| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Mini-Review
Volume 17, March 2022, pages 2-5
Selection of polyphenol oxidase affects biotransformation efficacy of targeted theaflavins
Weixin Wanga, Chi-Tang Hob, Shiming Lia, *
aCollege of Life Science, Huanggang Normal University, Huanggang 438000, Hubei, China
bDepartment of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
*Corresponding author: Shiming Li, College of Life Science, Huanggang Normal University, Huanggang 438000, Hubei, China. E-mail: shiming@rutgers.edu
DOI: 10.31665/JFB.2022.17297
Received: March 28, 2022
Revised received & accepted: March 30, 2022
| Abstract | ▴Top |
Theaflavins in black tea and other fermented tea have attracted many studies because of their stronger antioxidant and anti-inflammatory effects among bioactives other than catechins. However, within the four major theaflavins, namely theaflavin, theaflavin-3-O-gallate, theaflavin-3′-O-gallate and theaflavin-3,3′-O,O-digallate, their biological properties are different. A method to efficiently and selectively synthesize targeted theaflavins with desired property is a key condition for further evaluation. Herein, we have summarized the sources of polyphenol oxidase (PPO) and the yields of total and individual theaflavins based on some available publications. This overview lays the foundation for a comprehensive review in this area of researchin the near future.
Keywords: Theaflavins; Polyphenol oxidase; Catechins; Theaflavin monogallate; Theaflavin digallate; Anti-inflammation
| 1. Introduction | ▴Top |
Tea is the most consumed functional beverage in the world, among which black tea is the most popular tea and accounts for 78% of the overall tea industry. The major polyphenols in black tea consists of theaflavins and catechins. The chemical components of black tea have high amount of green tea catechins (4–15%) and theaflavins are usually between 0.5 and 2% by dry weight. Major theaflavins are theaflavin (TF1), theaflavin-3-O-gallate (TF2a), theaflavin-3′-O-gallate (TF2b) and theaflavin-3,3′-O,O-digallate (TF3). Although to a lesser degree as compared to green tea, black tea also contain major catechins, including (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC) and (−)-epigallocatechin gallate (EGCG) (Li et al., 2013; 2021). Catechins and theaflavins are reported to be the chief contributors to the biological activity of black tea by additive or synergistic effects.
The health-promotional properties of black tea and its major theaflavin components include antioxidant (de Majia, Ramirez-Mares and Puangpraphant, 2009; Xu, et al., 2021), anti-inflammatory (Arent et al., 2010; Gosslau et al., 2011), anti-cancer (Yang et al., 2009; Pan et al., 2013), cardioprotective (Stangl et al., 2007; Santesso and Manheimer, 2014) effects as well as controling of obesity and metabolic syndrome, among others (Lin et al., 2007; Tang et al., 2013; Vermeer et al., 2008).
| 2. Formation of theaflavins | ▴Top |
Despite the fact that manufacturing process of black tea is traditionally termed as fermentation of green tea, it is actually a first order oxidation reaction under the catalysis of polyphenol oxidase and peroxidase followed by a polymerization of the oxidized quinones. This is a completely different process from microbial fermentation that produces ethanol and vinegar from crops and soy sauce from soybean. As mentioned above, theaflavin formation undergoes oxidation and polymerization, two simplified steps (Li et al., 2013). The first step is the oxidation reaction (Figure 1), i.e. green tea catechins, particularly EGC and EGCG are partially oxidized to quinones under the catalysis of polyphenol oxidase (PPO, EC1.14.18.1) and/or peroxidase (POD, EC1.11.1.7). PPO and POD exist naturally in fresh tea leaves, fruits and vegatables.
