| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 17, March 2022, pages 73-82
Isorhamnetin at physiologically attainable nanomolar concentrations inhibits adipocyte differentiation and lipid droplet accumulation in vitro
Diana Carvajal-Aldaz, Karen McDonough, Jack N. Losso*
School of Nutrition and Food Sciences, Louisiana State University, Baton Rouge, LA 70803, USA
*Corresponding author: Jack N. Losso, School of Nutrition and Food Sciences, Louisiana State University, Baton Rouge, LA 70803, USA. Tel: 225-578-3883; E-mail: jlosso@agcenter.lsu.edu
DOI: 10.31665/JFB.2022.17305
Received: March 19, 2022
Revised received & accepted: March 30, 2022
| Abstract | ▴Top |
Differentiation of preadipocytes into adipocytes is a major step leading to obesity. This study examines the effects of isorhamnetin, a metabolite of quercetin, at physiological and supraphysiological concentrations on the differentiation of 3T3-L1 pre-adipocyte to adipocyte. Comparison was made with the effect of quercetin on 3T3-L1 differentiation under the same conditions. Cell viability during adipocyte differentiation for 8 days in the presence of isorhamnetin and quercetin was above 94 and 97%, respectively. Oil Red O staining showed significant differences (P < 0.05) between the effect of isorhamnetin or quercetin on cytoplasmic lipid droplet accumulation and control untreated cells. Isorhamnetin at physiologically attainable concentrations was more effective than quercetin in inhibiting cytoplasmic lipid droplet accumulation. Neither isorhamnetin nor quercetin had an effect on the expression of macrophage chemoattractant protein-1 (MCP-1). CCAAT/enhancer binding protein α (C/EBP-α) was down-regulated by isorhamnetin. Compared to the control, isorhamnetin or quercetin decreased PPAR-γ 1 and 2 expressions. The data indicate that isorhamnetin was more effective than quercetin at physiologically attainable concentrations in reducing lipid accumulation in 3T3-L1 pre-adipocytes.
Keywords: Isorhamnetin; Quercetin; 3T3-L1; Pre-adipocyte differentiation; MCP-1; PPAR-gamma; C/EBP-alpha
| 1. Introduction | ▴Top |
Isorhamnetin (3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one occurs naturally in seabuckthorn and berries (Lee et al., 2009). Isorhamnetin is also the major metabolite of quercetin (3,3′,4′,5,7-penthydroxyflavone). Quercetin is one of the most abundant dietary flavonoids present in fruits, vegetables and beverages and its antioxidative properties have been associated with cardiovascular health and anti-cancer activities (Jiang et al., 2019; Russo et al., 2012). The main sources of quercetin are red onions, red delicious apples, asparagus, red leaf lettuce, cranberry juice, red wine and tea (Dixon Clarke and Ramsay, 2011; Hertog et al., 1993). A diet rich in fruits and vegetables can provide 68 mg of quercetin per day; however, on the average 5–40 mg of quercetin is consumed per day (Harwood et al., 2007). It has been shown that the daily intake of quercetin can be increased to 200–500 mg per day in individuals who consume quercetin rich foods such as apples, onions and tomatoes (Harwood et al., 2007). Studies have shown that quercetin can act as an antioxidant at low doses, prooxidant at high doses, anti-inflammatory, and regulate lipid accumulation (Dajas, 2012; Fujimori and Shibano, 2013; Pandey et al., 2012; Vargas and Burd, 2010). In dietary sources, quercetin is found conjugated to a variety of carbohydrates. In onions, quercetin is conjugated to glucose and is found as quercetin-4′-O-β-D-glucoside and quercetin-3-4′-O-bis-β-D-glucoside; in apples quercetin is conjugated to galactose, arabinose, rhamnose, xylose and glucose while berries contain quercetin arabinosides (Erlund, 2004). Quercetin is absorbed as an aglycone and the deglycosylation of quercetin depends on the carbohydrate conjugated to it and the availability of the appropriate enzyme such as epithelial β-glucosidase or intestinal bacteria rhamnosidase in human astrointestinal tract (Dixon Clarke and Ramsay, 2011). Quercetin is absorbed as is or is metabolized and absorbed as isorhamnetin or tamaraxetin (Egert et al., 2008).
Isorhamnetin as an antioxidant, and an anti-inflammatory compound, inhibits monoamine oxidase A and has antitumor activity (Dixon Clarke and Ramsay, 2011; Manach et al., 2005; Yan et al., 2002). The antioxidative and anti-inflammatory activities of isorhamnetin are mediated via HO-1 induction through the NF-E2-related factor 2 (Nrf2) mechanism and a PPAR-γ activation pathway (Christensen et al., 2010; Ramachandran et al., 2012). Kong et al. (2012) reported that micromolar concentrations of isorhamnetin inhibit the adipogenic activity of 3T3-L1 cells (Kong et al., 2012). However, the fate of isorhamnetin or quercetin when incubated with 3T3-L1 cells in DMEM is not known.
Obesity is major risk factor for several chronic degenerative diseases including hypertension, type 2 diabetes, coronary heart diseases, and cancer. Plasma concentrations of metabolites such as isorhamnetin are in the nanomolar range (Dixon Clarke and Ramsay, 2011). The objective of this study was to evaluate the efficacy of isorhamnetin or its parent compound quercetin at physiologically attainable concentration on biomarkers of 3T3-L1 differentiation.
| 2. Materials and methods | ▴Top |
2.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM), Pre-adipocyte Expansion Medium (PEM) composed of 90% DMEM and 10% bovine calf serum, differentiation medium (DM) containing 90% DMEM and 10% FBS, and adipocyte maintenance medium (AMM) containing 90% DMEM, 10% FBS and 1.0 μg/ml Insulin were purchased form Gibco Life Technologies, Parsley, PA, USA). Calf serum and fetal bovine serum (FBS) were obtained from ATLANTA® Biological (Lawrenceville, GA, USA). Dexamethasone, insulin, isorhamnetin, oil red O, and the PeroxideDetect Kit were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methyl isobutylxanthine (IBMX) was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). Quercetin of more than 98% purity and isorhamnetin of more than 95% purity were purchased from LKT Labs (St. Paul, MN, USA). Anti-β-catenin (cat number 4977), β-actin (catalog number 8480), C/EBP-α (catalog number 8178), and PPAR-γ (catalog number 2443) monoclonal antibodies were obtained from Cell Signaling (Danvers, MA, USA). Alkaline phosphate-conjugated secondary antibody was from Santa Cruz Biotechnology® (Santa Cruz, CA, USA). SuperSignal® West Pico Chemiluminescent Substrate was from Thermo Scientific (Rockford, IL, USA).
