| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 17, March 2022, pages 49-55
The protective effect of Jerusalem artichoke polysaccharides against oxidative damage to biomolecules
Jing Wang*, Liangliang Fang, Jinfeng Yu, Lili Zhao, Jiaojiao Yao, He Li, Xuefeng Chen
School of Food and Biological Engineering, Shaanxi University of Science & Technology, Xi’an 710021, China
*Corresponding author: Jing Wang, School of Food and Biological Engineering, Shaanxi University of Science & Technology, Xi’an 710021, China. E-mail: wangjingliwang@126.com
DOI: 10.31665/JFB.2022.17303
Received: March 14, 2022
Revised received & accepted: March 24, 2022
| Abstract | ▴Top |
AAPH and hydroxyl radicals were used to induce oxidative damage to biomolecules (lipid, DNA, protein), and the inhibitory effect of Jerusalem artichoke polysaccharides (JAPS) on oxidative damage was evaluated. The results showed that Jerusalem artichoke polysaccharides had a good inhibitory effect on the oxidative damage of lipids and DNA caused by AAPH and hydroxyl radicals. In the concentration range of 0.5–50.0 μg/mL, the degree of inhibition of Jerusalem artichoke polysaccharides on lipid peroxidation and DNA oxidation increased at first and then decreased. The protective effect of Jerusalem artichoke polysaccharides was the best at a concentration of 5.0 μg/mL. However, Jerusalem artichoke polysaccharides performed weakly in inhibiting protein oxidation, and they even exerted prooxidative effect in hydroxyl radical reaction system. These results may provide some preliminary information to further study Jerusalem artichoke polysaccharides and to help in better utilization of Jerusalem artichoke.
Keywords: Antioxidant; Free radicals; Jerusalem artichoke; Polysaccharides; Oxidation
| 1. Introduction | ▴Top |
A large number of endogenous free radicals with high oxidative activity are generated by respiration and various normal metabolism or physiological activities. Radiation, sunlight exposure, environmental pollution, ultraviolet and other extreme environments may also lead the body to produce abundant free radicals (Castro and Freeman, 2001). Under normal circumstances, there is dynamic equilibrium between the oxidation and antioxidant system in the body. However, when a variety of factors disturb this balance and lead to oxidative stress, the excessive free radicals will attack biomolecules in the body (Aslani and Ghobadi, 2016). Biomolecules such as protein, lipid and DNA are the main targets of various free radicals. Biomembranes are rich in unsaturated fatty acids that play an extremely important role in maintaining membrane fluidity and many other functions of biomembranes. Unsaturated fatty acids in the cell membrane when attacked by reactive oxygen species (ROS) decrease membrane fluidity and ultimately affect membrane biological function, including the changes of membrane permeability, membrane receptor, and ion channel activity. In addition, ROS can induce intracellular protein oxidation (mainly at cysteine or methionine residues that are sensitive to free radicals), mitochondrial damage, cause structure damage to DNA, shorten telomeres, activate death signals, and finally affect cell life (Adibhatla and Hatcher, 2010; Berlett and Stadtman, 1997; Hu et al., 2021). These alteration and oxidation of biomolecules are common causes of various diseases. Thus, to prevent disease, it is very important to control in vivo free radicals and oxidative damage caused by them. To deal with excessive free radicals produced upon oxidative stress, humans have developed sophisticated mechanisms for maintaining redox homeostasis. In particular, antioxidants work effectively against various free radicals, exerting their protective effects against cellular damage. It has been well demonstrated that antioxidants can simultaneously scavenge one or more types of free radicals in vivo. Moreover, antioxidants can inhibit free radical generation by chelating prooxidant metal ions. In addition, enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-PX) can capture free radicals in cells. Antioxidants not only act on free radicals directly, but also indirectly enhance the scavenging effect of free radicals by improving the activity of endogenous enzymes, so as to prevent oxidative damage (Leopoldini et al.,2011; Masella et al., 2005). Therefore, research on the protective effect of antioxidants on free radical-induced oxidative damage of biomolecules has attracted increasing attention (Guan et al., 2021; Yeo and Shahidi, 2020).
Antioxidants may be synthetic or natural. Natural antioxidants, mainly derived from animals, plants or microorganisms, have the advantages of strong antioxidant capacity and good safety record (Liu, 2022; Miyashita et al., 2018). Among them, plant polysaccharides are substances with superior biological activity (Yarley et al., 2021). Jerusalem artichoke polysaccharides are active components derived from Jerusalem artichoke tubers. Jerusalem artichoke is also known as Yangjiang and Guizi ginger in China. In addition to its ecological, pharmaceutical, ornamental and energy-source, Jerusalem artichoke is also regarded as a nutritional food and a neotype feed resource (Wang et al., 2020; Yang et al., 2015). The content of Jerusalem artichoke polysaccharides in tubers accounts for about 80% of its total carbohydrate content. As heterogeneous blend of fructose polymers, Jerusalem artichoke polysaccharides are promising as additives in food processing. For example, Jerusalem artichoke polysaccharides might be used as a substitute for sugar, fat and flour in the production of cake, chip, meat stuffing, sausage and noodles (Takeuchi and Nagashima, 2011; Zhu et al., 2020). Jerusalem artichoke polysaccharides also have ideal food processing characteristics that are valuable for production of dairy and flour products (Yovchev and Le-Bail, 2021). Furthermore, Jerusalem artichoke polysaccharides have many bioactivities, responsible for reducing the risk of hyperglycemia in humans (Shoaib et al., 2016), improving the gastrointestinal function, and exerting anticancer activities (Shao et al., 2021; Yu et al., 2018). In addition, it is also a potential ingredient in functional foods (Shoaib et al., 2016). Jerusalem artichoke polysaccharides could well scavenge free radicals in vitro, thus acting as antioxidants (Mu et al., 2021). However, the effect of Jerusalem artichoke polysaccharides on biomolecule oxidation induced by free radicals has not yet been reported. In this study, two free radical systems (hydroxyl radicals, and AAPH to generate alkoxyl radicals) were applied to induce oxidative damage to biomolecules (lipid, protein and DNA) in vitro, and Jerusalem artichoke polysaccharides were used to evaluate their protective effect on biomolecules. The acquired information may provide the basis for further research and development for Jerusalem artichoke polysaccharides.