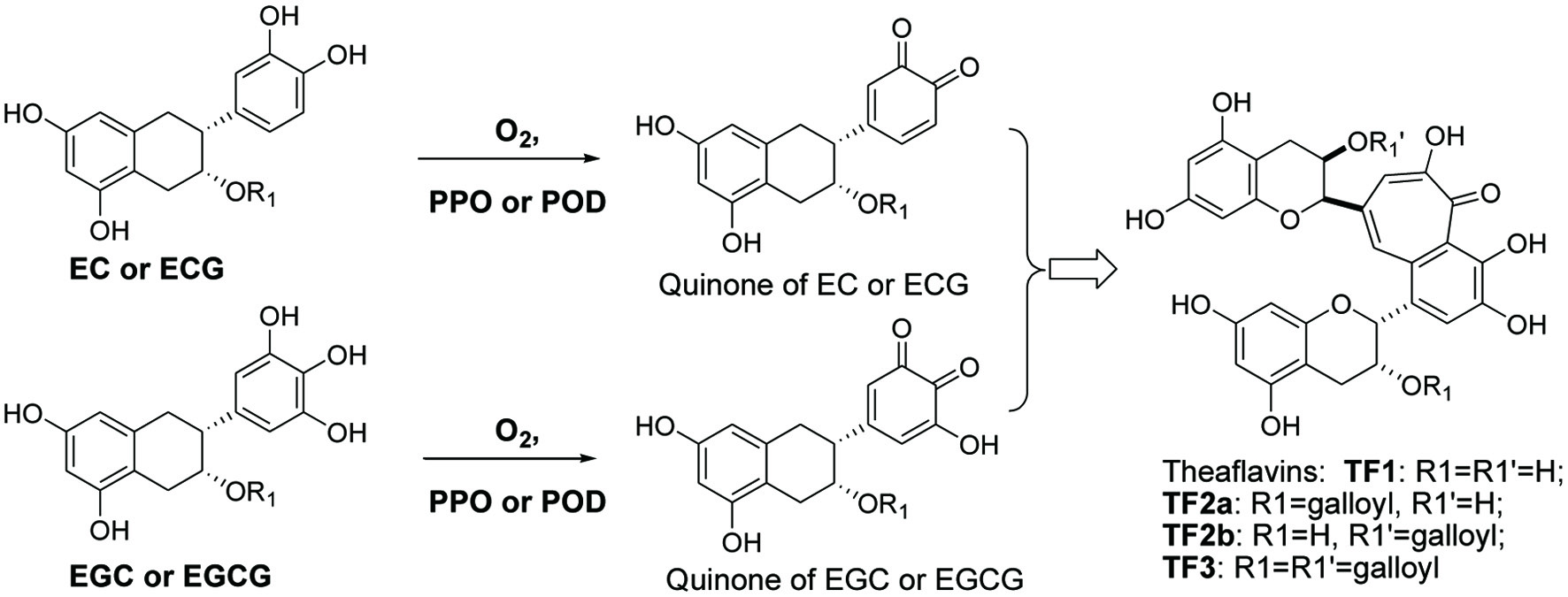 Click for large image | Figure 1. Formation of black tea theaflavins. |
| 3. Examples of PPO sources from plants and microorganisms | ▴Top |
The initial enzyme-catalyzed oxidation is a key step and, therefore the existence of PPO/POD in plants or microorganisms crucial for this process. Many studies have found that the sources of PPO and POD play very important roles in multiple aspects, such as yields, conversion rate and composition/ratio of individual theaflavins such as TF1, TF2a, TF2b and TF3. Traditionally, the source from fresh green tea leaves is used in the ‘fermentation’ process to produce black tea. However, there are limitations to rely solely on tea PPO/POD, such as the off season time of tea harvesting and low conversion yields from green tea catechins to black tea theaflavins. However, it is reported that PPO has greater efficacy than POD in catalyzing the oxidation of catechins to their corresponding quinones (Jiang et al., 2018). Therefore, other sources of PPO have been explored from vegetables, fruits, mushrooms and microbes (Lin et al., 2017; Sang et al., 2011). Examples of PPO derived from different sources are listed in Table 1. As seen in Table 1, the optimal conditions to obtain maximum yields of theaflavins, including temperature, pH, time and concentration, are different from one source of enzyme to another. For instance, between the two different tea leaves, the synthesized content of theaflavins catalyzed by the PPO of C. sinensis is 38.8% higher than that of C. sinensis var.assamica (Huang et al., 2017; Lin et al., 2017). Pear, Malus pumila and Ipomoea batatas, especially pear (Echeveria ‘Sulli’) affords the highest catalytic capacity (Wang et al., 2007).
