative food processing techniques may be used for value-added production of commodities from fruit waste with economic return to the processors. In several fruits, such as mango, carotenoids may not be available for intestinal absorption due to their association with dietary fiber. In order to overcome this hurdle, Mercado-Mercado et al. (2018) subjected mango by-products (peels and paste) to a probe-type ultrasound treatment, which increased the yield of β-cryptoxanthin and β-carotene compared to the control. Furthermore, the ultrasound technique significantly increased the bioaccessibility of lutein by 46.04%, β-cryptoxanthin by 44.16%, and β-carotene by 44.01%. The authors hypothesized that the cavitation process promoted changes to the matrix while the energy applied broke the bond between carotenoids and dietary fiber. In addition, with matrix re-structuring, bound carotenoids became more exposed to digestive enzymes, thus facilitating their release.
The lipophilic nature of carotenoids is a major limitation for their use in water-dominated environments. In order to overcome this hurdle and open new possibilities for carotenoid use in novel formulations, Santos et al. (2021) developed microcapsules of tucumã oil, a carotenoid-rich source, with gum Arabic by spray drying at two different temperatures (120 and 180 °C). Carotenoid retention was higher in the microcapsules produced at 120 °C (101.9–105.2%), with the ones produced at 180 °C retaining 84.8–86.5%. Regardless of spray drying temperature, the encapsulated tucumã oil showed significant water dispersibility (93.8–98.1%) and up to 177-fold greater oxidative stability compared to non-encapsulated oil. Moreover, an in vitro digestion study showed that 64% of carotenoids contained in the microcapsules were released upon complete digestion.
PEF can be used both as a treatment for carotenoid-rich fruit beverages and as a pre-treatment for drying fruit pulp. Kumar et al. (2019) compared the effects of pasteurization and PEF on the carotenoid content of mango nectar and detected that PEF-treated nectar exhibited a very similar total carotenoid content (17.4–21.0 μg/g) when compared with its untreated counterpart (20.1–21.6 μg/g) throughout the storage period (90 days at 5 °C and 30 days at room temperature). On the other hand, carotenoid levels dropped significantly in pasteurized samples (13.6–16.4 μg/g). Lammerskitten et al. (2020) used PEF as a pre-treatment before subjecting mango pulp to convection and vacuum drying. Maximum carotenoid yields were obtained upon PEF treatment at 1 kJ/kg, regardless of the drying method that followed. However, the authors also pointed out that higher PEF intensities may cause degradation of bioactives. Therefore, process optimization with an emphasis on the bioactive content should always be performed as a first step.
The nutritional value of fruit juice partly relies on the extractability of bioactives, which means that these compounds should be released from the fruit matrix and further dissolved into the juice. HPP also aids the extractability of phytoene (by 40%) and phytofluene (by 10-fold) from Navel orange for juice production. The process increased the release of some carotenoids form the cell structures where they are located. However, this was not observed for lycopene from Cara-Cara orange, which presented a 36–50% decrease upon HPP application. Lycopene is a highly non-polar carotene, and it occurs in crystal form in chromoplasts of juice vesicle cells. This arrangement causes higher environmental exposure, which can lead to increased instability and susceptibility to oxidation (de Ancos et al., 2020).
HPP is a preferred processing option for carotenoid protection over heat treatment. Barba et al. (2012) subjected an orange juice-milk beverage to various conditions of HPP and heat treatment at 90 and 95 °C. Higher carotenoid levels were detected when HPP was applied for 420 and 540 sec, surpassing the concentration recorded for the untreated control and thermal-treated samples. The authors hypothesize that this could be due to HPP being able to avoid carotenoid thermo-oxidation and also by pressure-induced changes in the matrix structure, allowing a higher carotenoid release.
| 4. Conclusion | ▴Top |
The nutritional value of fruits goes far beyond their vitamins and macronutrients, such as fiber. Traditional and exotic fruits are excellent sources of several classes and individual bioactive compounds, including ascorbic acid, phenolics, and carotenoids. Besides acting as antioxidants in a number of ways in order to mitigate oxidative stress, these substances have also been reported to carry other biological activities, such as reduction of pro-inflammatory cytokines, decreasing the viability of cancerous cells, and inhibiting enzymes related to type 2 diabetes. Additionally, lesser-known fruits, namely buriti, mamey, camapu, and tucumã have vitamin C contents comparable to highly popular ascorbic acid sources (e.g., oranges). Pitanga, another exotic fruit, is one of the richest sources of lycopene in nature. Besides, it should be emphasized that fruits must be used as a whole, as in many cases, their by-products are very rich in bioactives, which in many cases surpass their edible counterparts. This can be seen for proanthocyanidins in grape seeds and anthocyanins in grape skins.
Processing can be used as a means to expand the use of fruits beyond their raw consumption. However, specific treatments need to be carefully considered. Drastic thermal treatments can significantly reduce vitamin C, phenolic, and carotenoid levels. Meanwhile, mild thermal processes and non-conventional techniques, such as high-pressure processing, ohmic heating, and pulsed electric field, may increase the release of bound phenolics and enhance the bioaccessibility of selected bioactives.
| References | ▴Top |