| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 16, December 2021, pages 34-47
Blackberry phenolic and volatile extracts inhibit cytokine secretion in LPS-inflamed RAW264.7 cells
Pauline Morina, Luke Howarda, *, John Tiptonb, Laura Lavefvea, Cindi Brownmillera, Sun-Ok Leea, Inah Gua, Rohana Liyanagec, Jackson O. Layc
aDepartment of Food Science, University of Arkansas, 2650 N. Young Ave., Fayetteville, AR 72704, USA
bMathematical Sciences, Science Engineering Hall, 850 West Dickson Street, Fayetteville, AR 72701, USA
cUniversity of Arkansas Statewide Mass Spectrometry Facility, 1260 West Maple Street, Fayetteville, AR 72701, USA
*Corresponding author: Luke R. Howard, Department of Food Science, University of Arkansas, 2650 N. Young Ave., Fayetteville, AR 72704, USA. Tel: (479) 575-2968; Fax: (479) 575-6936; E-mail: lukeh@uark.edu
DOI: 10.31665/JFB.2021.16291
Received: December 6, 2021
Revised received & accepted: December 30, 2021
| Abstract | ▴Top |
The anti-inflammatory activity of blackberries has been attributed to phenolic compounds, especially anthocyanins. The present study hypothesized that volatiles could contribute to anti-inflammatory activity as well. The anti-inflammatory properties of three blackberry genotypes varying in total volatile and phenolic contents were assessed by measuring concentrations of nitric oxide (NO), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) within LPS-inflamed RAW264.7 murine macrophage cells after a preventive treatment of either a phenolic or a volatile extract. Extracts from blackberry genotypes A2528T, A2587T and Natchez had total phenolic contents of 4,315, 3,369 and 3,680 µg/mL, respectively, and total volatile contents of 283, 852 and 444 ng/mL, respectively. Phenolic and volatile extracts of all genotypes significantly lowered the secretion of NO, IL-6 and TNF-α in ranges varying between 20–42%, 34–60% and 28–73% inhibition, respectively. Volatile extracts exhibited greater anti-inflammatory properties than phenolic extracts, despite being present at much lower concentrations in the berries. Further research is needed to assess bioavailability and anti-inflammatory effect of blackberry volatiles in vivo.
Keywords: Anti-inflammatory; Blackberries; Cytokines; Inflammation; In vitro; Phenolics
| 1. Introduction | ▴Top |
With 67% of their worldwide acreage based in the United States, blackberries (Rubus subgenus Rubus Watson) are becoming more common in the American diet (Strick, 2007). Blackberries are known for many health-promoting properties such as antioxidant, anticancer and anti-inflammatory capacities (Seeram et al., 2006; Bowen-Forbes et al., 2010; Van de Velde et al., 2016; Van de Velde et al., 2019). Blackberries contain a variety of phenolic compounds, anthocyanins, flavonols, ellagitannins, and phenolic acids responsible for bioactive properties and health benefits (Kaume et al., 2012). Anthocyanins in particular, which are responsible for berry colors, are often the focus of the studies since they are the major blackberry phytochemical (Strick, 2007).
Blackberries contain a wide array of volatile compounds including acids, alcohols, esters, monoterpenes and sesquiterpenes that contribute to the berries aroma (Du et al., 2010; Qian and Wang, 2005). Little research has been conducted on the anti-inflammatory effect of blackberry volatiles, but studies have demonstrated the anti-inflammatory capacities of essential oils that contain common blackberry volatiles (Hirota et al., 2010; Huo et al., 2013; Plastina et al., 2018). Moreover, in a recent study by Moore et al. (2019), phenolic and volatile extracts isolated from cranberries, as well as each of the four most abundant volatiles in cranberries, α-terpeniol, linalool oxide, eucalyptol, and linalool were tested at concentrations found in fresh cranberries for their anti-inflammatory capacities, before or after inflammation of RAW264.7 murine macrophage cells. When applied as a treatment after induction of inflammation in the cells, not only did the cranberry volatiles decrease nitric oxide (NO) production by the inflamed cells, but their effect was comparable to that of cranberry phenolics, even though they were 353 times less concentrated. When used as a preventive treatment, before induction of inflammation in the cells, both phenolic and volatile extracts showed stronger NO reduction in comparison with treatment after inflammation, exerting comparable NO production between the two extracts. In another recent study by Gu et al. (2020), phenolic and volatile fractions from blackberries, blueberries, red raspberries, strawberries, cranberries and black raspberries showed comparable ability to inhibit lipopolysaccharide (LPS)-induced production of NO, prostaglandin E2, cyclooxygenase-2, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Additionally, the berry phenolic and volatile extracts reduced nuclear translocation of nuclear factor kappa B (NF-κB) by blocking the degradation of IκBα and subsequent phosphorylation of p65. Based on these findings, our hypothesies were 1) blackberry volatile compounds contribute to the anti-inflammatory capacities of blackberries, and 2) blackberry genotypes varying in volatile composition will possess varying anti-inflammatory capacities. To investigate these hypotheses we evaluated three blackberry genotypes varying in phenolic and volatile composition for their ability to inhibit the secretion of NO, IL-6 and TNF-α after inflammation of RAW264.7 murine macrophage cells.
| 2. Materials and methods | ▴Top |
2.1. Chemicals and reagents
2-heptanol, ethyl acetate, α-terpineol, terpinen-4-ol, α-p-dimethylstyrene, 1-octanol, 1-hexanol, geraniol, β-myrcene, 3-hexan-1-ol, perillic alcohol, myrtenol, linalool, thymol, D-limonene, decanal, 2-hexenal, nerol, nonanal, α-terpinene, 1-pentanol, borneol, hexanal, citronellol, pentanal, benzaldehyde, 1-butanol-3-methyl, octanal, ethyl hexanoate, toluene, 2-decaenal, 1-nonanol, cymene, 1-heptanol, 2-heptanone, 1-octen-3-ol, 3-hexen-1-ol, 1-decanol, 5-hepten-2-one, methyl, methyl octanoate, β-ionone, 2-octanal, heptanal, Folin-Ciocalteu reagent, gallic acid, rutin, alkane standards (C5-C20), HPLC grade methanol, acetone, acetic acid, formic acid, Tween-20, trolox, 2,2 diphenyl-1-picrylhydrazyl (DPPH) and lipopolysaccharide (E. Coli 0111 :B4) were obtained from Sigma-Aldrich (St. Louis, MO). Cyanidin 3-glucoside was obtained from Polyphenols AS (Sandnes, Norway). Sep-Pak® C18 cartridges were obtained from Waters Corp. (Milford, MA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate buffered saline (PBS, pH 7.2), and other cell culture reagents were purchased from Thermo Fischer Scientific (Waltham, MA). 3-(4,5-dimethylthiazol-2-yl)-5-(3 carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and Griess reagent system were obtained from Promega Co. (Madison, WI). Enzyme-linked immunosorbent assays (ELISA) kits used to measure levels of IL-6 and TNF-α (mouse IL-6 ELISA and mouse TNF-α ELISA) were obtained from RayBiotech Inc. (Norcross, GA).
2.2. Plant material
One named-variety (Natchez) and two advanced University of Arkansas breeding lines (A2528T, A2587T) were harvested at the University of Arkansas Fruit Research Station in Clarksville, AR. Fruits were picked when they were fully black and based on ease of abscission from pedicel, according to the recommendation of Perkins-Veazie et al. (1996). The berries were transported in coolers to the Department of Food Science, and upon arrival were placed into sealable Ziploc bags and stored at −20 °C until further analysis.