2.2. Cell culture
3T3-L1 cell line was obtained from American Type Culture Collection (ATCC) (Rockville, MD, USA). 3T3-L1 is a continuous substrain of 3T3 (Swiss albino) developed through clonal isolation (Zebisch et al., 2012). 3T3-L1 pre-adipocyte differentiation was performed following the ATCC protocol. Cells were seeded and maintained with PEM for 48 h by incubating at 37 °C and 5% CO2, or after reaching 100% confluence. Next, identical volume of medium was replaced with DM, 1.0 μM dexamethasone, 0.5 mM isobutylmethylxanthine (IBMX), and 1.0 μg/ml Insulin) (Day 0) and incubated for 48 h at 37 °C in humidified atmosphere containing 5% CO2. Then, the differentiation medium was replaced with AMM. AMM was changed every 72 h. Finally, the cells were fully differentiated on day 10 after induction. Pre-adipocyte cells were fully differentiated between 8 and 12 days after DM application. To examine the effect of quercetin or isorhamnetin on adipocyte differentiation, 3T3-L1 cells were treated with PEM until day 10 in the absence or presence of 20 nM to 10 µM of isorhamnetin or quercetin. In addition, a flask of cells containing PEM (non-differentiated cells) was maintained until day 10.
2.3. MTS cell viability assay
The 3T3-L1 cells were seeded in 96-well plates at a density of 1.0 × 104 cells per well. The cells were initiated for differentiation as described earlier. Stock solutions of 100 μM and 10 μM of pure isorhamnetin or quercetin were prepared with PEM, DM, and AMM. Different concentrations of isorhamnetin or quercetin (25 nM to 10 μM) were tested during the differentiation period. The cells were incubated for 1, 3, 5, 7, and 8 days at 37 °C in an incubator with 5% CO2. After incubation, cell viability was determined according to the protocol provided by the supplier of CellTiter 96 AQueous One solution (Promega, Madison, WI, USA). Twenty microliters of MTS reagent were added to every well and incubated under the same conditions for 4h. Absorbance of the plates was read at 490 nm in a BioRad Model 680 micro plate reader (Hercules, CA, USA). The number of viable cells was directly proportional to the absorbance of formazan formed due to the reduction of MTS. Cell viability was expressed as the percentage of control cells. Treatments were tested in triplicates.
2.4. Oil red O staining intracellular triacylglycerols
The 3T3-L1 cells were seeded in 6-well plates at a density of 8 × 104 cells per well. Cells were subjected to differentiation following the protocol described above including non-differentiated, control, and different concentrations of isorhamnetin or quercetin (25 nM to 10 μM) in triplicates. On day 10 of differentiation, 3T3-L1 adipocytes were washed with PBS and fixed with 10% formalin for 30 min. The cells were washed with distilled water twice, stained for 45 minutes in a 37 °C incubator with diluted oil red O solution. Treatments were photographed with a microscope OLYMPUS Model DP72 (Center Valley, PA, USA). Finally, the dye retained in 3T3-L1 cells was eluted with isopropanol and pipetted in a 96 well-plate to measure the absorbance by a microplate spectrophotometer BIORAD Benchmark PlusTM (Philadelphia, PA, USA) at 510 nm. Briefly, the oil red O solution was prepared from 0.05 g of oil red O dissolved in 100 ml of propanol (stock solution), then six parts of stock solution were mixed with four parts of distilled water, and this mixture was vacuum filtered.
2.5. Analysis of biomarkers during 3T3-L1 differentiation
In order to study the inhibitory effects of isorhamnetin or quercetin on differentiation, the prevention approach was tested. Cells were treated in the presence or absence of isorhamnetin or quercetin before and during adipocyte differentiation. The 3T3-L1 cells were seeded in T25 flasks at a density of 2 × 105 cells per flask and subjected to differentiation following the protocol previously described. Cells were treated with isorhamnetin or quercetin at concentrations of 0 to 10 μM from the expansion period until the completion of adipocyte differentiation on day 10. Finally, the supernatant and the cell lysates were collected for further analysis.
The inhibitory effect of isorhamnetin or quercetin on cell differentiation was determined by collecting the supernatants on days 0, 2, 5, 7, and 10 that were used to measure the MCP-1 levels by ELISA using commercial ELISA kits from eBioscience® (San Diego, CA, USA). All analyzes were performed in triplicate.
The anti-adipogenic properties of isorhamnetin or quercetin were measured during 3T3-L1 differentiation. The levels of β-catenin, PPAR-γ, and C/EBP-α were determined on day 10 after differentiation by Western blot. 3T3-L1 cells were rinsed once with ice-cold phosphate buffered saline (1X PBS), then lysed in 240 µl of radioimminoprecipitation assay buffer (RIPA) supplemented with protease inhibitor cocktail, sodium orthovanadate and PMSF for 30 min at 4 °C. Cells were harvested by scraping and transferred into an Eppendorf, incubated on ice for 20 min, centrifuged at 10,500 ×g for 30 min, and the supernatant was saved in an Eppendorf. Protein concentration in the supernatant was determined by the BCA method using BCA protein Assay Reagents (Pierce Chemical, Rockford, IL). Extracts containing 20 µg protein from control, quercetin or isorhamnetin treated 3T3-L1 cells were mixed with LDS sample buffer (Invitrogen, Carlsbad, CA, USA), water, boiled for 3 minutes for protein denaturation, and 20 µl of samples were loaded onto a 12 well 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA, USA), ran for 35 min at 200 V and 125 mA. After protein separation, they were transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA) for 1h 10 min at 30 V and 170 mA. Five percent bovine serum albumin (BSA) in 1X PBS containing 0.05% Tween 20 (PBS-Tween) was used to block the membrane for 1h at room temperature. Then the membrane was incubated with primary antibody (dissolved in PBS-Tween containing 5% BSA as manufacturer’s instructions) overnight at 4 °C with gentle agitation. The next day, the membrane was washed 3 times for 10 min each with PBST, secondary antibody (dissolved in PBS-Tween containing 5% BSA as manufacturer’s instructions) was incubated for 1h at room temperature and washed again 3 times for 10 min each with PBST. Finally, the blots were developed by enhanced chemiluminescence with SuperSignal® West Pico Chemiluminescent substrate and exposed to X-ray film (Kodak X-omat 1000A processor).
2.6. Statistical analysis
Data were expressed as mean ± standard deviation from triplicates of each experiment. Statistical analysis was performed using the Statistical Analysis Software (SAS) (version 9.2). Differences between control and treatments were determined by analysis of variance (ANOVA) and followed by Tukey analysis. A P-value of < 0.05 was considered statistically significant.
| 3. Results and discussion | ▴Top |
3.1. Cell viability
The effects of isorhamnetin or quercetin on 3T3-L1 cell viability during the eight-day pre-adipocyte differentiation period were determined using 3T3-L1 cells treated with 0 to 10 µM of isorhamnetin, quercetin or non-differentiated cells maintained only on PEM. Data were collected on day 1, 3, 5, 7, and 8; cell viability results are expressed as the percentage of the control cells. The results for isorhamnetin are shown in Figure 1a. There was no significant difference in viability among non-differentiated cells, the control, and 25 nM to 10 µM isorhamnetin treatments. The cell viability results for quercetin are also shown in Figure 1b. Similar to isorhamnetin, there was no significant difference among non-differentiated cells, control, and 25 nM to 10 µM quercetin treated cells. During adipocyte differentiation in the presence of isorhamnetin or quercetin, average cell viability was 94.84 and 97.63%, respectively. Isorhamnetin or quercetin at the concentrations used was not cytotoxic to 3T3-L1 during the differentiation from pre-adipocytes to adipocytes.