| 2. Materials & Methods | ▴Top |
2.1. Chemicals and reagents
Bovine serum protein (BSA), calf thymus DNA and lecithin were purchased from Beijing Solarbio Technology Co. Ltd (Beijing, China). Linoleic acid was bought from Shanghai Macklin Biochemical Technology Co. Ltd (Shanghai, China). The compounds 1,10-phenanthroline hydrochloride (Phen) and 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH) were bought from Tianjin Kemio Chemical Reagent Co. Ltd (Tianjin, China) and 2-thiobarbituric acid (TBA) was purchased from Shanghai Yuanye Chemical Reagent Co. Ltd (Shanghai, China). Trichloroacetic acid (TCA) was procured from Shanghai Shanpu Chemical Co. Ltd (Shanghai, China). Other chemicals were of analytically grade.
2.2. Preparation of Jerusalem artichoke polysaccharides (JAPS)
Jerusalem artichoke tuber powder was mixed in a ratio of 1:25 (w/v) with distilled water, stirred and extracted in 65 °C water bath (DK-98, Shanghai Precision Instrument Co., Ltd) for 70 min. The supernatant was then collected after filtration. The impurities were removed by lime milk-phosphoric acid method. First, adjusting pH to 11 with lime milk solution, after 50 °C water bath for 20 min, the protein will be coagulated and precipitated with calcium ion under the action of strong alkali. Then the pH was adjusted to 6 with phosphoric acid followed by 80 °C water bath for 15 min for further precipitation of protein, repeating 3 times. De-colorization was carried out with D301-G ion exchange resin. Ethanol was added to the solution and let it stand at 4 °C for 12 h, and the precipitation was collected. The precipitation was pre-freeze at minus 80 °C, then lyophilized (FD-1A-80, Shanghai Bilon Instrument Manufacturing Co., Ltd, Shanghai, China).
2.3. Linoleic acid oxidation
The hydroxyl radical produced by Fe2+/Vitamin C (Vc) reaction system was used to induce lipid peroxidation of linoleic acid (LA) (Xiang et al., 2013). In the reaction system, different concentrations of JAPS were mixed evenly with linoleic acid (1.0 mmol/L), FeSO4 (50.0 µmol/L) and Vc (1.0 mmol/L), followed by heating in a water bath at 37 °C for 24 h. Then TBA (1% w/v) and TCA (28% w/v) (TBA assay) were added and heated at 100 °C for 15 min, and the final absorbance (A) of the reaction solution was recorded at 532 nm (Varioskan flash, Thermo Fisher Scientific). The degree of oxidation of linoleic acid was calculated using Equation 1 (Fagali and Catalá, 2009). In AAPH induction system, AAPH was preheated for 2 min before use, and AAPH (25.0 mmol/L) instead of hydroxyl radical induction solutions was used (Liu and Osawa, 2007).
2.4. Lipid peroxidation
Lecithin is an important component of cell membranes, alveolar surfactant, lipoproteins and bile. Lipid peroxidation process is considered to be associated with many physiological and pathological events. The effect of JAPS on lipid peroxidation was assessed with lecithin as a model (Fagali and Catalá, 2009). Lecithin was sonicated in an ultrasonic cleaner bath (KQ3200E, Suzhou Kunshan Ultrasonic Instrument Co., Ltd, China) in phosphate buffer (0.05 mmol/L, pH 7.4) for 30 min. Different concentrations of JAPS were mixed evenly with the sonicated solution and FeSO4 (50 mmol/L), and the mixtures were then placed in a water bath at 37 °C for 40 min accompanied by vortexing every 5 min. At the end of reaction, TBA and TCA were added and heated (100 °C for 15 min) followed by centrifugation (2,400 g for 10 min) (HC-1014, Beijing Shidai Beili centrifuge Co., Ltd, Beijing, China), and the final absorbance of the reaction supernatant was recorded (532 nm). The degree of lipid peroxidation was calculated using the Equation 1.
2.5. DNA oxidative damage
Evidence suggests that reactive oxygen species are responsible for damage to the DNA molecule. A mechanism for the formation of malondialdehyde during deoxyribose degradation (Figure 1) has been proposed (Cheeseman et al., 1988). In addition, it is reported that this DNA damage by ROS leads to the release of oligonucleotides, free bases and a molecule with aldehyde function. This oxidation product with aldehyde function can react with 2-thiobarbituric acid (TBA) to give colour reaction similar to that given by the three-carbon-atom molecule malondialdehyde, and has therefore been referred to as a malondialdehyde-like substance (Gutteridge, 1979). Hydroxyl radical was produced by phen-CuSO4-Vc system (Stoewe and Phitz, 1987). Various concentrations of JAPS were mixed into the reaction solutions of CuSO4 (1.0 mmol/L), phen (1.75 mmol/L) and DNA (3.0 mg/mL). Then the reaction was started by Vc (17.5 mmol/L) followed by incubation at 37 °C for 90 min. TBA assay was used for detection and the absorbance was recorded at 532 nm. AAPH-induced oxidative damage was analyzed following the same procedure except that AAPH was used instead of hydroxyl radical (Xiang et al., 2013). The results were calculated according to Equation 1.