2.3. Chemical characterization
2.3.1. Extraction and purification of blackberry phenolics
Due to varying berry sizes among genotypes, blackberries were sliced longitudinally and a sample of 50 g of half-fruits was weighted. Then, blackberries were homogenized with an IKA T-18 Ultra-Turrax mixer (Wilmington, NC) in 100 mL of extraction solvent containing methanol, water and formic acid (60:37:3 v/v/v) and then centrifuged at 6,000 g for 5 min. The supernatant was filtered through MiraCloth (CalBiochem, LaJolla, CA) into a flask, and the pellet was re-extracted with 100 mL of acetone, water and acetic acid (70:29.5:0.5 v/v/v) before being centrifuged at 6,000 g for 5 min. These steps were repeated until the sediments recovered from the MiraCloth and the pellet following centrifugation had no visible color. The sediments and MiraCloth were then rinsed with the solvents into the flask containing all supernatants. Samples were then adjusted with extraction solvent to 500 mL in a volumetric flask. The extraction was repeated three times for each genotype.
The phenolic extracts were subjected to Solid Phase Extraction (SPE) in order to remove non-phenolic compounds prior to use for cell culture. A volume of 50 mL of phenolic extract, which equates to 5 g of fresh berries, was dried using a Speed-Vac® Plus SC210A concentrator overnight. The samples were then solubilized in 5 mL of DI water. The SPE unit was equipped with Sep-Pak® C18 cartridges (Waters, Milford, MA) for each sample. Each cartridge was preconditioned with 20 mL of 80% methanol and 40 mL of DI water before the sample (5 mL) was loaded. The cartridge was then rinsed with 40 mL DI water and the eluate was discarded. Next, the cartridge was rinsed with 80 mL of 80% methanol. The eluate was collected, dried overnight in a Speed-Vac® Plus SC210A concentrator, and residue resolubilized in 5 mL of DI water.
2.3.2. Identification of blackberry phenolics
Aliquots (5 mL) of phenolic extracts obtained by the previous solvent extraction were dried using a Speed-Vac® Plus SC210A concentrator and brought back to a volume of 1 mL in 5% formic acid for anthocyanins analysis and 50% methanol for flavonols analysis. The solutions were then filtered through a 0.45 μm filter (Whatman, Marlborough, MA) prior to High Performance Liquid Chromatography (HPLC) and High Performance Liquid Chromatography Mass Spectrometry (HPLC-MS) analysis.
Anthocyanins were identified according to the HPLC-MS method described by Cho et al. (2004). The HPLC system consisted of a Hewlett Packard 1100 HPLC (Hewlett Packard, Palo Alto, CA) equipped with an autosampler, binary HPLC pump and UV/VIS detector. A 250 × 4.60 mm Symmetry 5 µm C18 column (Waters Corp., Milford, MA) was used for separation with compounds monitored at 510 nm. The mobile phase consisted of a 5% formic acid solution (solvent A) and methanol (solvent B). At a solvent flow rate of 1 mL/min, the concentration of solvent A varied from 98% to 40% from 0–60 min, and then to 98% from 61–85 min. The system was interfaced to a Bruker Esquire HPLC-MS ion trap mass spectrophotometer with the following conditions: positive ion mode, capillary voltage of 4,000 V, nebulizing pressure of 30 psi, drying gas flow of 9 mL/min, 300 °C, and a full scan mode comprising a mass range of m/z 50 to 1,000 at 1 s/cycle. Compounds were identified using the m/z data, retention time and previous identifications reported by Cho et al. (2004).
Flavonols were identified according to the HPLC-MS method described by Cho et al. (2005) using the same HPLC-MS system described above, except peaks were monitored at 360 nm and a 250 × 4.60 mm Aqua® C18 column (Phenomenex, Torrance, CA) was used for separation. The mobile phase consisted of a 2% aqueous solution of acetic acid (solvent A) and 0.5% acetic acid in water and acetonitrile at a ratio of 1:1 (v/v, solvent B). Using a solvent flow rate of 1 mL/min, the concentration of solvent A started at 90% from 0–50 min, then decreased to 45% from 51–60 min and ended at 90% from 61–75 min. The system was interfaced to a Bruker Esquire HPLC-MS ion trap mass spectrophotometer with the following conditions: negative ion mode, capillary voltage of 4,000 V, nebulizing pressure of 30 psi, drying gas flow of 9 mL/min, 300 °C, and a full scan mode comprising a mass range of m/z 50 to 1,000 at 1 s/cycle. Compounds were identified using the m/z data, retention times and previous identifications reported by Cho et al. (2005).
2.3.3. Quantification of blackberry phenolics
Anthocyanins were quantified by injecting 50 μL of filtered phenolic extract into a Waters HPLC system (Milford, MA) equipped with a model 600 pump, model 717 autosampler and model 996 photodiode array detector. The same HPLC conditions described above were used. External calibration curves of a cyanidin 3-glucoside (C3G) standard (10–100 μg/mL, Polyphenol AS, Norway) were used to quantify anthocyanins, with results expressed as µg of C3G equivalents (C3GE) per mL extract.
Flavonols were quantified by injecting 50 μL of filtered phenolic extract into the same Waters HPLC system described above for quantification of anthocyanins and the HPLC-MS conditions described above for identification of flavonols. External calibration curves of a rutin standard (10–100 μg/mL, Millipore Sigma, St. Louis, MO) were used to quantify flavonols, with results expressed as µg of rutin equivalents (RE) per mL extract.
2.3.4. Low temperature vacuum distillation of blackberry volatiles
For each genotype, 150 g of fresh blackberries, 150 mL of deionized water and 50 g of NaCl were blended and then vacuum distilled for 30 min at 28 in. Hg in a 50 °C water bath, 0 °C condenser using a Buchi Rotavapor R-114 (Buchi, Flawil, Switzerland). The first 100 mL were collected in a flask stored in an ice water bath and frozen at −20 °C until use. The extraction was repeated three times for each genotype.
In order to verify that no phenolic compounds were present in the volatile extracts, they were analyzed by HPLC following the protocol described above, except the detection wavelength was 280 nm.
2.3.5. Identification and quantification of blackberry volatiles
Two samples (4 mL) of low temperature vacuum distillates from each genotype were placed in vials and preheated to 65 °C before a 85 μm DVB/CAR/PDMS Solid Phase Microextraction (SPME) fiber was placed in the headspace above the sample. The vials were held for 30 min on a stir plate set at 65 °C before removing the SPME fiber and GC injection.
Gas chromatography analysis was performed using a Shimadzu GC-2010 Plus Gas Chromatograph equipped with a Flame Ionization Detector (GC-FID) and a GCMS-QP2010 SE Mass Spectrometer (GC-MS). Samples were analyzed by both GC-FID and GC-MS and separation was performed on each using a HP-5 (30 m × 0.25 mm inner diameter, 5% phenyl-methylpolysiloxane, 1.0 µm film thickness) capillary column (Agilent, Santa Clara, CA). For both GC-MS and GC-FID analysis, the injector temperature was 250 °C. Helium was used as the carrier gas and column flow rate was 1.92 mL/min for GC-FID and 1.20 mL/min for GC-MS. The oven temperature was programmed for a 4-min hold at 30 °C, then 30 °C to 180 °C at 6 °C/min, then from 180 °C to 280 °C at 8 °C/min, and a final 3 min hold at 280 °C. The GC-FID detector temperature was 280 °C and the interface temperature for the GC-MS had an ion source temperature of 230 °C and an interface temperature of 250 °C. GC-MS was performed in full scan mode, with a scan range of 20–300 m/z. The volatiles were identified by comparison of their mass spectra with the National Institute of Standards and Technology NIST17 spectral library, literature data, and retention indices. The retention indices were performed after running C5-C20 alkane standards (Millipore Sigma, St. Louis, MO) and online searches of similar work with HP5 or DB5 columns. Individual compounds were quantified using external calibration curves of authentic standards. Compounds with no standard available were quantified using calibration curves of a compound of similar class that eluted in close proximity. The standards used to quantify each compound are listed in Table 1.