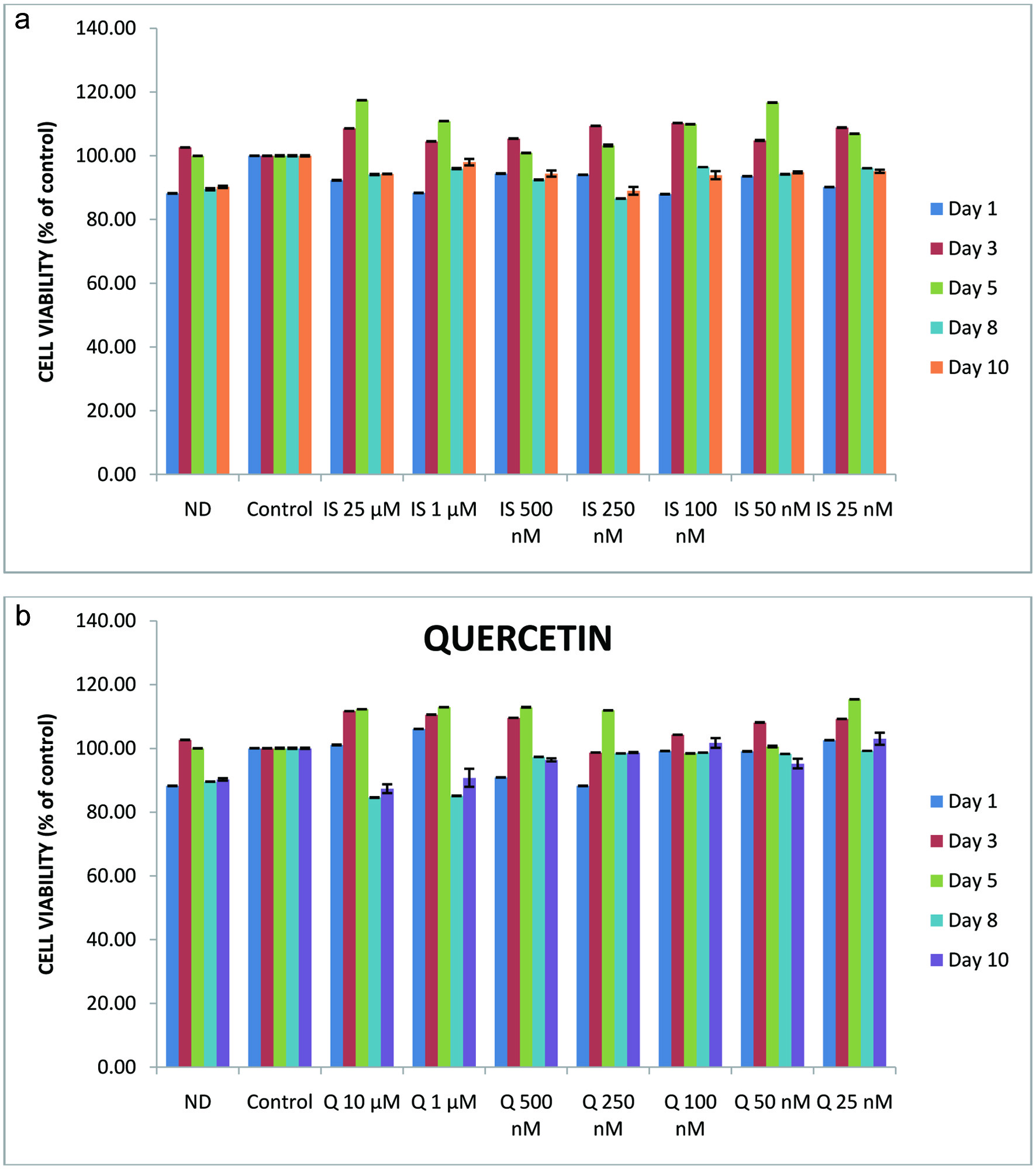 Click for large image | Figure 1. Effects of isorhamnetin (a) or quercetin (b) on 3T3-L1 cell viability. Non-differentiated 3T3-L1 cells were treated with isorhamnetin (0 to 25 µM) or quercetin (0 to 10 µM) for 0 to 8 days. The values are expressed as percentage of control cells. |
3.2. Effect of isorhamnetin and quercetin on lipid accumulation
Obesity is characterized by increased adipose tissue mass that results from increased fat cell size (hypertrophy) or number of adipocytes (hyperplasia). Adipose tissue serves as the major energy reservoir in the body, storing excess energy as lipids and releases the lipids for energy on demand. Adipocytes also constitute an endocrine system which secrete TNF-α and IL-6 adipokines (Kanda et al., 2012). Recent studies have shown that phytochemicals can down regulate adipose tissue mass accumulation by inhibiting adipogenesis from fibroblastic pre-adipocytes to mature adipocytes (Ejaz et al., 2009; Ju et al., 2011; Kim et al., 2012; Yang et al., 2008).
Results in Figure 2 clearly show the difference between non-differentiated cells and the control on day 10. Non-differentiated cells (ND) maintained the initial fibroblast morphology and showed no lipid accumulation except for the control, where the intracellular lipid accumulation is observed in oil red O staining. The addition of isorhamnetin showed a significant reduction (P = 0.001; P < 0.05) in lipid accumulation among non-differentiated cells, the control and 25 nM to 10 µM isorhamnetin-treated cells (Figure 2a and b). Control differentiated cells were significantly (P < 0.05) different from all other treatments. There was a significant difference between cells treated with 25 nM isorhamnetin and the control cells (P < 0.05). Tukey’s test results showed that cells treated with 50 nM to 10 µM isorhamnetin were not significantly different from non-differentiated cells. Isorhamnetin anti-adipogenic effect was observed at a concentration that did not significantly reduce 3T3-L1 cell viability and is physiologically attainable. While Lee et al. (2009) reported that at concentrations between 25 and 50 µM isorhamnetin significantly lowered lipid accumulation (Lee et al., 2009). Our results show that at physiologically attainable concentration in the 50–500 nanomolar range isorhamnetin can exhibit a potent anti-adipogenic effect and significantly inhibit lipid accumulation in 3T3-L1.