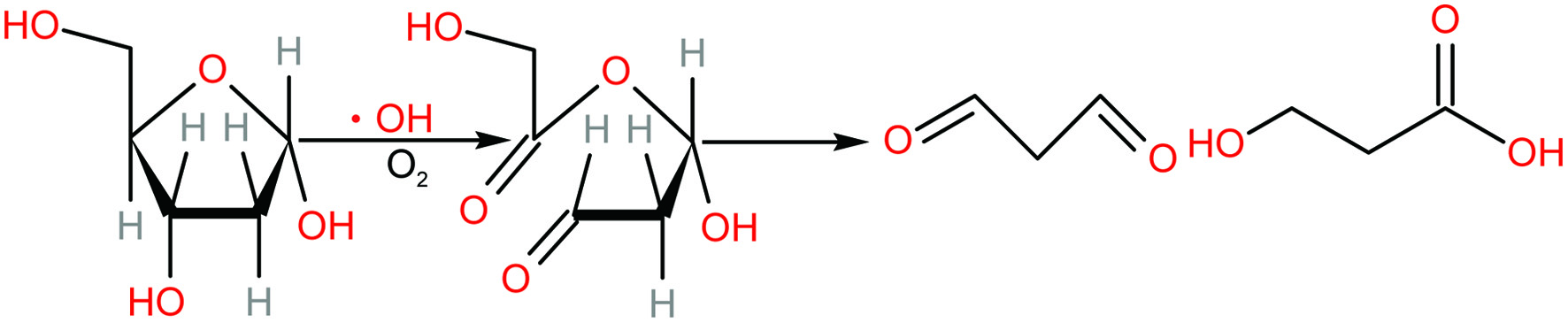 Click for large image | Figure 1. Proposed mechanism for the formation of malondialdehyde after 2-deoxyribose was attacked by hydroxyl radical (adapted from Cheeseman et al., 1988). |
2.6. Protein oxidative damage
Hydroxyl radical was produced by CuSO4/H2O2 induction system. The effects of polysaccharides on protein (BSA) oxidation were evaluated according to the method of Xiang et al. (2012). The reaction mixture containing JAPS sample, BSA (0.5 mg/mL), CuSO4 (100 μmol/L) as well as H2O2 (2.5 mmol/L) were incubated at 37 °C for 90 min. The results were detected by Coomassie blue R250 dyeing after sodium dodecylsulfate-polyacrylamide gel ectrophoresis (SDS-PAGE) (Bio-Rad). For AAPH-induced BSA oxidative damage, AAPH was used instead of CuSO4/H2O2 (Xiao et al., 2012). The results were presented as the residue of intact proteins.
| 3. Results | ▴Top |
3.1. Jerusalem artichoke polysaccharides (JAPS) protect linoleic acid from oxidation induced by hydroxyl radicals
The membrane structures of mitochondria, cell membranes, endoplasmic reticulum, lysosomes, peroxisomes and other organelles contain a large number of polyunsaturated fatty acids which are readily oxidized by free radicals. Excessive lipid peroxidation is involved in the occurrence and development of stroke, atherosclerosis, chronic inflammation, diabetes, Alzheimer’s disease (AD), Parkinson’s disease (PD) and other ailments. Linoleic acid (LA) is a ω-6 fatty acid that is easily oxidized. Thus, it serves as an ideal model to study lipid peroxidation. A Fe2+/Vc reaction system was used to generate hydroxyl radicals to induce oxidation of linoleic acid, and the protective effect of JAPS on linoleic acid was analyzed.
The effects of different concentrations of JAPS on the oxidation of linoleic acid are shown in Figure 2. JAPS inhibited the oxidation of linoleic acid within the concentration range of 0.5, 2.5, 5.0, 25.0, 50.0 µg/mL. However, the degree of oxidation did not show a simple decreasing trend with increasing concentration of JAPS. The lowest oxidation degree (about 60%) was detected at a concentration of 5.0 µg/mL. Thus, JAPS could effectively protect linoleic acid from oxidation.
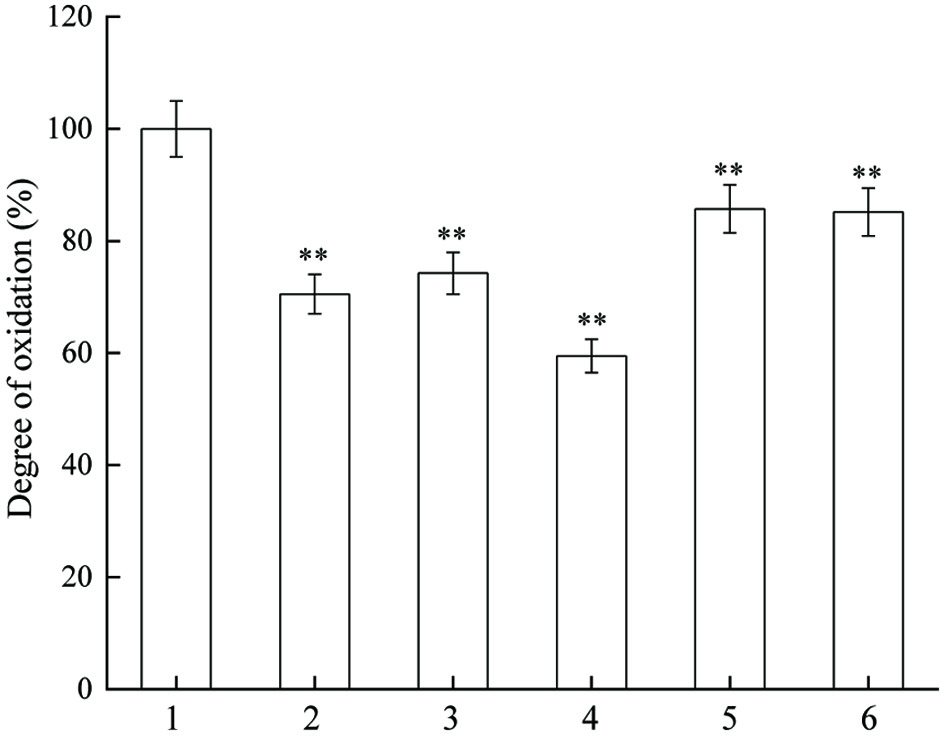 Click for large image | Figure 2. Inhibitory actvity of JAPS on linoleic acid oxidation in FeSO4/VC system. 1: FeSO4/VC + Linoleic acid, 2: FeSO4/VC + Linoleic acid+ 0.5 μg/mL of JAPS, 3: FeSO4/VC + Linoleic acid+ 2.5 μg/mL of JAPS, 4: FeSO4/VC + Linoleic acid+ 5.0 μg/mL of JAPS, 5: FeSO4/VC + Linoleic acid+ 25.0 μg/mL of JAPS, 6: FeSO4/VC + Linoleic acid+ 50.0 μg/mL of JAPS. **p < 0.01, group with JAPS vs. group without JAPS. |
3.2. Jerusalem artichoke polysaccharides (JAPS) protect linoleic acid from oxidation induced by AAPH
AAPH after thermal decomposition produces alkoxyl radicals. The results in Figure 3 suggest that JAPS inhibited AAPH-induced oxidation of linoleic acid. The degrees of oxidation decreased significantly in groups treated with JAPS compared with that of the group without the addition of JAPS. The oxidation degree displayed a trend similar to that of Fe2+/Vc system, and the most protective effect was observed at a concentration of 5.0 µg/mL.