 Click to view | Table 1. Volatile composition (ng/mL) of low temperature vacuum distillates obtained from three blackberry genotypes |
2.3.6. Total phenolic assessment
The total phenolic content of blackberry phenolic and volatile extracts was assessed using the Folin-Ciocalteu assay (Slinkard and Singleton, 1977). Phenolic extracts were diluted 50-fold and volatile extracts were not diluted. The absorbance was read at 760 nm, with results expressed as µg of Gallic Acid Equivalents (GAE) per mL of extract.
2.3.7. Antioxidant capacity assessment
The antioxidant capacity of blackberry phenolic and volatile extracts was assessed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay (Gorinstein et al, 2004). A standard solution of 1,000 μM Trolox was diluted to obtain a range of concentrations from 31.25 to 1,000 μM. A volume of 10 μL of Trolox dilutions, methanol blank, non-diluted blackberry essence, and 50-fold diluted phenolic extracts was added in a 96-well microplate in triplicate. Then, a volume of 140 µL of 0.1 mM DPPH was added to each well. The plate was incubated in the dark for 30 min before reading the absorbance at 517 nm. Results are expressed as µM of Trolox equivalents (TE) per mL of extract.
2.3.8. Preparation of the standard volatiles mix
In order to confirm that the anti-inflammatory effect observed within the blackberry volatile extracts was due to the volatiles and not other compounds co-extracted via the hydrodistillation process, a mix of standard volatiles was prepared based on the list of volatiles previously identified in the blackberry volatile extracts obtained by low temperature vacuum distillation. Only one solution was prepared, and the concentration of each volatile standard was based on the average concentration among the three genotypes, which resulted in a list of 43 volatiles (Table 2).
 Click to view | Table 2. Standard volatiles used to prepare the simulated blackberry volatile mixture |
2.4. Cell culture conditions and treatments
2.4.1. Cell culture conditions
RAW 264.7 murine macrophage cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in Dulbecco’s modified eagle’s medium (DMEM) enriched with 1% penicillin-streptomycin, 1% L-glutamine and 10% fetal bovine serum (FBS). The cells were incubated at 37 °C in a 5% CO2 environment in 75 cm2 culture flasks. All reagents used for cell culture were purchased from Promega® (Madison, WI) and Thermo Fischer Scientific (Waltham, MA).
2.4.2. Cell culture treatments
For each genotype, three replicates of phenolic extracts were pooled, as well as the three replicates of volatile extracts. Targeting a cell density of 1.0x104 cells/well, 0.5 mL of RAW264.7 cells in DMEM media were seeded in a 24-well plate and incubated at 37 °C and 5% CO2 for 24 hr. After incubation, the media was replaced by fresh media containing 0.02% of Tween 80 and either a 2-fold, 4-fold or 8-fold dilution of volatile extract, or 10-fold, 20-fold or 40-fold dilution of phenolic extract. The positive (i.e. LPS-induced cells, no treatment) and negative (i.e. no inflammation, no treatment) controls were incubated with working media containing 0.02% Tween 80. After incubating for one hr, the treatment was replaced by media containing 100 ng lipopolysaccharides (LPS)/mL for 24 hr, except in the case of the negative control in which the media was replaced by fresh working media. Then, the supernatants were centrifuged for 1 min at 140 g, aliquoted and frozen at −80 °C until all samples required for the next step were collected. The experiment was repeated over three wells per treatment, and three times per genotype, each time using a different set of RAW264.7 cells.
2.4.3. Measurement of cell viability
The effect of phenolic and volatile extracts on cell viability was measured by the MTS assay using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega Co., Madison, WI). Cells were seeded in three different 96-well plate and incubated for 24 hrs. Then, cells were treated with a 10-, 20-, or 40-fold diluted phenolic extract or a 2-, 4-, or 8-fold diluted volatile extract. After an hr of exposure to the extracts, each well was rinsed twice with working media before adding a volume of 100 μL of working media and 20 μL of MTS reagent in each well. The plate was incubated for one hr before reading the absorbance of wells at 490 nm. The manipulation was repeated after the second and third hr of exposure to the blackberry extracts with the remaining plates. The concentration in formazan obtained by absorbance measurement was directly proportional to the number of living cells. Consequently, the viability of cells within one, two and three hrs of exposition with the blackberry extracts was calculated as a percentage with a non-treated control as reference.
2.4.4. Measurement of nitric oxide
The Griess Reagent System was used to measure NO production as a marker of inflammation. Using a 96-well plate, a nitrite standard reference curve was obtained through serial dilutions leading to a concentration range of 0 to 100 μM. Then, 50 μL of sulfanilamide solution was added to 50 μL of each supernatant and incubated for 10 min in the dark, followed by 50 μL of N-1-napthylethylenediamine dihydrochloride (NED) for 10 min in the dark. Finally, the absorbance was read by the plate reader at 540 nm, and the absorbance values were converted into NO levels using the nitrite standard curve and after deducting the background noise absorbance measured by the sample control media.
2.4.5. Measurement of IL-6 and TNF-α
Enzyme-linked immunosorbent assays (ELISA) kits were used to measure levels of IL-6 and TNF-α (mouse IL-6 ELISA and mouse TNF-α ELISA, RayBiotech Inc., Norcross, GA). Prior to testing, cell culture supernatants were diluted 20-fold. The measurements were then performed according to the manufacturer’s instruction.
2.5. Experimental design and statistical methods
Significant differences in total phenolics, anthocyanins, flavonols, volatiles and DPPH among genotypes were assessed using Tukey’s HSD test with a critical value of 5%. Significant differences in NO, IL-6 or TNF-α concentrations between the controls and phenolic or volatile extract treatments were assessed through a nested ANOVA with pairwise differences determined using Tukey’s HSD multiple comparison adjustments. A variable “plate_ID” was attributed to each replicate based on the plate it was tested on. The samples tested on one specific plate had the same cell line batch and cell passage, which made them dependent. To correct for this condition, a random effect was applied to the model based on the plate_ID variable. Differences between means were determined by Tukey’s HSD test. The critical value of all tests was 5%.
| 3. Results and discussion | ▴Top |
3.1. Blackberry phenolic composition
The composition of the phenolic extracts of A2528T, A2587T and Natchez are presented in Table 3. The total phenolic concentrations were 4,315 ± 121 µg/mL (GAE) for A2528T, 3,369 ± 92 µg/mL for A2587T, and 3,680 ± 171 µg/mL for Natchez, which fell within concentration ranges (1,140–10,560 µg/mL) reported in previous studies (Kaume et al., 2012).
 Click to view | Table 3. Composition and antioxidant activity of blackberry phenolic extracts |
The typical blackberry anthocyanins, i.e. cyanidin 3-glucoside, cyanidin 3-rutinoside, cyanidin 3-xyloside, cyanidin 3-malonylglucoside and cyanidin 3-dioxalylglycoside dioxalylglycoside (Cho et al., 2004, Kaume et al., 2012, Wu and Prior, 2005) were detected in all genotypes, except Natchez in which cyanidin 3-xyloside was not detected (Table s1). The ranges of concentration in anthocyanins varied between 1,484 ± 96 and 2,263 ± 144 µg/mL (fresh weight) C3G equivalents, which was in accordance with previous studies (Cho et al., 2004, Kaume et al., 2012, Wu and Prior, 2005).