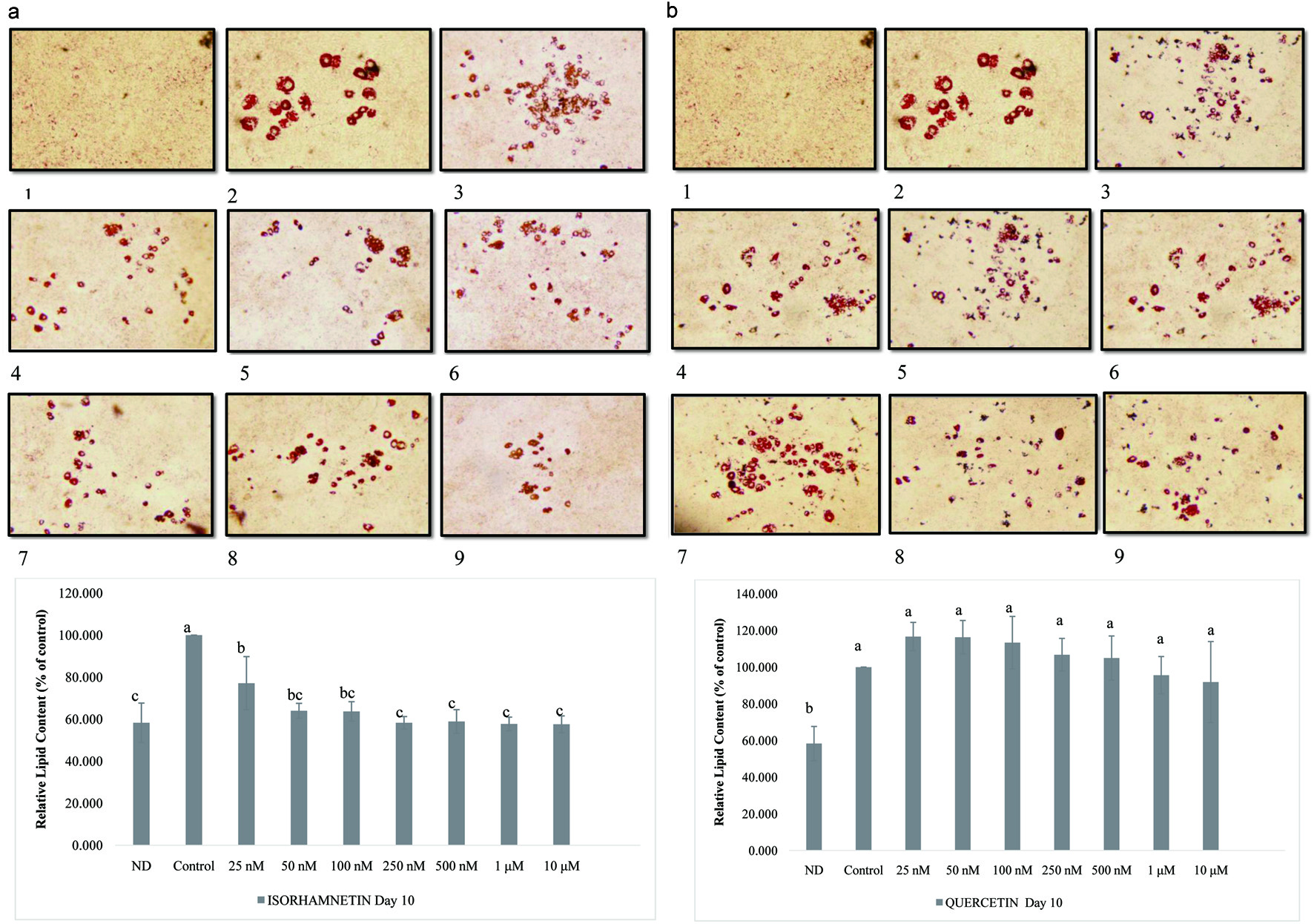 Click for large image | Figure 2. Effect of isorhamnetin (a) or quercetin (b) on lipid accumulation in 3T3-L1 cells. The values were expressed as percentage of control cells. Results are presented as mean ±S.D. (n = 3). Letters with different superscripts are significantly different (P < 0.05) among groups. (1) non-differentiated cells, (2) control, and from (3) to (10) 25 nM to 25 µM isorhamnetin- or quercetin-treated 3T3-L1 cells, respectively. Pictures were obtained with an OLYMPUS microscope Model DP72 and taken at 5X magnification. |
The physiological concentration of quercetin is ≤ 1 µM (Nicholson et al., 2010). The addition of quercetin reduced intracellular lipid accumulation in a dose-dependent manner that resulted in a significantly (P = 0.0002; P < 0.05) lower lipid accumulated in cells treated with 25 nM to 10 µM quercetin (Figure 2). There was significant difference (P = 0.0002; P < 0.05) between non-differentiated cells, the control, and 25 nM to 10 µM quercetin-treated cells. Ex vivo incubation of quercetin aglycone with gastrointestinal fluids from healthy individuals showed minimal quercetin degradation (Hollman et al., 1995). Quercetin aglycone uptake is very low (Hollman et al., 1995). However, an in vitro study using human hepatocarcinoma cell line Hep G2 showed that quercetin is rapidly metabolized into metabolites including isorhamnetin (Boulton et al., 1999), suggesting the anti-adipogenic effects observed with low concentrations of isorhamnetin may be ascribed to its viability inside the cells as opposed to quercetin. Yang et al. (Yang et al., 2008) reported that quercetin at 25 µM decreased lipid accumulation by 15.9 ± 2.5% while at 12.5 µM there was no significant difference compared to the control. Furthermore, it was found that combinations of quercetin with resveratrol down regulated lipid accumulation by 68.7 ± 0.7% (Yang et al., 2008). Yang et al. (Yang et al., 2012) reported that concentrations of quercetin from 0.1 to 20 µM showed a concentration-dependent inhibition of adipocyte differentiation.