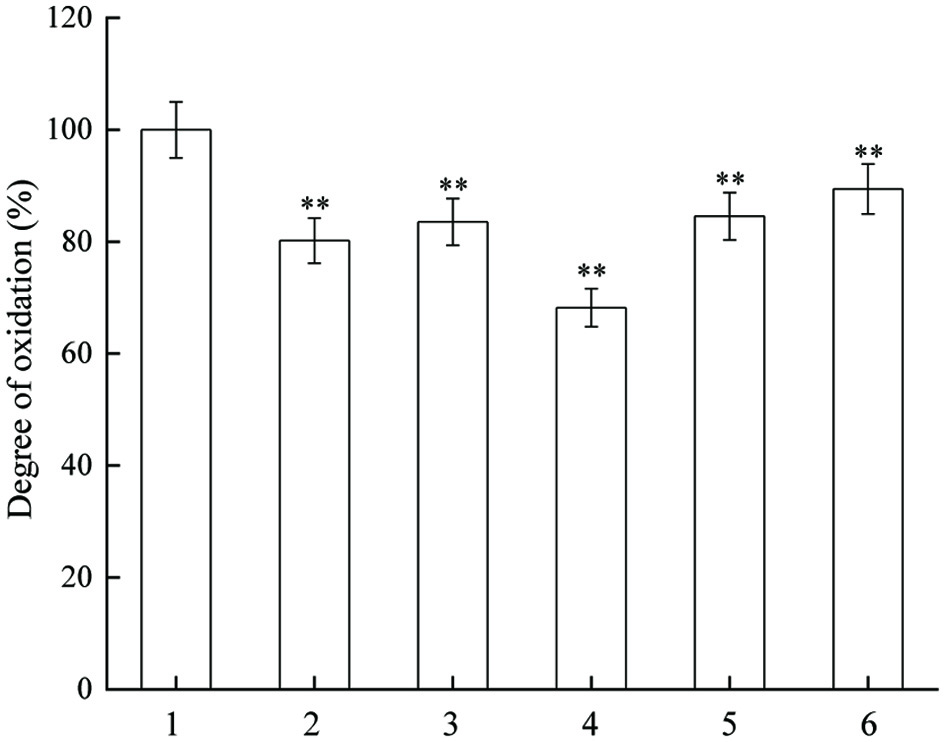 Click for large image | Figure 3. Inhibitory actvity of JAPS on linoleic acid oxidation in AAPH system. 1: AAPH + Linoleic acid, 2: AAPH + Linoleic acid+ 0.5 μg/mL of JAPS, 3: AAPH + Linoleic acid+ 2.5 μg/mL of JAPS, 4: AAPH + Linoleic acid+ 5.0 μg/mL of JAPS, 5: AAPH + Linoleic acid+ 25.0 μg/mL of JAPS, 6: AAPH + Linoleic acid+ 50.0 μg/mL of JAPS. **p < 0.01, group with JAPS vs. group without JAPS. |
3.3. Jerusalem artichoke polysaccharides (JAPS) inhibit lipid peroxidation
The inhibitory effects of different concentrations of JAPS on lipid peroxidation are shown in Figure 4. JAPS at various concentrations showed different inhibitory effect on lipid peroxidation. With the increase of JAPS concentration, the degree of lipid peroxidation showed a downward trend first and then an upward one. The degrees of lipid peroxidation were significantly lower in groups treated with JAPS compared with that of the group without the addition of JAPS. The degree of lipid peroxidation decreased to the lowest (less than 10%) when 5.0 µg/mL of JAPS was used. The results showed that JAPS had an excellent effect in inhibiting lipid peroxidation. In addition, JAPS performed much better in lecithin peroxidation (the lowest degree of oxidation, less than 10%) than in linoleic acid model (the lowest degree of oxidation, around 60%).