A total of six flavonols were identified in A2528T, seven in A2587T and nine in Natchez (Table s2), leading to total flavonol concentrations of 163 ± 13, 105 ± 16, and 129 ± 13 µg/mL (fresh weight) RE, for A2528T, A2587T and Natchez, respectively. These profiles and concentrations were consistent with previous studies (Kaume et al., 2012, Cho et al., 2005, Kolniak-Ostek et al., 2015, Mikulic-Petkovsek et al., 2012).
3.2. Blackberry volatile composition
The composition of the volatile extracts of A2528T, A2587T and Natchez obtained by low temperature vacuum distillation is presented in Table 1. The concentrations of total volatiles were 283 ± 45 ng/mL for A2528T, 852 ± 49 ng/mL for A2587T, and 444 ± 35 ng/mL for Natchez. These concentrations were much lower than the values obtained from fresh berries of the same genotypes by SPME analysis, 2,419 ng/mL for A2528T, 5,574 ng/mL for A2587T, and 3,882 ng/mL for Natchez (data not shown). Qian and Wang (2005) reported total volatile concentrations of 3,950 and 24,540 ng/mL for Marion and Thornless Evergreen blackberries, respectively and total volatile concentrations of 12,885 ng/mL and 17,045 ng/mL were reported for Marion and Black Diamond blackberries, respectively (Du et al. 2010). The lower volatile concentrations of extracts obtained by low temperature vacuum distillation compared with those of fresh blackberries are due to a dilution effect, i.e. addition of water to the berries to facilitate blending into a homogeneous puree and/or incomplete recovery of the volatiles at the low distillation temperure of 50oC. Although the low temperature distillation method resulted in lower recovery of volatile compounds compared with fresh berry extracts measured by SPME, the technique helped prevent thermal degradation and iomerization of volatile compounds, especially monoterpenes. Thermal degradation of terpenes can occur via four different oxidative reactions, cleavage of double bonds, epoxidation, dehydrogenation into aromatic systems, and allylic oxidation into alcohols, ketones, and aldehydes (McGraw et al., 1999).
In the present study, the major class of volatiles were monoterpenes, alcohols, and esters, with volatile composition varing among the three genotypes. Monoterpenes accounted for 28%, 46% and 38% of total volatiles in A2528T, A2587T and Natchez, repectively, alcohols accounted for 26%, 34% and 31% of total volatiles in A2528T, A2587T and Natchez, repectively and esters accounted for 30%, 6%, and 16% of total volatiles in A2528T, A2587T and Natchez, repectively. Gu et al. (2020) reported acids and alcohols as the major classes in an unknown blackberry genotype, while Du et al. (2010) and Qian and Wang (2005) reported acids, alcohols, monoterpenes and furanones to be the major classes of volatiles in Marion, Black Diamond and Thornless Evergreen blackberries grown in Pacific Northwest. The differences in volatile composition between studies were not surprising since they can vary due to differences in genetics, maturation, sample preparation and GC conditions.
A total of 80 volatiles were identified among the three genotypes (Table 1). In A2528T, the five major volatiles were ethyl acetate (76 ± 11 ng/mL), hexanol (26 ± 1 ng/mL), α-terpineol (24 ± 13 ng/mL), 2-hexen-1-ol (17 ± 2 ng/mL) and β-damascenone (11 ± 7 ng/mL). In A2587T, they were 2-heptanol (190 ± 28 ng/mL), α-terpineol (69 ± 14 ng/mL), terpinen-4-ol (53 ± 4 ng/mL), δ-carvone (52 ± 8 ng/mL) and ethyl acetate (50 ± 38 ng/mL). In Natchez, they were ethyl acetate (67 ± 13 ng/mL), 6-camphenol (40 ± 4 ng/mL), α-terpineol (36 ± 4 ng/mL), terpinen-4-ol (35 ± 5 ng/mL) and 1-hexanol (35 ± 5 ng/mL).
3.3. Antioxidant capacity of phenolic and volatile extracts
The antioxidant capacities of the blackberry phenolic extracts were 30 ± 1 μmol/mL TE for A2528T, 24 ± 1 μmol/mL TE for A2587T and 25 ± 2 μmol/mL TE for Natchez (Table 2). These values were higher than the previous value of 19.8 μmol/mL TE reported for an unknown blackberry variety (Gu et al., 2020). The volatile extracts had no antioxidant activity, which was expected since they are present in low concentration, and did not contain phenolics, especially anthocyanins that are highly correlated with antioxidant activities of blackberry fruits (Kaume et al., 2012).
3.4. Effect of blackberry extracts on cell viability
The potential cytotoxicity of the phenolic and volatile extracts was measured with the MTS assay (Figure 1). None of the extracts significantly reduced cell viability compared with the non-treated cells, demonstrating that the extracts were not cytotoxic and that the effects observed in the following experiments were not due to a cytotoxic effect of blackberry phenolics or volatiles.
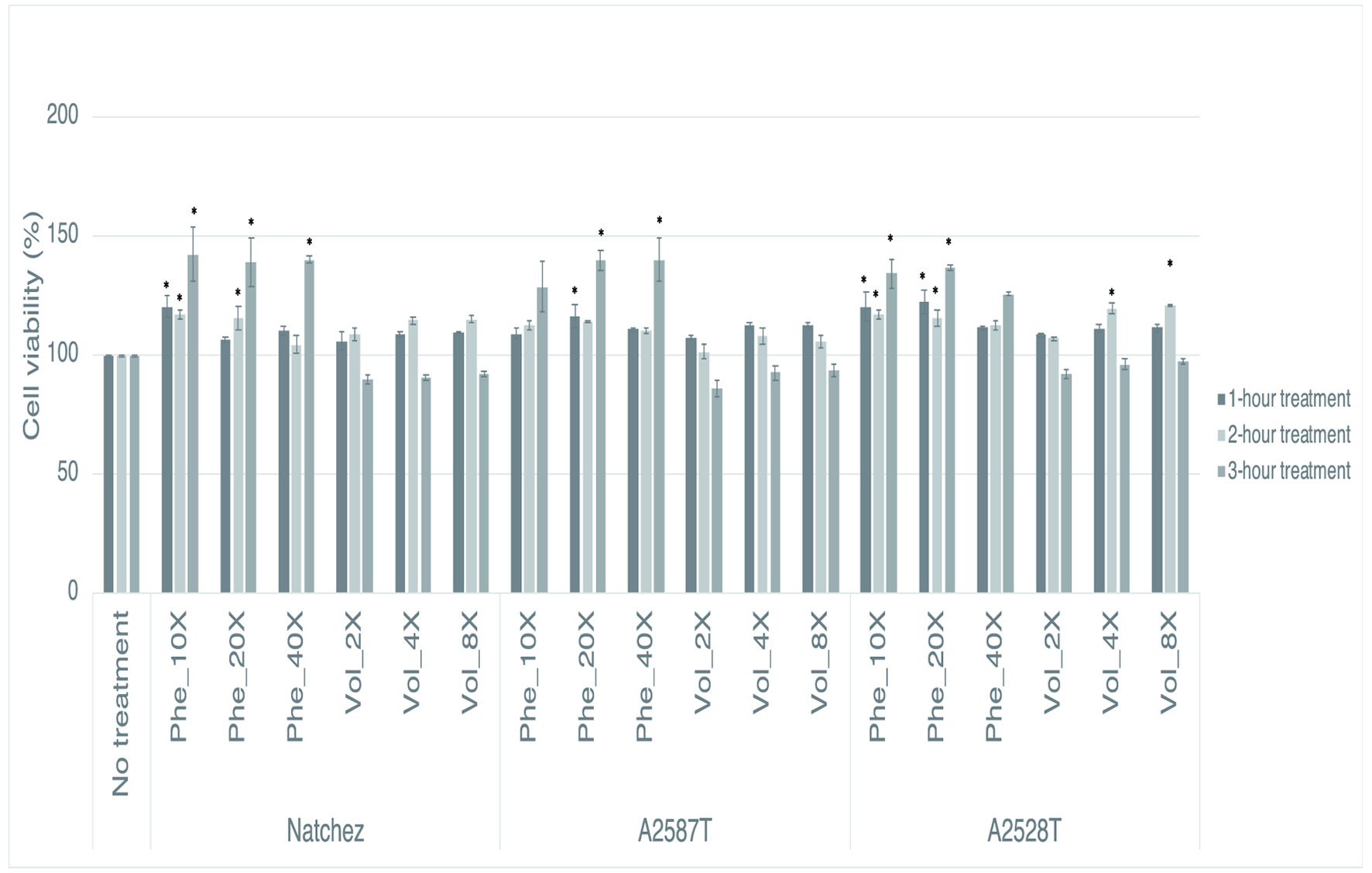 Click for large image | Figure 1. Percentage of viability of RAW264.7 cells after a preventive treatment of one, two and three hrs with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold. Values represent mean ± SEM (n = 3). * Denotes significant difference from cells that received no treatment (P<0.05). |
3.5. Effect of blackberry extracts on NO production
NO is an inflammatory mediator released by immune cells. Macrophages can release high levels of NO following pro-inflammatory cytokine or pathogen activation, whose roles include vasodilatation, modulation of the immune response and regulation of apoptosis (Sharma et al., 2007). The concentration in NO observed within the control cells and the cells treated with blackberry extracts are presented in Figure 2. The negative control corresponded to cells that were not treated and not inflamed with LPS, the positive control corresponded to cells that were not treated but inflamed. The average concentrations of NO in all dilutions of both phenolic and volatile extracts of all three genotypes were significantly lower than the positive control (p<0.05).