3.3. Effects of isorhamnetin and quercetin on the secretion of MCP-1 during pre-adipocyte differentiation
During adipogenesis pro-inflammatory adipokines such as macrophage chemoattractant protein-1 (MCP-1) are released. MCP-1 plays an important role in the development of obesity-associated insulin resistance (Olefsky and Glass, 2010; Sartipy and Loskutoff, 2003; Tateya et al., 2010). The expression of MCP-1 by adipose tissue induces inflammatory reactions and insulin resistance, suggesting that the suppression of MCP-1 is important for the management of adipose accumulation (Kuroyanagi et al., 2008; Tateya et al., 2010). The levels of MCP-1 in the supernatants of 3T3-L1cells treated with vehicle, undifferentiated, or isorhamnetin are shown in Figure 3. There was no significant difference between non-differentiated cells, the control and 25 nM to 10 µM isorhamnetin treatments. These data also suggest that isorhamnetin did not affect MCP-1 levels during adipocyte differentiation. Studies in human umbilical cells stimulated with TNF-α for 24 h in order to increase MCP-1 secretion showed that quercetin at 2 to 10 µM decreased the secretion of MCP-1 by 18 and 67%, respectively (Winterbone et al., 2009). However, in these umbilical cells, isorhamnetin which is a metabolite of quercetin did not affect MCP-1 secretion. The effects of quercetin on the secretion of MCP-1 in the supernatants were also determined (Figure 3). The supernatants were collected on day 0, 2, 5, 7, and 10. Only samples from days 0, 2, and 10 were measured by ELISA. Treatments were compared to the control and non-differentiated cells. There were significant statistical differences (P < 0.0001; P < 0.05) between non-differentiated cells, the control and quercetin or isorhamnetin treatments. The means separation by Tukey’s suggested that there were differences between non-differentiated – the control and quercetin treated – non-differentiated cells. Results in Figure 3 show that there was no significant difference among non-differentiated cells, the control and 25 nM to 10 µM quercetin-treated cells suggesting that quercetin did not affect MCP-1 levels during adipocyte differentiation. Extracts rich in p-coumaric acid, quercetin, and resveratrol changed the levels of MCP-1 in 3T3-L1 cells (Yen et al., 2010) suggesting that quercetin alone does not inhibit MCP-1.
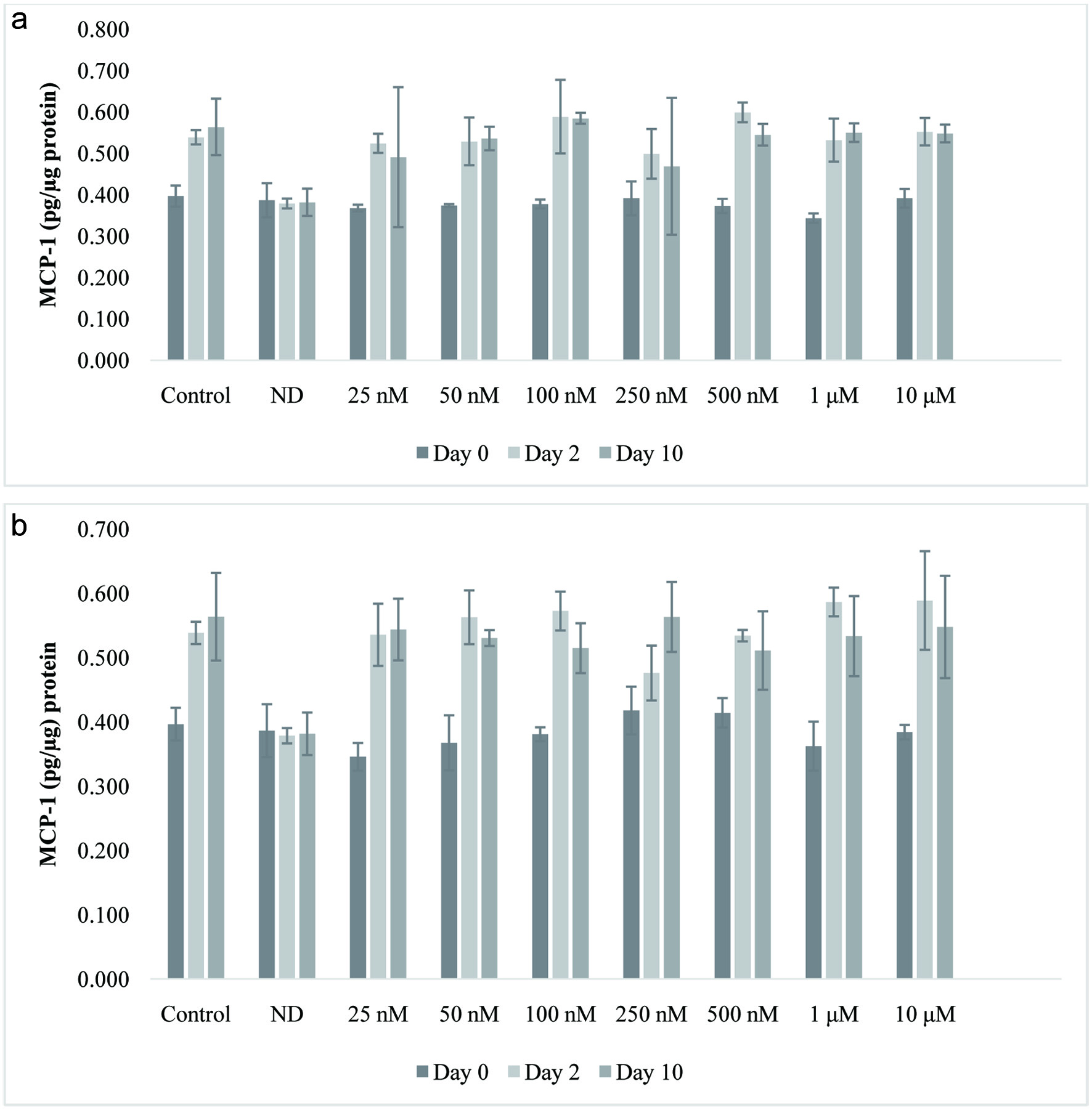 Click for large image | Figure 3. Effect of isorhamnetin (a) or quercetin (b) on MCP-1 levels (pg/µg protein) during 3T3-L1 adipocyte differentiation. Results are presented as mean ± S.D. (n = 3). Letters with different superscripts are significantly different (P < 0.05). |
3.4. Expression of β-catenin, PPAR-γ, or C/EBP-α during 3T3-L1 cell differentiation
The Wnt/β-catenin signaling pathway has been identified as a negative regulator of adipogenesis (Kennell and MacDougald, 2005; Prestwich and Macdougald, 2007; Ross et al., 2000). β-catenin has been identified as the central mediator of the Wnt/β-catenin pathway. Cell lysates were collected after PEM was replaced with DM. That was considered day 0 (D0); next the samples were collected after 5, 16, and 24 h. Subsequently after 48 h, DM was replaced with AMM every 72 h, samples were taken on day 2 (D2), day 5 (D5), day 7 (D7), and day 10 (D10). Finally, a sample from non-differentiated cells was collected on day 10. Total cell lysates were analyzed by western blot analysis as described under the materials and methods. Results are shown in Figure 4. β-catenin level decreased until day 5 and increased slightly on day 7 and 10 (Figure 4). There was significant difference for total cell lysate β-catenin expression (P < 0.0001; P < 0.05) during adipocyte differentiation. β-catenin expression decreased to 41.90 ± 2.43% of the control during early period of adipocyte differentiation. Lee et al. (2009) showed that β-catenin level decreased during the early period after DM was added.