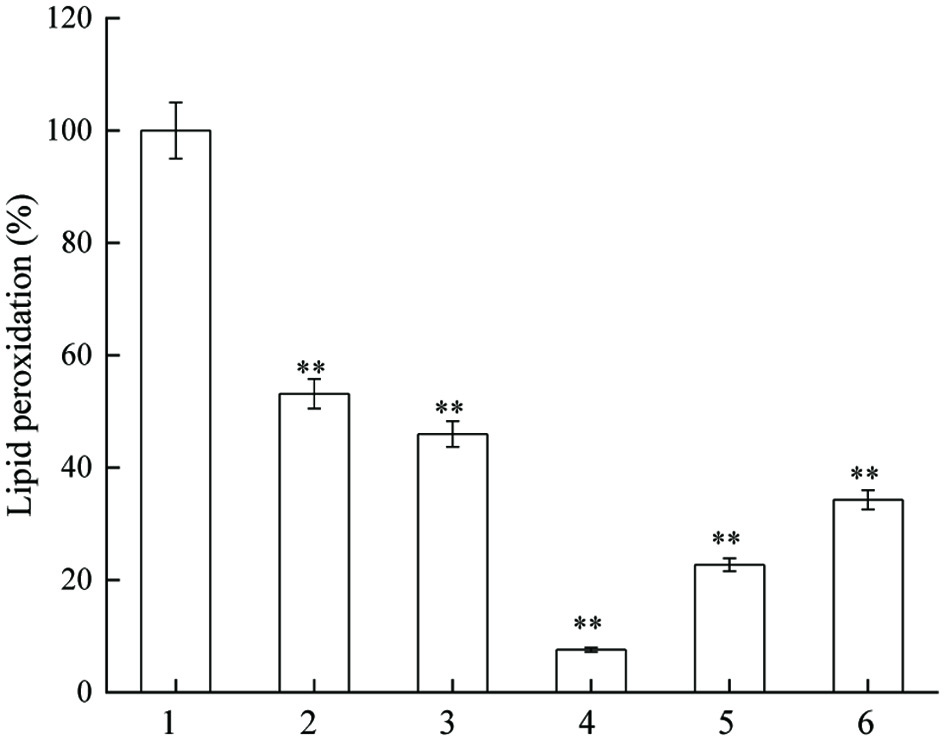 Click for large image | Figure 4. Inhibitory actvity of JAPS on lipid peroxidation. 1: Fe2+ + Lecithin, 2: Fe2+ + Lecithin + 0.5 μg/mL of JAPS, 3: Fe2+ + Lecithin + 2.5 μg/mL of JAPS, 4: Fe2+ + Lecithin + 5.0 μg/mL of JAPS, 5: Fe2+ + Lecithin + 25.0 μg/mL of JAPS, 6: Fe2+ + Lecithin + 50.0 μg/mL of JAPS. **p < 0.01, group with JAPS vs. group without JAPS. |
3.4. Jerusalem artichoke polysaccharides (JAPS) inhibit DNA oxidation induced by hydroxyl radicals
Oxygen free radical attacks to desoxyribose of DNA and forms malondialdehyde which can react with TBA under certain conditions (Figure 1), and thus form products (TBARS) for easy detection. As indicated in Figure 5, JAPS within tested concentrations could significantly inhibit DNA oxidative damage caused by phen-CuSO4-Vc system. When using 0.5 µg/mL of JAPS for protection, the degree of DNA oxidation decreased by about 20%, but it decreased to about 60% when DNA was protected by 5.0 µg/mL or 25.0 µg/mL of JAPS. However, the protective effect did not increase with the increase of JAPS concentration. Compared to the group with 5.0 µg/mL of JAPS, 50.0 µg/mL of JAPS worked less effectively in inhibiting DNA oxidation. Thus, 5.0 µg/mL and 2.5 µg/mL of JAPS exerted the best protective effect.
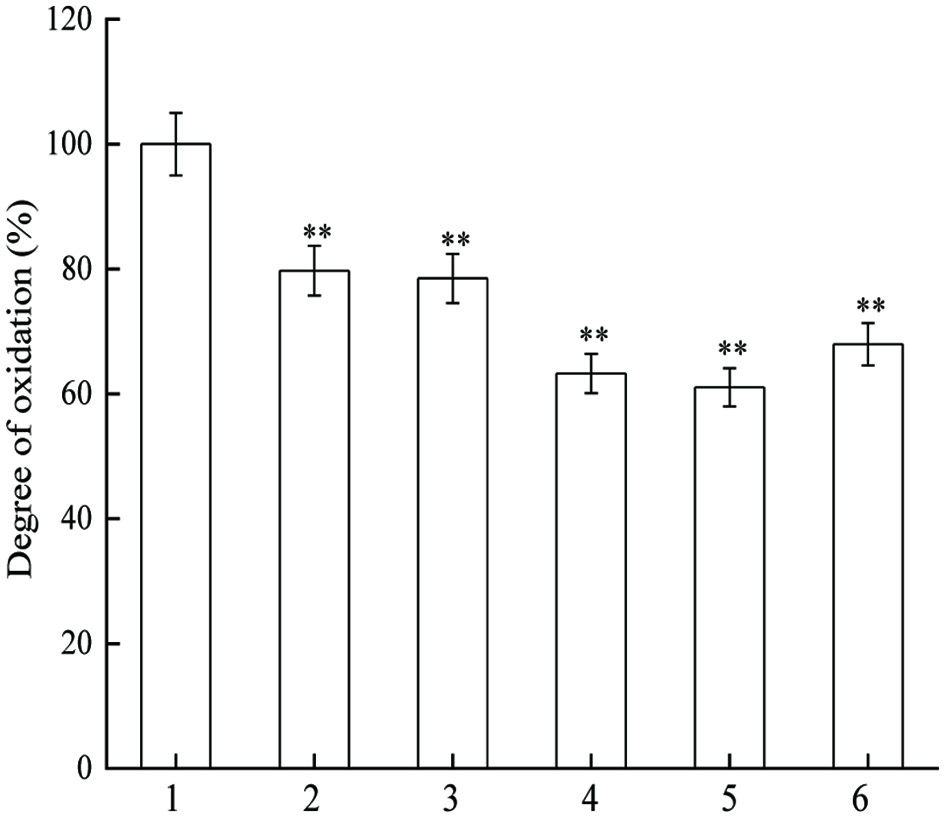 Click for large image | Figure 5. Inhibitory effect of JAPS on DNA oxidation in phen-CuSO4-Vc. 1: phen-CuSO4-Vc + DNA, 2: phen-CuSO4-Vc + DNA + 0.5 μg/mL of JAPS, 3: phen-CuSO4-Vc + DNA + 2.5 μg/mL of JAPS, 4: phen-CuSO4-Vc + DNA + 5.0 μg/mL of JAPS, 5: phen-CuSO4-Vc + DNA + 25.0 μg/mL of JAPS, 6: phen-CuSO4-Vc + DNA + 50.0 μg/mL of JAPS. **p < 0.01, group with JAPS vs. group without JAPS. |
3.5. Jerusalem artichoke polysaccharides (JAPS) inhibit DNA oxidation induced by AAPH
Different concentrations of JAPS dramatically inhibited DNA oxidative damage induced by AAPH (Figure 6). The overall inhibition effect of JAPS in AAPH system was better than that in the phen-CuSO4-Vc system. The degree of oxidation was reduced by nearly 80% with 0.5 µg/mL or 25.0 µg/mL of JAPS, whereas it was only reduced by about 50% if higher concentration of JAPS was applied. Lower concentrations of JAPS exerted better protection than higher concentrations.