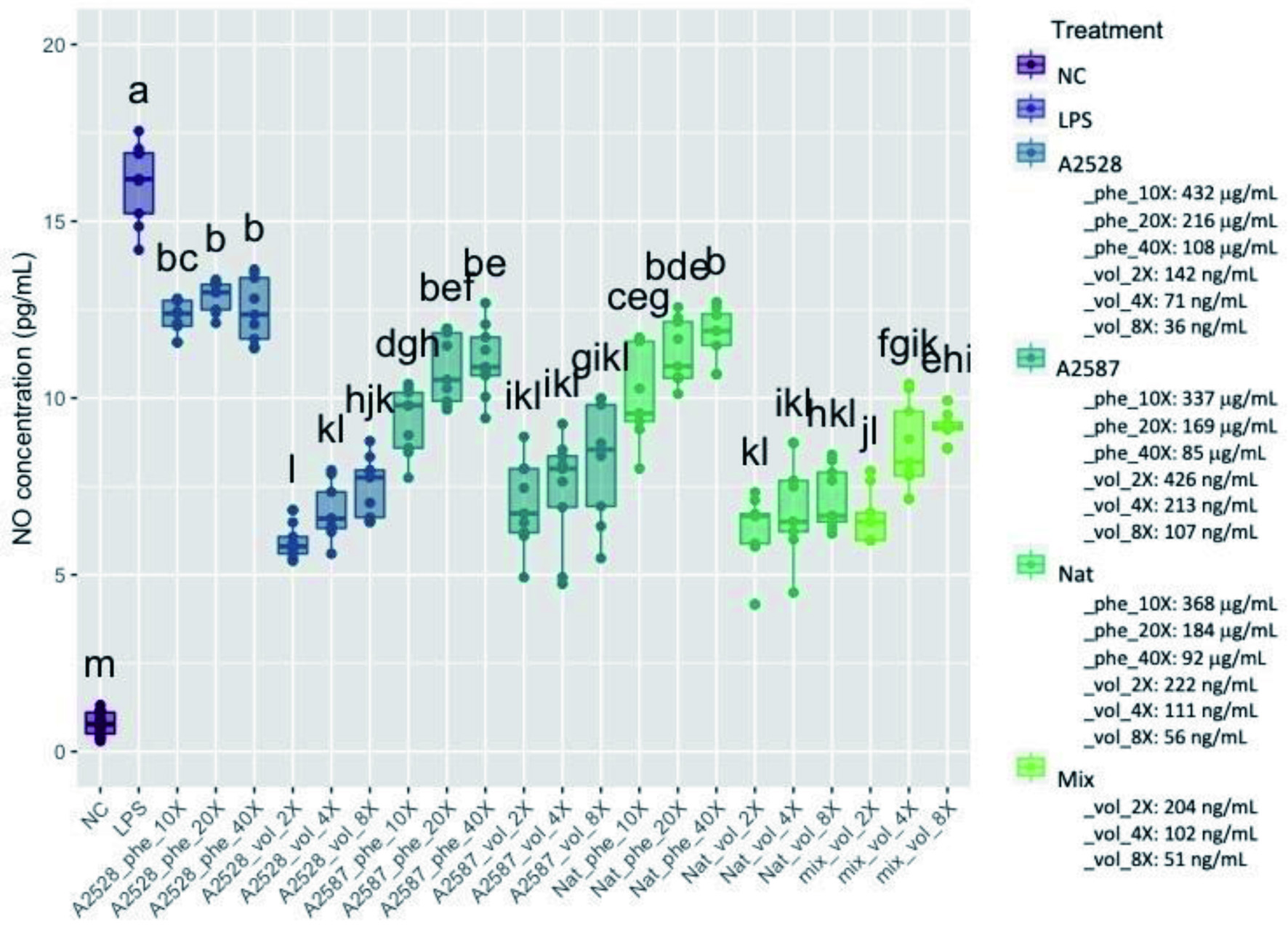 Click for large image | Figure 2. Average concentration of nitric oxide (NO, μmol/L) produced by RAW264.7 cells after a preventive treatment of one hr with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold, followed by LPS stimulation for 24 hr. The negative control (“NC”) corresponds to cells without treatment nor LPS stimulation. The positive control (“LPS”) corresponds to cells with LPS stimulation, but no treatment. The “Mix” is a lab-made solution of standard volatiles based on the average composition of the three blackberry volatile extracts. Conditions not connected by the same letter are significantly different (P<0.05). |
The percentage inhibition of NO was 24%, 42% and 37% by the phenolic extracts at 10-fold dilution for A2528T, A2587T and Natchez, respectively, and 63%, 51% and 61% within the volatile extracts at the 2-fold dilution, respectively. The average total phenolic concentrations in the 10-fold diluted phenolic extracts were 432, 337 and 368 µg/mL for A2528T, A2587T and Natchez, respectively, while the average total volatile concentrations in the 2-fold diluted volatile extracts were 142, 426, and 222 ng/mL.