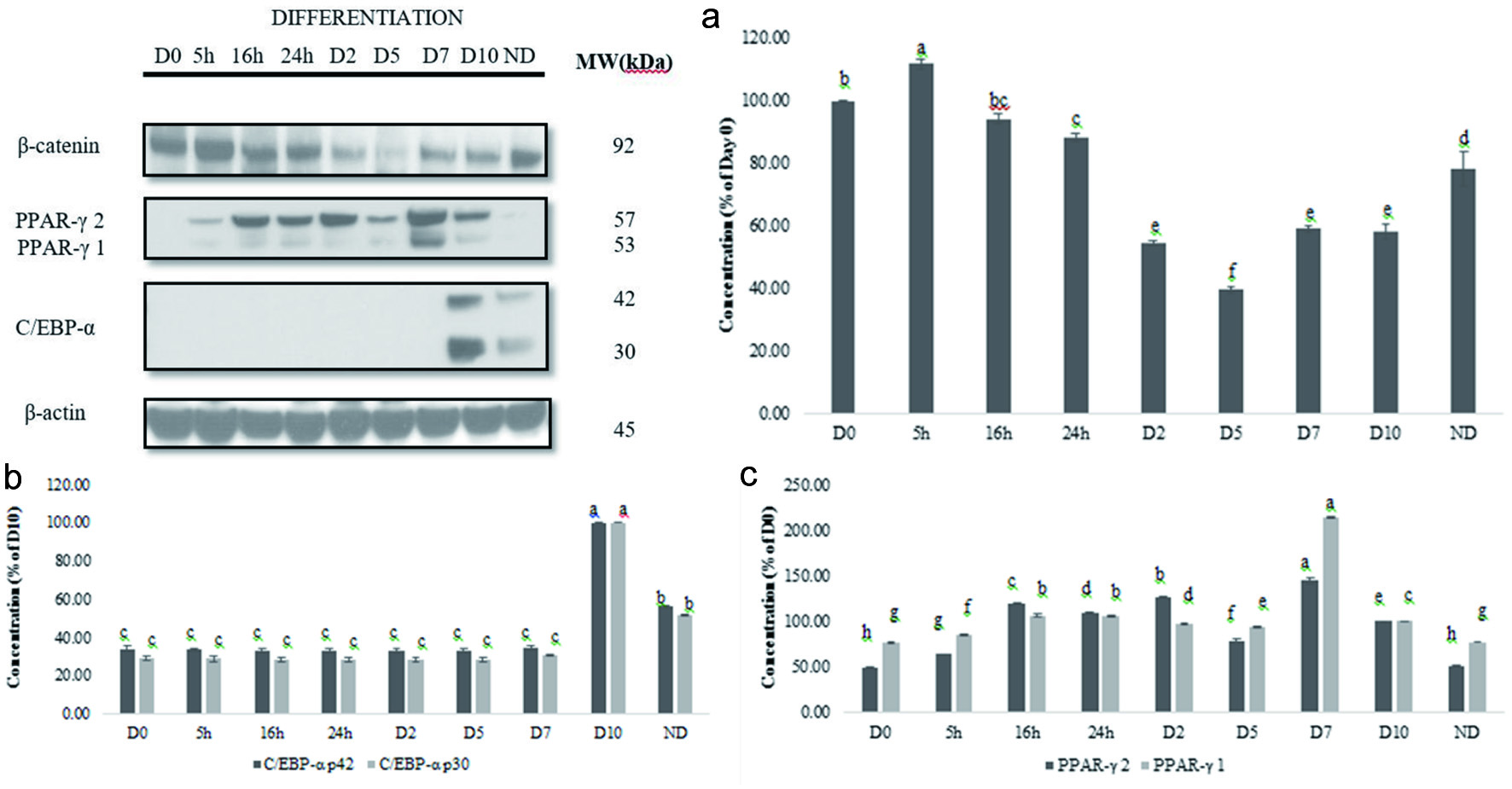 Click for large image | Figure 4. Expression of β-catenin, PPAR-γ1 and 2, and C/EBP-α during 3T3-L1 cell differentiation (a). Graphical representation of the expression of β-catenin (b), PPAR-γ 1 and 2 or C/EBP (c) during 3T3-L1 cell differentiation. Results are presented as mean ± S.D, (n = 3). Letters with different superscripts are significantly different (P < 0.05). |
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is the downstream target of β-catenin and expressed and involved in later stage of adipocyte differentiation (Cristancho and Lazar, 2011). The expression of (PPAR-γ1 and 2) during 3T3-L1 differentiation was examined using Western blot. We observed that PPAR-γ levels increased by day 10 (D10) (Figure 4). There was significant difference in PPAR-γ levels (P < 0.0001; P < 0.05). PPAR-γ 1 and 2 levels increased until D2, decreased by D5, and increased on D7 and D10. PPAR-γ 1 and 2 levels of non-differentiated cells on D10 were not significantly different from D0.
The CCAAT/enhancer binding protein α (C/EBP α) is a master regulator of adipogenesis (Christy et al., 1991). C/EBPα is critical to the progression of the final stage of adipocyte differentiation (Christy et al., 1991; Gregoire et al., 1998). The expression of C/EBP α was visible on day 10 (D10) (Figure 4). C/EBP-α p30 and p42 did not change from D0 to D7 but increased significantly from D7 to D10 (P < 0.0001; P < 0.05). C/EBP-α p30 and p42 level in non-differentiated cells was higher than D0.
3.5. Effects of isorhamnetin on β-catenin, PPAR-γ or C/EBP-α during differentiation
To evaluate the inhibition of adipocyte differentiation by isorhamnetin, the expression of β-catenin, PPAR-γ, and C/EBP-α in cells treated with 25 nM to 10 µM was examined on day 10 and compared with control and non-differentiated cells as described under material and methods. The expression of β-catenin, PPAR-γ and C/EBP-α on 3T3-L1 on D10 are shown in Figure 5. β-catenin was highly expressed in non-differentiated cells and isorhamnetin-treated cells (Figure 5). The highest expression of β-catenin level was in the control cells, and the lowest in 100 nM isorhamnetin-treated cells. There was significant difference for β-catenin expression (P < 0.0001; P < 0.05) among isorhamnetin treatments. Isorhamnetin at nanomolar concentration that is physiologically attainable can inhibit β-catenin accumulation in 3T3-L1 cells.