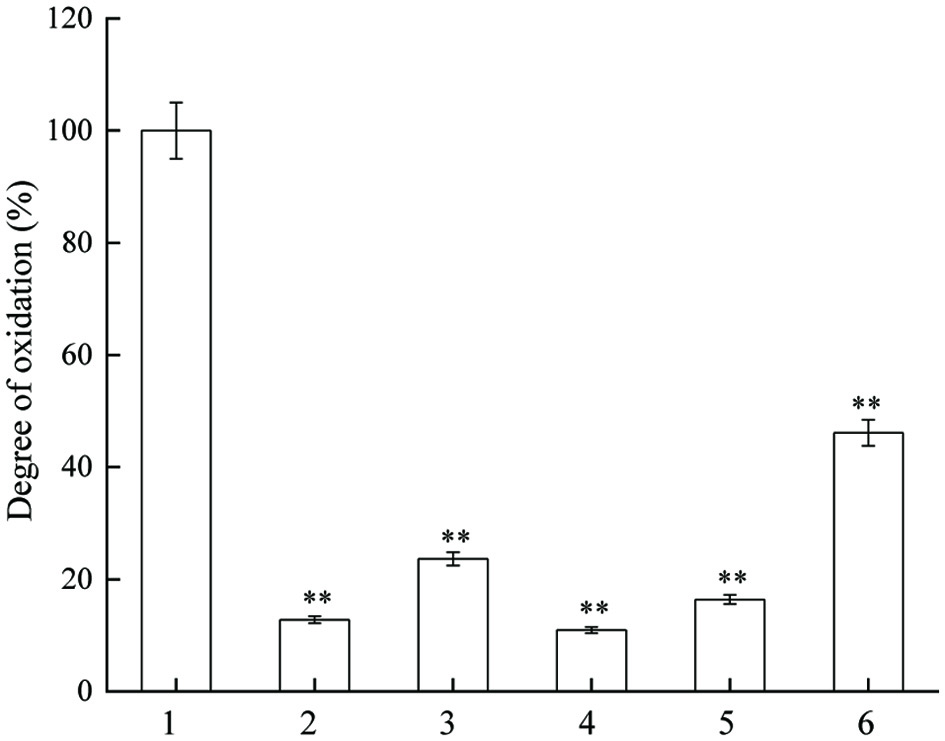 Click for large image | Figure 6. Inhibitory effect of JAPS on DNA oxidation in AAPH system. 1: AAPH + DNA, 2: AAPH + DNA + 0.5 μg/mL of JAPS, 3: AAPH + DNA + 2.5 μg/mL of JAPS, 4: AAPH + DNA + 5.0 μg/mL of JAPS, 5: AAPH + DNA + 25.0 μg/mL of JAPS, 6: AAPH + DNA + 50.0 μg/mL of JAPS. **p < 0.01, group with JAPS vs. group without JAPS. |
3.6. The effect of Jerusalem artichoke polysaccharides (JAPS) on protein damage induced by hydroxyl radicals
The oxidative degradation of protein was detected with Coomassie blue R-250 staining. When attacked by free radicals, the polypeptide chain of protein breaks, and the original protein band signal (residual protein) decreased with Coomassie blue R-250 staining. The effects of different concentrations of JAPS on the oxidation of BSA in Cu2+/H2O2 system were illustrated in Figure 7. Part of BSA was degraded after oxidation, which was indicated by the reduced content of residual BSA. Interestingly, lower or higher concentration of JAPS somehow exerted promotive effect other than suppressive effect on protein oxidation. More importantly, JAPS of 0.5 µg/mL did not work effectively in inhibiting protein damage, and only slightly increased the level of residual BSA. It seems that JAPS failed in inhibiting oxidative damage induced by hydroxyl radicals.