Treatments with blackberry phenolics effectively reduced NO synthesis in LPS-activated macrophages (Cuevas-Rodríguez et al., 2010; Pergola et al., 2006; Van de Velde et al., 2016, 2019). The potency of the NO lowering effect of blackberry phenolic extracts differed between genotypes, with better inhibition reported for genotypes containing higher phenolic concentrations (Cuevas-Rodríguez et al., 2010; Van de Velde et al., 2016). Blackberry anthocyanins, and particularly cyanidin 3-glucoside which is the dominant anthocyanin in blackberry, were particularly effective at reducing NO production of J774 murine monocyte/macrophages cells in a dose-dependent manner (Pergola et al., 2006). However, a later study comparing blackberry phenolic fractions demonstrated a better NO reduction effect of the proanthocyanidins (25% inhibition) compared to the anthocyanin fraction (14% inhibition) in RAW 264.7 cell induced with LPS (Van de Velde et al., 2019). Reduction of NO production by blackberry phenolic extracts is associated with the downregulation of the expression and/or activity of iNOS (Cuevas-Rodríguez et al., 2010; Pergola et al., 2006; Van de Velde et al., 2019), an enzyme responsible for the production of NO and upregulated during inflammation (Lowenstein and Padalko, 2004). iNOS synthesis itself can be initiated through the activation of the NF-κB pathway (Taylor and Geller, 2000), which has been shown to be partially inhibited by blackberry phenolic extracts (Gu et al., 2020; Pergola et al., 2006). Part of the NO reduction in the stimulated cells may also be attributed to the antioxidant activity of phenolic compounds and their capacity to scavenge reactive oxygen species (Basu and Maier, 2016).
Volatiles, in particular monoterpene compounds, have been studied for their anti-inflammatory effects although the literature mostly focused on tree terpenes and essential oil matrices (de Cássia da Silveira e Sá et al., 2013; Kim et al., 2020). Few studies have been conducted regarding food volatiles, but papers report the anti-inflammatory effects of individual monoterpenes. In our blackberry volatile extracts, α-terpineol was one of the major monoterpenes in all three genotypes. α-terpineol has been used as treatment in LPS-induced murine macrophages, and was effective at reducing NO production at all concentrations tested (1, 10 and 100 µg/ml), although no dose-response relationship was established (de Oliveira et al., 2012). α-terpineol tested in RAW 264.7 cells at 1.16 µg/ml also significantly reduced NO production by LPS-induced cells, although the volatile extract from cranberry was more effective than the monoterpenes tested alone (Moore et al., 2019). α-terpineol has also been reported to inhibit NF-κB translocation in cancer cells (Hassan et al., 2010), along with other terpenoid compounds (Salminen et al., 2008). Similarly to the phenolic compounds, the ability of volatiles to reduce NO synthesis may be related to the capacity of few or several volatile compounds to inhibit the NF-κB pathway, and is supported by Gu et al. (2020) results who reported similar NO and NF-κB p65 inhibition between blackberry phenolic and volatile extracts. However, the decrease of NO synthesis by blackberry volatile extract through scavenging activity is not supported by our results, which showed no antioxidant effect of the blackberry volatile extracts.
Although our three blackberry genotypes varied in phenolic and volatile concentrations, all three showed comparable ability to inhibit secretion of NO induced by LPS. Our findings also suggest a much higher capacity for blackberry volatiles than phenolics to reduce NO synthesis, because they showed similar NO reduction activity, but at much lower concentration. This trend was previously observed in RAW 264.7 cells treated with phenolic and volatile fractions extracted from cranberries (Moore et al., 2019) and multiple berries including blackberries (Gu et al., 2020). This could suggest different mechanisms of action through which polyphenols and volatiles modulate pro-inflammatory pathways and ultimately the production of NO by macrophages, with a better efficacy of the volatiles. The lower molecular weight and reduced polarity of monoterpenes compared to phenolics make them better candidates to potentially interact with or enter the cells, where they could modulate different pathways involved in inflammation regulation.
The mixture of volatile standards simulating the major volatiles in the blackberry extract diluted 2-fold showed comparable ability to inhibit NO (59%) as the volatile extracts from the three blackberry genotypes diluted 2-fold. Additionally, the mixture of volatile standards diluted 4 and 8-fold resulted in 47% and 43% inhibition of NO production, respectively. This finding supports the hypothesis that the inhibitory effect of the blackberry volatile extracts on LPS-stimulated NO production was due to volatiles and not other compounds co-extracted with volatiles during the low temperature distillation protocol used to prepare the volatile extracts.
3.6. Effects of blackberry extracts on IL-6 production
IL-6 is a proinflammatory cytokine whose production can be induced in macrophages by LPS through the activation of the NF-κB pathway (Lee et al., 2015). The concentrations in IL-6 observed within the control cells and the cells treated with blackberry extracts are presented in Figure 3. The average concentrations in IL-6 in all dilutions of both phenolic and volatile extracts obtained from all three genotypes were significantly lower than the positive control (p<0.05). The mix of standard volatiles which was made of volatile standards based on the volatile composition of the blackberry extracts showed the greatest ability to inhibit IL-6.
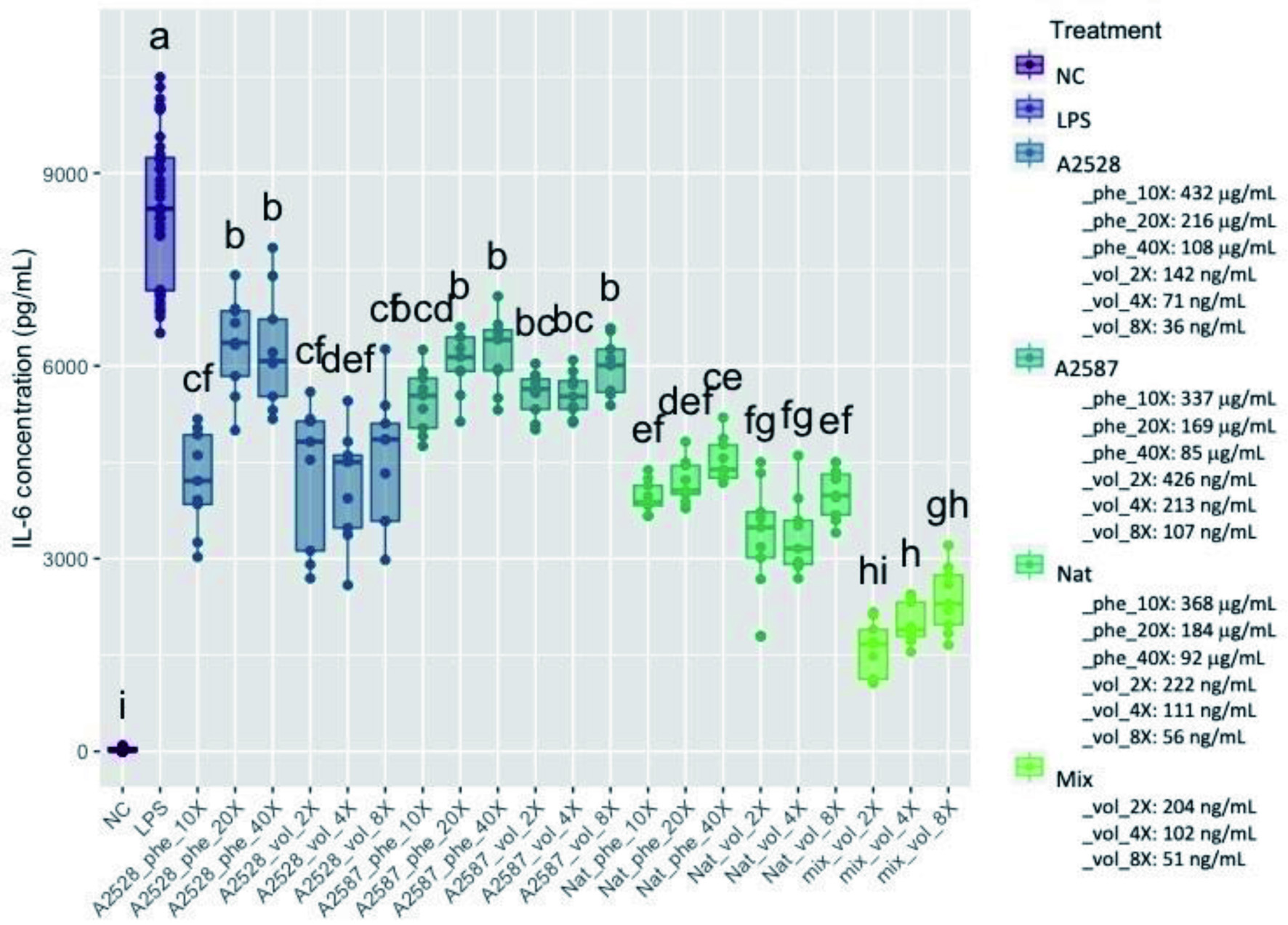 Click for large image | Figure 3. Average concentration of IL-6 (pg/ml) produced by RAW264.7 cells after a preventive treatment of one hr with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold, followed by LPS stimulation for 24 hr. The negative control (“NC”) corresponds to cells without treatment nor LPS stimulation. The positive control (“LPS”) corresponds to cells with LPS stimulation, but no treatment. The “Mix” is a lab-made solution of standard volatiles based on the average composition of the three blackberry volatile extracts. Conditions not connected by the same letter are significantly different (P<0.05). |
The percentage inhibition of IL-6 within the phenolic extracts at the 10-fold dilution of A2528T, A2587T and Natchez was 50%, 35% and 53%, respectively, and 49%, 34% and 60% within the volatile extracts at the 2-fold dilution, respectively. The inhibitory effect of both phenolic and volatile extracts was similar despite marked differences in bioactive compounds concentration. For example, the average bioactive compound concentration was of 337 µg/mL in phenolic extracts (10-fold dilution) of A2587T was 791-fold higher than the 426 ng/mL in the respective volatile extract (2-fold dilution), yet both extracts showed comparable ability to prevent IL-6 production. The mix of standard volatiles showed remarkable ability to inhibit secretion of IL-6, with 81%, 77% and 72% inhibition at 2, 4 and 8-fold dilutions, respectively.