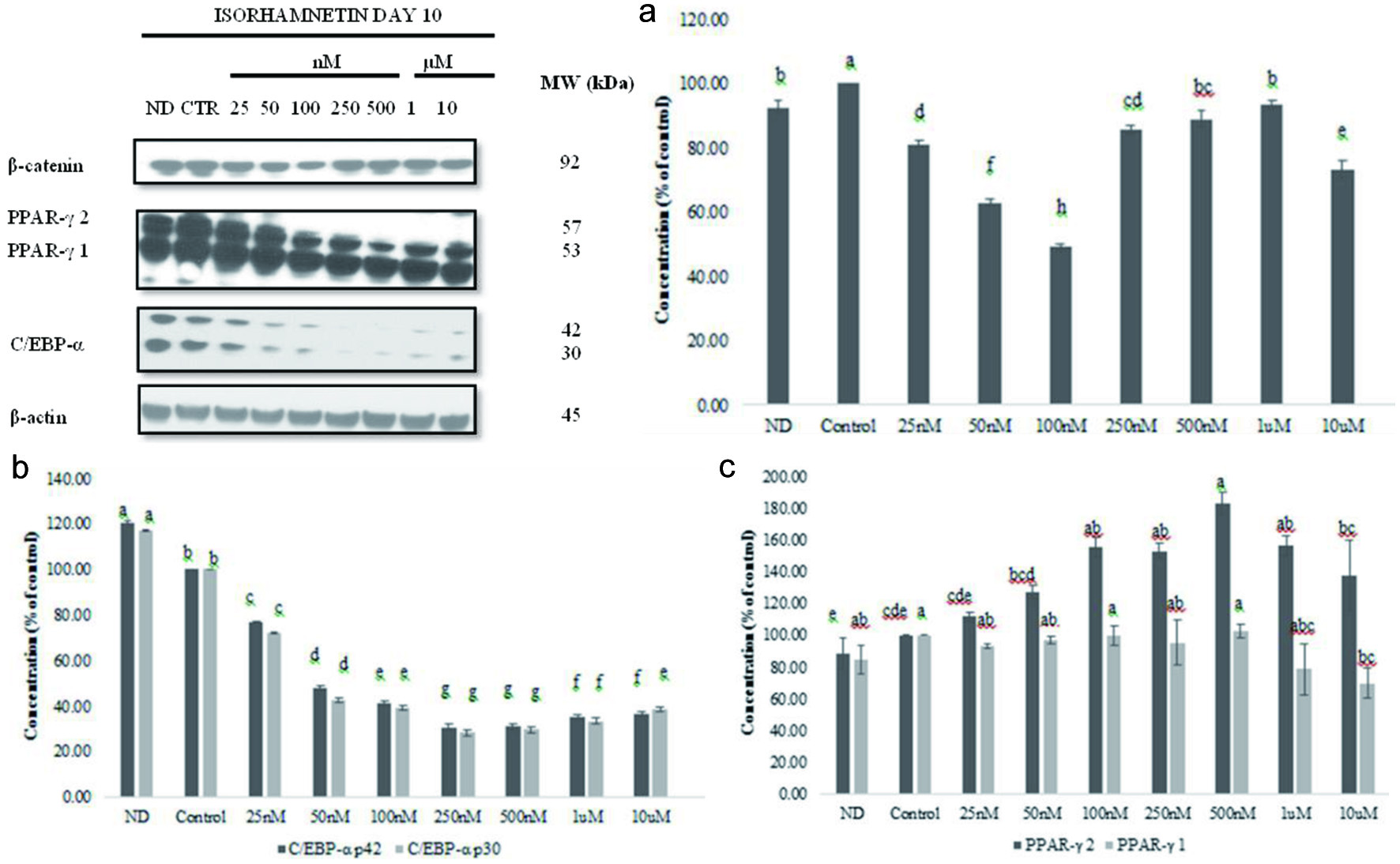 Click for large image | Figure 5. Effect of isorhamnetin on β-catenin, PPAR-γ1 and 2, or C/EBP during 3T3-L1 cell differentiation (a). Graphical representation of the effect of isorhamnetin on β-catenin (b), PPAR-γ1 and 2 (c) or C/EBP (b) (c) expression during 3T3-L1 cell differentiation. Results are presented as mean ± S.D, (n = 3). Letters with different superscripts are significantly different (P < 0.05). |
The PPAR-γ 1 and 2 expressions decreased with increasing concentrations of isorhamnetin (Figure 5). PPAR-γ 1 and 2 levels were low compared to levels in non-differentiated cells. Isorhamnetin had significant effects on PPAR-γ 1 and 2 levels (P < 0.0001; P < 0.05) (Figure 5). PPAR-γ 1 and 2 lowest levels were observed at 10 µM isorhamnetin. Lee et al. (2009) reported a decreased expression of PPAR-γ 1 and 2 by 25 or 50 µM isorhamnetin.
The results of C/EBP-α level in cells treated with 25 nM to 10 µM isorhamnetin are shown in Figure 5. The C/EBP-α p30 and p42 levels dose-dependently decreased with increasing concentration of isorhamnetin. There was significant difference (P < 0.0001; P < 0.05) among cells at increasing concentrations of isorhamnetin from 25 nM to 10 µM. Lee et al. (2009) reported significant decrease of C/EBP-α p30 and p42 levels in 3T3-L1 cells that were treated with 25 µM or 50 µM isorhamnetin during adipocyte differentiation compared to control cells (Lee et al., 2009).
3.6. Effects of quercetin on β-catenin, PPAR-γ or C/EBP-α during 3T3-L1 cell differentiation
Because of β-catenin central role in adipogenesis is through the Wnt/β-catenin pathway, it has been suggested that its targeting could prevent obesity. To evaluate the effect of quercetin on 3T3-L1 adipocyte differentiation, we determined the expression of β-catenin, PPAR-γ, and C/EBP-α in lysates of quercetin-treated cells on day 10 and compared with the control and non-differentiated cells as described under materials and methods. Results in Figure 6 show that β-catenin level was more intense in non-differentiated cells than in differentiated cells and decreased after quercetin treatment. The results of β-catenin expression in cells treated with vehicle, non-differentiated or 25 nM to 10 µM quercetin are shown in Figure 6. The highest expression of β-catenin level was in non-differentiated cells and the lowest when quercetin at 1 µM was added to the 3T3-L1 cells. Level of β-catenin did not follow a trend, suggesting that β-catenin expression was not dose dependent. There was significant difference in β-catenin expression (P < 0.0001; P < 0.05) with increasing concentrations of quercetin from 25 nM to 10 µM. The levels of β-catenin in non-differentiated cells were higher than in the control cells. These data suggest that the expression of β-catenin is not regulated by quercetin in a concentration dependent manner.