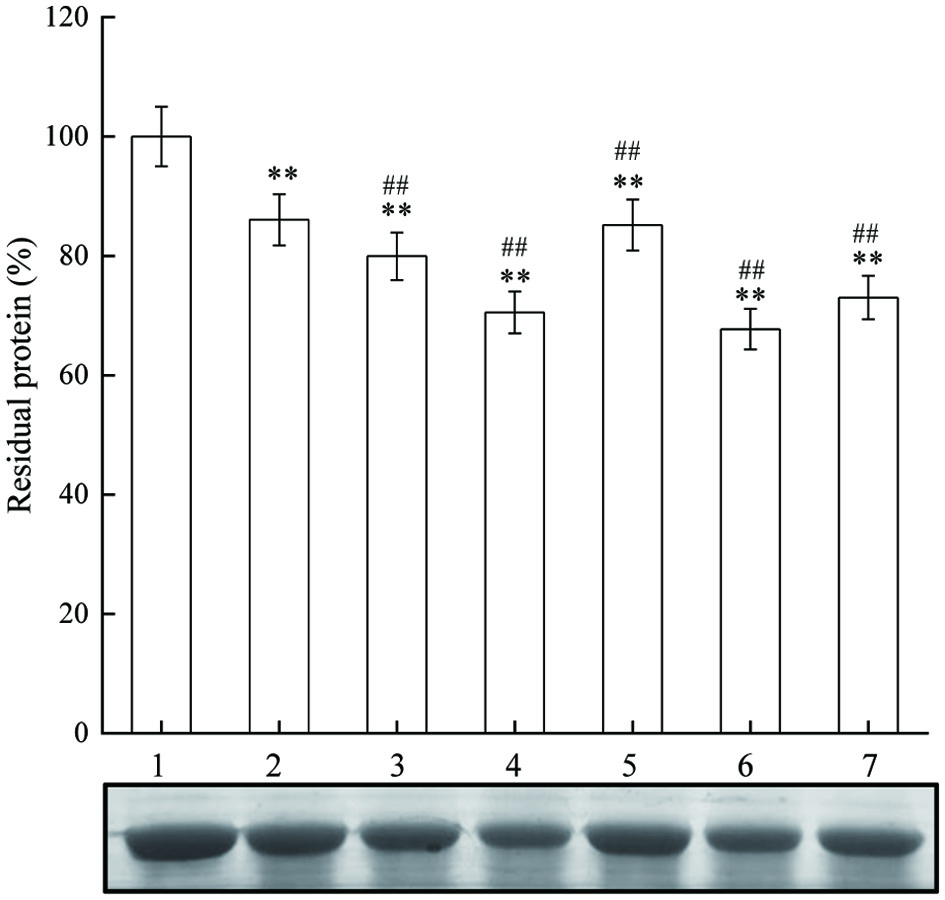 Click for large image | Figure 7. The effect of JAPS on BSA oxidation in Cu2+/H2O2 system. Lane 1: BSA, Lane 2: Cu2+/H2O2 + BSA, Lane 3: Cu2+/H2O2 + BSA + 0.5 μg/mL of JAPS, Lane 4: Cu2+/H2O2 + BSA + 2.5 μg/mL of JAPS, Lane 5: Cu2+/H2O2 + BSA + 5.0 μg/mL of JAPS, Lane 6: Cu2+/H2O2 + BSA + 25.0 μg/mL of JAPS, Lane 7: Cu2+/H2O2 + BSA + 50.0 μg/mL of JAPS. **p < 0.01 vs. Lane 1. ##p < 0.01 vs. Lane 2. |
3.7. The effect of Jerusalem artichoke polysaccharides (JAPS) on protein damage induced by AAPH
The results in Figure 8 suggest that there was a significant decrease of residual BSA in AAPH-induction group. However, AAPH-induced BSA oxidation was hardly affected by JAPS at low concentrations. A significant protection was observed only in groups treated with 5.0 µg/mL or 25.0 µg/mL of JAPS. Overall, the antioxidative capacity of JAPS in AAPH system was better than that in the Cu2+/H2O2 system.
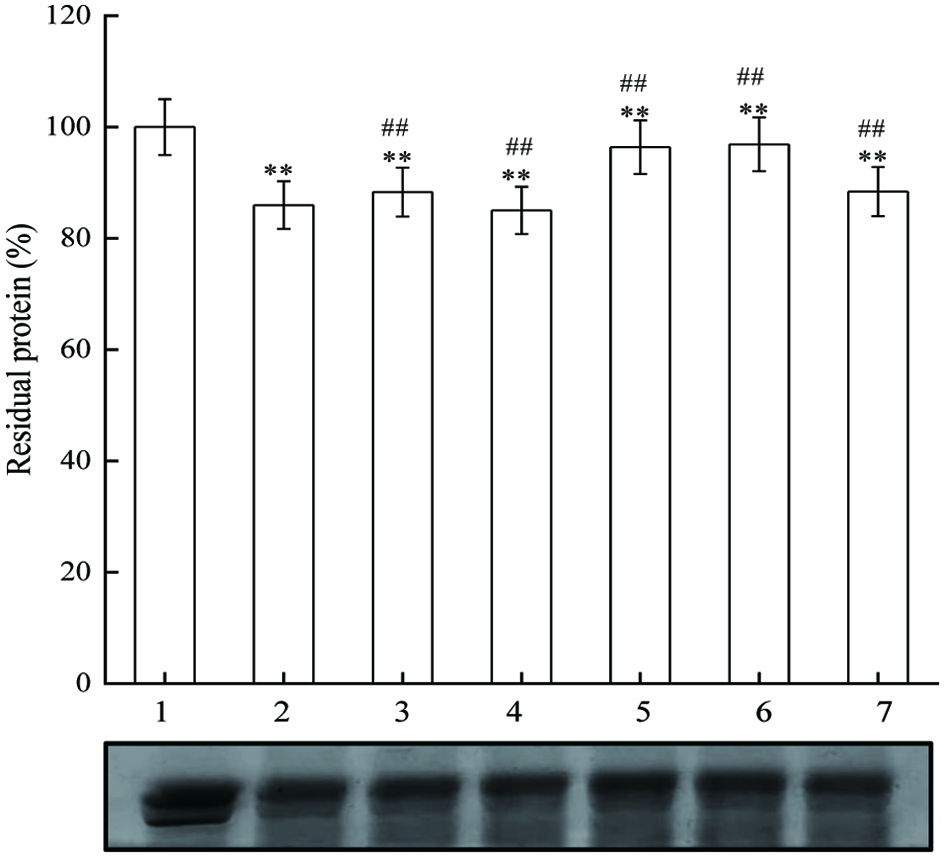 Click for large image | Figure 8. The effect of JAPS on BSA oxidation in AAPH system. Lane 1: BSA, Lane 2: AAPH + BSA, Lane 3: AAPH + BSA + 0.5 μg/mL of JAPS, Lane 4: AAPH + BSA + 2.5 μg/mL of JAPS, Lane 5: AAPH + BSA + 5.0 μg/mL of JAPS, Lane 6: AAPH + BSA + 25.0 μg/mL of JAPS, Lane 7: AAPH + BSA + 50.0 μg/mL of JAPS. **p < 0.01 vs. Lane 1. ##p < 0.01 vs. Lane 2. |
| 4. Discussion | ▴Top |
Persistent oxidative damage of biomolecules results in various diseases such as cancer, diabetes, cardiovascular and cerebrovascular diseases. Supplements of antioxidants which slow down or prevent oxidation is an effective means to reduce oxidative damage in vivo (Abdul-Latif et al., 2021; Pisoschi et al., 2022).