As for NO, the inhibition of IL-6 secretion by blackberry phenolics has been demonstrated in LPS-induced macrophages (Van de Velde et al., 2016). The comparison of IL-6 inhibition by three blackberry cultivars showed better inhibitory effect for the two genotypes higher in total polyphenolics, as already observed for the NO marker (Van de Velde et al., 2016). When investigating different blackberry phenolic fractions, however, the authors did not report a significant difference between blackberry crude extract, anthocyanin and proanthocyanidin fractions on IL-6, while the latter fraction was the most effective at reducing NO (Van de Velde et al., 2016). These observations suggest a similar potency of different blackberry phenolic fractions on the pro-inflammatory IL-6 marker, while the blackberry proanthocyanidins may have exerted better scavenging activity towards NO.
α-terpineol, found in all three blackberry genotypes, and terpinen-4-ol, a major volatile in the A2587T and Natchez blackberries, are also two main components of tea tree essential oils (Nogueira et al., 2014) and have been studied for their anti-inflammatory effects on LPS-induced human macrophages. Terpinen-4-ol inhibited the production of IL-6 in macrophages induced with LPS from Escherichia coli at the two doses tested (551 and 68.1 µg/ml), but α-terpineol was only effective at the highest dose tested (551 µg/ml) and had no effect at the lowest dose (68.1 µg/ml) (Nogueira et al., 2014). The effect of terpinen-4-ol was later tentatively explained through its modulatory effect towards the ERK mitogen-activated protein kinase (MAPK) pathway, while the whole tea tree extract slightly inhibited both NF-κB and MAPK (Nogueira et al., 2014). However, in KB cancer cell line, an epidermal carcinoma of the mouth, α-terpineol effectively reduced the production of IL-6, as well as decreased the expression level of the IL-6 receptor gene in the cells. The other monoterpenes tested in this study, limonene and linalool, either increased (limonene) or did not impact IL-6 production (linalool) (Held et al., 2007). Our results support the modulation of IL-6 production by blackberry phytochemicals, and particularly volatile compounds. The inhibitory effect of the volatiles (either extracted from blackberry or made from standards) towards IL-6 may be carried out by a few individual compounds with high potency towards IL-6 inhibition, and/or a synergistic effect between several compounds. More research is needed to identify the specific phenolic and volatile compounds that inhibit secretion of IL-6 and to determine potential synergistic effects among compounds, although the complexity of the volatile composition of blackberry complicates the task.
Similarly to what was previously observed within the NO assay, our results indicate a potentially much higher capacity for volatiles to prevent inflammation. A similar conclusion was made by Gu et al. (2020) after a blackberry volatile extract (35% inhibition, average volatiles concentration of 244 ng/mL in cell media) exerted a higher IL-6 inhibitory effect than a blackberry phenolic extract (15% inhibition, average phenolics concentration of 77 ug/mL in cell media). Despite differences in the chemical profiles of the three blackberry genotypes tested in this study, all three genotypes showed comparable ability to inhibit secretion of IL-6 during the inflammatory process, strengthening the hypothesis of the compounds having synergistic effects rather than the extracts having only one main bioactive molecule.
3.7. Effects of blackberry extracts on TNF-α production
TNF-α is a cytokine that is rapidly produced after exposition to proinflammatory stimuli, and has various functions in coordinating the inflammatory response (Parameswaran and Patial, 2010), including the induction of iNOS expression in the liver in response to LPS (Salkowski et al., 1997). The NF-κB pathway is involved in the transcription of the TNF-α gene in LPS-induced macrophages (Shakhov et al., 1990).
The concentration in TNF-α observed within the control cells and the cells treated with blackberry extracts is presented in Figure 4. The average concentration in TNF-α in all conditions except the 40-fold-diluted phenolic extracts was significantly lower than the positive control (p<0.05). The percentage of inhibition of TNF-α secretion within the phenolic extracts at the 10-fold dilution of A2528T, A2587T and Natchez was 31%, 44% and 28%, respectively, and 61%, 73% and 67% within the volatile extracts at the 2-fold dilution, respectively. The mix of standard volatiles again showed remarkable ability to inhibit secretion of TNF- α, with 76%, 74% and 64%, inhibition at 2, 4 and 8-fold dilutions, respectively, which confirmed that the observed results were due to the volatiles present in the extracts.