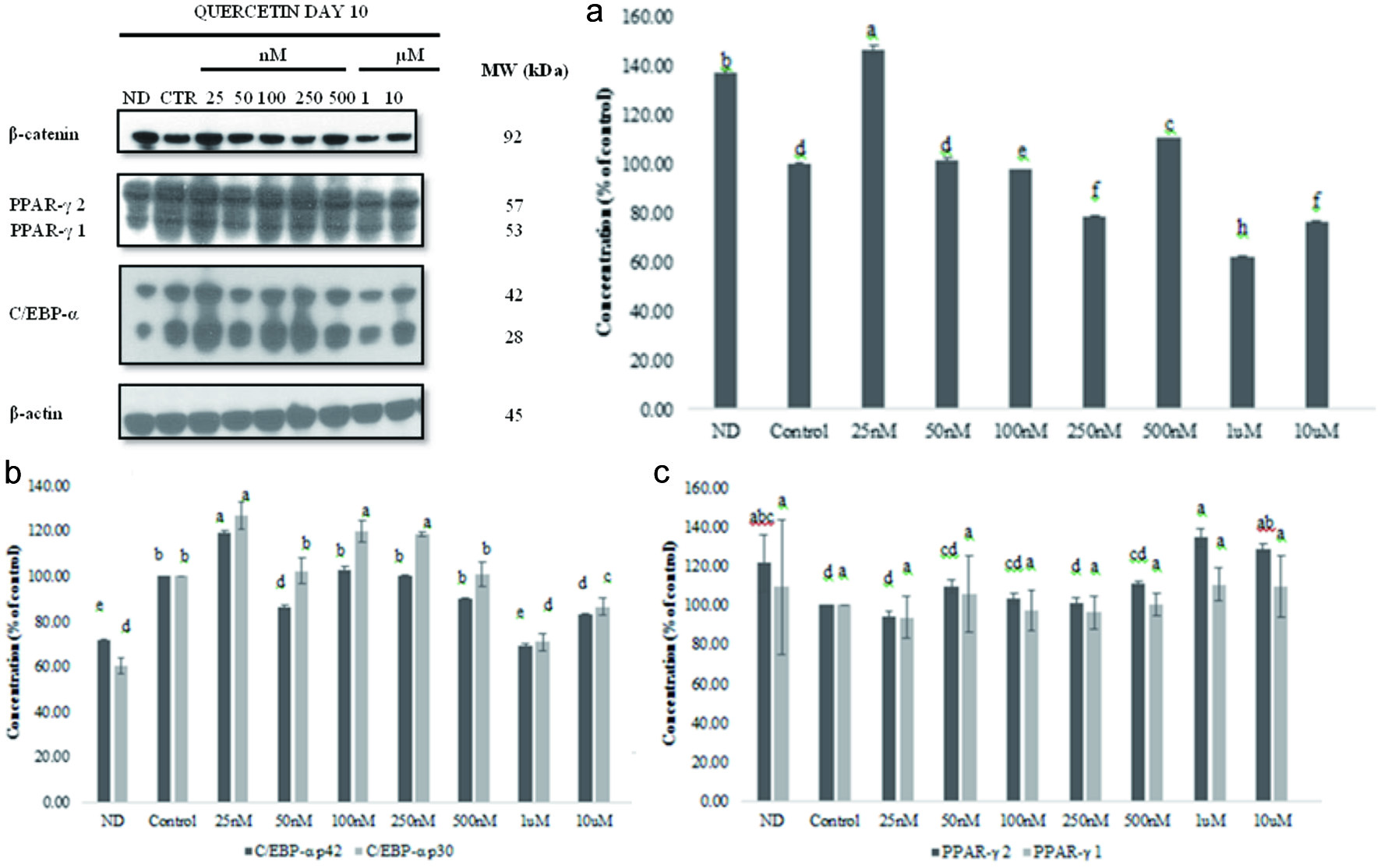 Click for large image | Figure 6. Effect of quercetin on β-catenin, PPAR-γ1 and 2, or C/EBP expression during 3T3-L1 cell differentiation (a). Graphical representation of the effect of quercetin on β-catenin (b), PPAR-γ 1 and 2 (c), or C/EBP (d) expression during 3T3-L1 cell differentiation. Results are presented as mean ± S.D, (n = 3). Letters with different superscripts are significantly different (P < 0.05). |
PPAR-γ expression decreased with increasing concentration of quercetin; at 10 µM quercetin the levels of PPAR-γ 1 and 2 were lower than in non-differentiated cells. PPAR-γ levels in cells treated with 25 nM to 10 µM quercetin are shown in Figure 6; there was significant difference (P < 0.0001; P < 0.05) compared to the control cells. PPAR-γ level decreased as quercetin concentration increased. PPAR-γ 1 and 2 lowest levels were observed at 10 µM quercetin. Quercetin at 25 µM decreased PPAR-γ 1 by 27.6 ±1.4% (p < 0.001) but had no effect on PPAR-γ 2 expression (Yang et al., 2008). At physiologically attainable concentration, quercetin had no effect on PPAR-γ 1 and 2 expression.
Quercetin reduced the expression of C/EBP-α levels compared to that in the control differentiated cells. Results in Figure 6 show a significant difference (P < 0.0001; P < 0.05) compared to control untreated cells. C/EBP p30 and p42 levels decreased at 25 µM quercetin but did not have a specific trend in response to quercetin concentrations. Quercetin alone at 25 µM did not have any effect on the expression of C/EBP-α p30 and p42 on 3T3-L1 cells, whereas quercetin at 25 µM and resveratrol at 25 µM significantly decreased the expression by 60.2 and 45.4%, respectively (Yang et al., 2008).
In this study, we investigated the effect of isorhamnetin or quercetin on 3T3-L1 differentiation with the aim of finding the physiological concentration range at which these bioactive compounds could be useful for preventing the pre-adipocyte from differentiating without any cytotoxic effect. We concluded that isorhamnetin or quercetin at physiologically attainable concentrations of 25 nM to 10 µM was not cytotoxic to 3T3-L1 cells. Isorhamnetin suppressed lipid accumulation more than quercetin during 3T3-L1 cell differentiation. Isorhamnetin more than quercetin down regulated lipid accumulation. Isorhamnetin or quercetin did not have any effect on MCP-1 levels during adipocyte differentiation. Isorhamnetin more than quercetin reduced β-catenin in 3T3-L1 cells. Both isorhamnetin and quercetin decreased C/EBP-α p30 and p42 and PPAR-γ 1 and 2 expressions compared to the control. Taken together, the results from this study indicate that isorhamnetin, more than quercetin, at physiologically attainable concentrations inhibited adipogenesis through down regulation of lipid droplet accumulation during pre-adipocyte differentiation, expression of β-catenin, and down regulation of the expression of C/EBP-α and PPAR-γ suggesting the beneficial effect of consuming foods rich in quercetin such as apple skin, cranberry, onion, garlic, and ginger because quercetin metabolizes into isorhamnetin which in turn can at physiologically attainable concentration be effective against adipogenesis. A direct translation of this study to humans is premature. However, the results reported herein suggest that isorhamnetin or quercetin at physiologically attainable concentration should be investigated for their efficacy on human pre-adipocyte differentiation.
| 4. Conclusion | ▴Top |
This study has demonstrated that certain dietary bioactives can be effective at nanomolar concentrations. Consumption of foods rich in dietary bioactives such as quercetin or isorhamnetin which are bioavailable and effective at nanomolar concentration should be promoted. The health benefits of these compounds are so many that even nanomolar concentrations can enhance health. In vivo investigation of the health benefits of quercetin and isorhamnetin and consumption of foods and beverages rich in these compounds such as onion, citrus fruits, berries, apples, parsley, tea, and red wine should be promoted.
Acknowledgments
This research was entirely funded by the LSU AgCenter.
| References | ▴Top |