 Click to view | Table 1. Examples of different sources of PPO used in theaflavin synthesis with mixed catechins as substrates |
| 4. Yields and ratios of major theaflavins influenced by different sources of PPOs | ▴Top |
Theaflavins in black tea or related products are important for their biological activities as demonstrated by a proprietary 40% theaflavins-containing black tea extract to induce a strong inhibition of pro-inflammatory markers. The markers considered were COX-2, TNF-α, ICAM-1, IL-1β, IL-6, IL-8, NF-κB, C-JUN and p53 and up-regulation of anti-inflammatory IL-10 in a cDNA and oligomicroarray analysis by the use of a human cell-based monocyte-macrophage differentiation model (Arent et al., 2010; Li et al., 2019). Therefore, sufficient content of theaflavins is a key for black tea or its products to demonstrate efficacy against inflammation, among others. Furthermore, it is also evidenced that TF2 and TF3 are more effective in their bioactivities than TF1 (Gosslau et al. 2011; Zhou et al., 2022). Hence, the content of total theaflavins and proportions of TF2 and/or TF3 are of great importance in order to reach the desired functional bioactivity. The exploration has generally focused on the conversion yield of total theaflavins starting with green tea or catechins. Table 2 shows several examples of the impact on the conversion efficiency to theaflavins with different sources of PPO. It is evident that PPOs from pear genus (Pyrus family) has high catalytic efficiency in producing total theaflavins regardless of enzyme activity. However, more than 65% of the theaflavins was TF1, which does not show any outstanding biofunctionality, indicating that enzymes from pear are not good catalysts for theaflavin products with high percentage of TF2 and TF3 (Wang et al., 2007). The combined contents of TF2 and TF3 are over 50% from tea PPOs (Camellia family), representing natural ‘fermentation’ of green tea, but overall transformation yield of theaflavins is much lower than that of pear PPOs (Wang et al., 2007; Li, 2006; Lin et al., 2017). Thus, more exploration and optimization are needed to obtain high yield of total theaflavins from tea PPOs. It is worth to pay special attention that two sources of PPO can catalytically transfer catechins to very high percentages of TF2 and TF3. In particular, 54% of TF2a, 28% of TF2b were obtained with the PPO from Malus pumila Mill (Lin et al., 2017) and nearly 60% of TF2 and 37% of TF3, a total of 97% of TF2 and TF3 combined, were generated with the catalysis of PPO from Dioscoreae Rhizoma (Lin et al. 2017). Therefore, we have learned from the limited reports that there are options to selectively produce high percentages of TF1, TF2a, TF2b or TF3 with a careful selection of PPOs from different sources, but further optimization of conversion yield and reaction conditions are warranted to reach the desired goals.
 Click to view | Table 2. Examples for contents of four major theaflavins produced by different sources of PPO with catechins as substrates |
| 5. Conclusion | ▴Top |
Theaflavins as a whole have a multitude of biological activities, but different theaflavins, i.e. TF1, TF2 and TF3, have demonstrated different potency in various biological assays. TF2 and TF3 have possess better efficacy than TF1). In preparation of theaflavin-rich black tea or its related products, aside from the ratios of natural catechins, PPO plays a key role in the synthesis of theaflavins. From the knowledge gained from only limited publications available, we recommend a careful source selection of PPOs as a priority in producing theaflavin-rich products with targeted individual theaflavins prior to further optimization for mass production of tea products.
Acknowledgments
This research is funded by the grant from Hubei Province, China (2019ABA100).
| References | ▴Top |