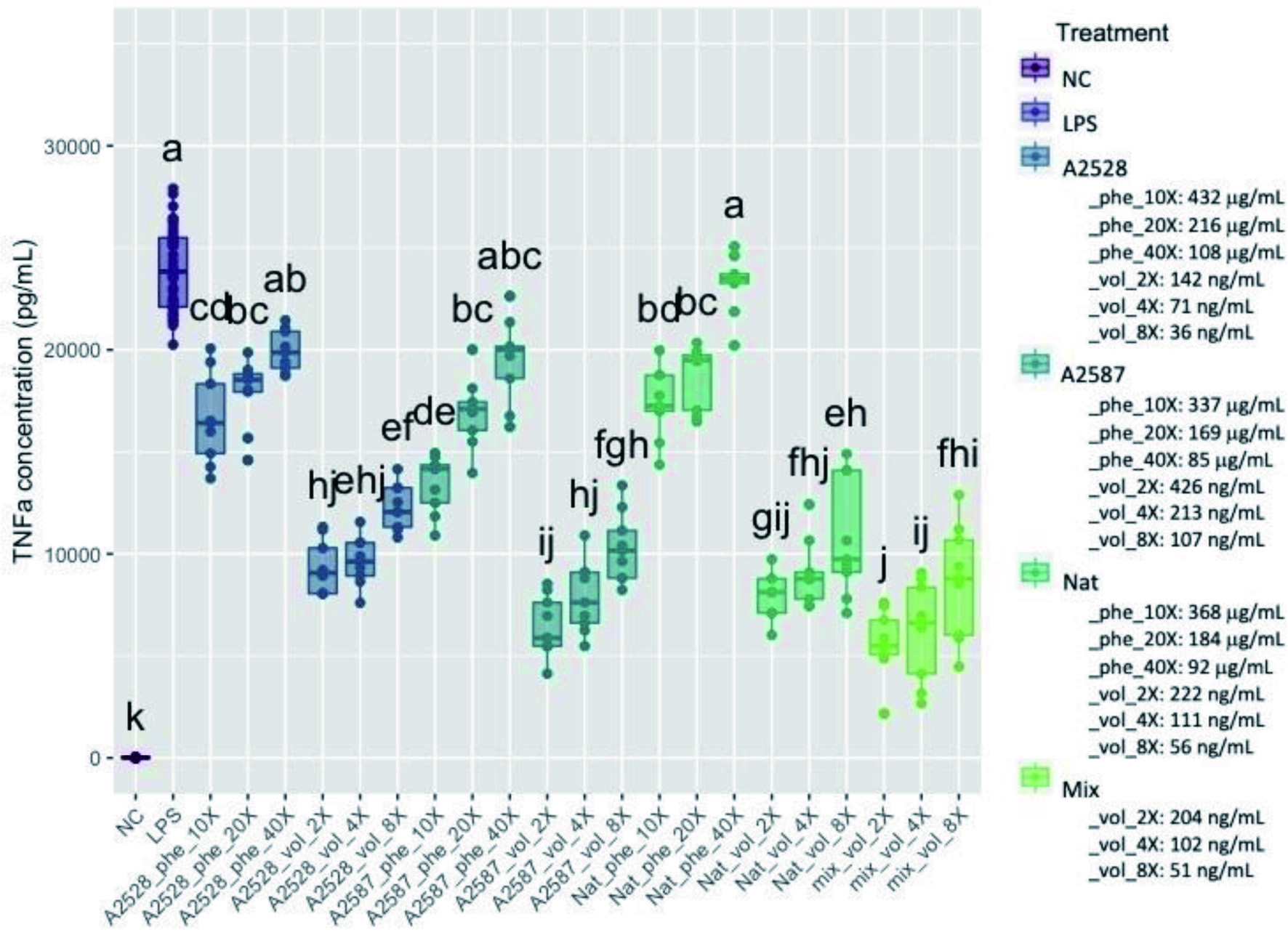 Click for large image | Figure 4. Average concentration of TNF-α (pg/ml) produced by RAW264.7 cells after a preventive treatment of one hr with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold, followed by LPS stimulation for 24 hr. The negative control (“NC”) corresponds to cells without treatment nor LPS stimulation. The positive control (“LPS”) corresponds to cells with LPS stimulation, but no treatment. The “Mix” is a lab-made solution of standard volatiles based on the average composition of the three blackberry volatile extracts. Conditions not connected by the same letter are significantly different (P<0.05). |
The effect of blackberry phenolic compounds on inflammation has mostly been measured using IL-6, IL-1β, PGE2 and NO markers, but few studies have reported the effect of blackberry phenolic fractions on TNF-α in macrophages in vitro. Gu et al. (2020) demonstrated a slight but significant reduction in TNF-α produced by LPS-induced RAW 264.7 cells following a treatment with blackberry phenolic fraction (77 µg/ml). Cyanidin 3-glucoside, the major anthocyanin reported in blackberry, significantly decreased TNF-α gene expression and protein synthesis in THP-1 human macrophages (Zhang et al., 2010). The inhibition effect was dose-dependent at concentrations ranging from 0.005 to 0.5 µM, but the effect was lost when the concentration was increased to 10 µM (Zhang et al., 2010).
LPS-induced human monocytes/macrophages treated with α-terpineol did not show a reduction in TNF-α production (Hart et al., 2000; Nogueira et al., 2014). Terpinen-4-ol significantly decreased the production of TNF-α in human monocytes (Hart et al., 2000), but failed to regulate the same cytokine in U937 activated macrophages, which contradicted the observations reported in the same study on IL-6 (Nogueira et al., 2014). Although these two compounds show no or moderate activities towards TNF-α, other monoterpenes were effective at reducing cytokine production in RAW 264.7 cells induced by LPS. Terpinolene and α-phellandrene decreased TNF-α production by about 20 to 40% at 14 and 28 µg/ml, respectively (de Christo Scherer et al., 2019), and linalool used between 40 and 120 µg/ml dampened TNF-α production in a dose-dependent manner, up to 70% inhibition (Huo et al., 2013).
Similarly to NO and IL-6 results, the TNF-α inhibitory effect of our blackberry volatile extracts was stronger than phenolic extracts for all three genotypes, demonstrating a much higher capacity for volatiles to prevent inflammation. The conclusion was consistent with a previous study by Gu et al. (2020) where blackberry volatile extract showed higher ability to inhibit TNF-α than blackberry phenolic extract. Our results show a strong decrease in TNF-α production by RAW 264.7 cells treated with the blackberry volatile extract and volatile standard mix. Similarly to the previous tests, there was not enough evidence to claim a difference in effect between the three blackberry genotypes despite differences in phenolic and volatile composition among genotypes. The particular effect of one or several volatile compounds remain to be investigated, as the most bioactive compounds may not be the ones present at higher concentration in the extracts.
| 4. Conclusion | ▴Top |
Blackberry phenolic and volatile fractions and a standard mix of blackberry volatile standards effectively inhibited the secretion of NO, IL-6 and TNF-α following LPS-induced inflammation of RAW264.7 cells. The volatile fraction showed greater ability to inhibit secretion of inflammatory cytokines than the phenolic fraction, despite being present at a much lower concentration. The three blackberry genotypes that varied in total volatile content all showed comparable ability to inhibit secretion of cytokines, indicating that volatile composition may be more important than total volatile content in modulating production of inflammatiory cytokines. Additional research is needed to determine the specific volatiles or class of volatiles responsible for anti-inflammatory activity, potential synergism existing between volatiles and phenolics, and the difference in respective mechanisms of action explaining the higher anti-inflammatory potency reported for volatiles. Future animal and human trials are also needed to determine bioavailability of volatile compounds, identification of metabolites and confirmation of anti-inflammatory effects in vivo.
C3GE: Cyanidin 3-glucoside equivalents; DPPH : 2,2 diphenyl-1-picrylhydrazyl; ELISA: Enzyme linked immunosorbent assay; GAE: Gallic acid equivalents; GC: Gas chromatography; GC-FID: Gas chromatography - flame ionization detection; GC-MS: Gas chromatography – mass spectrometery; HPLC: High pressure liquid chromatography; HPLC-MS: High pressure liquid chromatography – mass spectrometry; IL-6: Interluekin 6; LPS: Lipopolysaccharide; MAPK: Mitogen-activated protein kinase; NC: Negative control; NED : N-1-napthylethylenediamine dihydrochloride ; NF-κB: Nuclear factor of kappa light polypeptide gene enhancer in beta cells; NO: Nitric oxide; Phe: Phenolic extract; RE: Rutin equivalents; SPE: Solid phase extraction; SPME: Solid phase microextraction; TNFα : Tumor necrosis factor alpha; Vol: Volatile extract
| Supplementary material | ▴Top |
Acknowledgments
We thank Dr. John Clark, Horticulture Department, University of Arkansas for providing samples of blackberry genotypes for the study.
Conflict of interest
There are no conflicts of interest to declare.
| References | ▴Top |