ROS in the body include superoxide radical anion (O2−•), hydrogen peroxide (H2O2), hydroxyl radical (•OH), singlet oxygen (1O2), and their derivatives such as alkoxyl radical (RO•), and alkylperoxyl radical (ROO•), among others (Castro and Freeman, 2001). Much of the damage resulting from oxidative stress has been attributed to the highly reactive hydroxyl radical which has the capacity to degrade DNA, lipids and proteins by oxidation. Certain substances such as vitamin C, iron, and copper ions, which widely exist in biological systems, could readily trigger hydroxyl radical generation in aerobic metabolism (Keshtkar Vanashi and Ghasemzadeh, 2022). The metal-mediated production of hydroxyl radicals is one important mechanism of biological deterioration. Therefore, hydroxyl radicals should be well managed to help maintain homeostasis in normal and healthy tissues.
A large body of evidence has show that the antioxidant effect of polysaccharides is exerted via a variety of mechanisms. For example, the alcohol hydroxyl group in the polysaccharide structure can inhibit the formation of hydroxyl radical via complexation with transition metal ions, such as those of iron and copper. In addition, polysaccharides can directly capture ROS produced in the peroxidation chain reaction, blocking or slowing down lipid peroxidation. The antioxidant effect of polysaccharides also involves scavenging of free radicals, enhancing the activity of antioxidant enzymes, and regulating antioxidant signaling pathways in cells (Wang et al., 2016; Yarley et al., 2021; Mu et al., 2021). Based on the results in this study, it could be summarized that JAPS could inhibit the oxidation of lipids and DNA induced by hydroxyl radicals and AAPH. However, JAPS worked less effectively in inhibiting protein damage using BSA as a model. In general, the antioxidant performance of JAPS in inhibiting lipid peroxidation and AAPH-induced DNA oxidative damage were much better than that tested in other systems, such as hydroxyl radical-induced DNA and linoleic acid oxidation, AAPH-induced linoleic acid and protein oxidation. In addition, the protective effect of JAPS did not rise steadily with the increase of JAPS concentration, and peaked at the concentration of 5.0 µg/mL. However, there was a decline in the protective effect of JAPS when its concentration was too low or too high.
We also noted that the protective effect of JAPS on protein was not as obvious as that on lipid and DNA. Contrary to expectations, low concentration of JAPS even promoted BSA oxidation in Cu2+/H2O2 system. Although 5.0 µg/mL of of JAPS seemed to play a certain protective role in AAPH system, the protection effect was still minimal. Thus, the antioxidant activity of components may be closely related to the reaction conditions and their concentration.
What is particularly noteworthy is the negative effect on antioxidation in some cases. With the increase of concentration, the inhibitory effect of JAPS on oxidative damage caused by free radicals increased, but the effect decreased at higher concentrations. For hydroxyl radical-induced protein damage, higher and lower concentrations of JAPS even promoted oxidation. Some studies have confirmed that many antioxidants have prooxidative effects at high concentrations (Lange et al., 2020). For example, the level of serum lipid peroxidation in guinea pigs increases after high-dose injection of vitamin C (Kapsokefalou and Miller, 2001). Flavonoids can react with transition metals and accelerate the formation of hydroxyl radicals (Procházková et al., 2011). High concentration of tea polyphenols can promote oxidation of proteins in emulsions (Tian et al., 2021). At present, the mechanism of antioxidant promoting oxidation has not been fully elucidated but it may be related to the structure of antioxidants. For example, the antioxidant or prooxidant activities of flavonoids largely depend upon the number of hydroxyl substituents in their backbone structure (Cao et al., 1997). System composition and environmental conditions are also important factors. Moreover, higher concentrations of tea polyphenols resulted in prooxidant activity of proteins at pH 7 (Tian et al., 2022). Therefore, it might also be related to the properties of free radicals, the different action sites on biomolecules as well as the type of in vitro research models employed (Eren-Guzelgun et al., 2018). Further relevant research are still needed to fully clarify these interesting preliminary findings.
| 5. Conclusion | ▴Top |
Jerusalem artichoke polysaccharides (JAPS) within certain concentrations effectively inhibited the oxidation of DNA and linoleic acid induced by hydroxyl and alkoxyl radicals, and also inhibited lipid peroxidation. However, JAPS exhibited poor effect in inhibiting protein oxidation. Furthermore, higher concentrations of JAPS may exert a prooxidative effect. This study provides evidence for the antioxidant activity of JAPS. However, comprehensive antioxidative and prooxidative mechanisms of JAPS are still needed to shed light on details of their effects.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21707086), Shaanxi Provincial Natural Science Foundation (No.2019JQ-453), Shaanxi Weiyang District Science and Technology Plan (202044) and National innovation and entrepreneurship training program for college students (202110708045).
Conflict of interest
The authors declare that there is no conflict of interest.
| References | ▴Top |