Figures
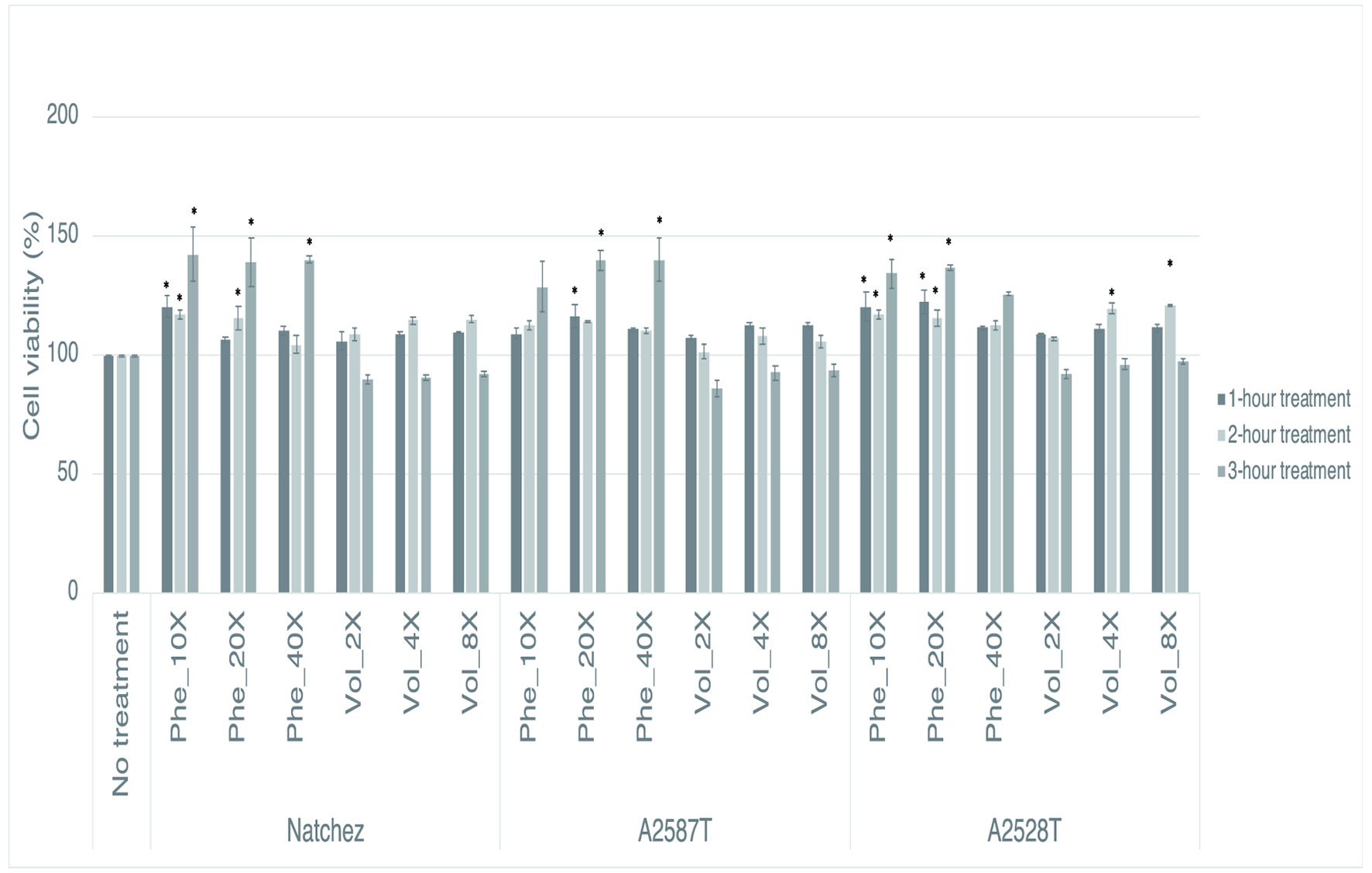
Figure 1. Percentage of viability of RAW264.7 cells after a preventive treatment of one, two and three hrs with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold. Values represent mean ± SEM (n = 3). * Denotes significant difference from cells that received no treatment (P<0.05).
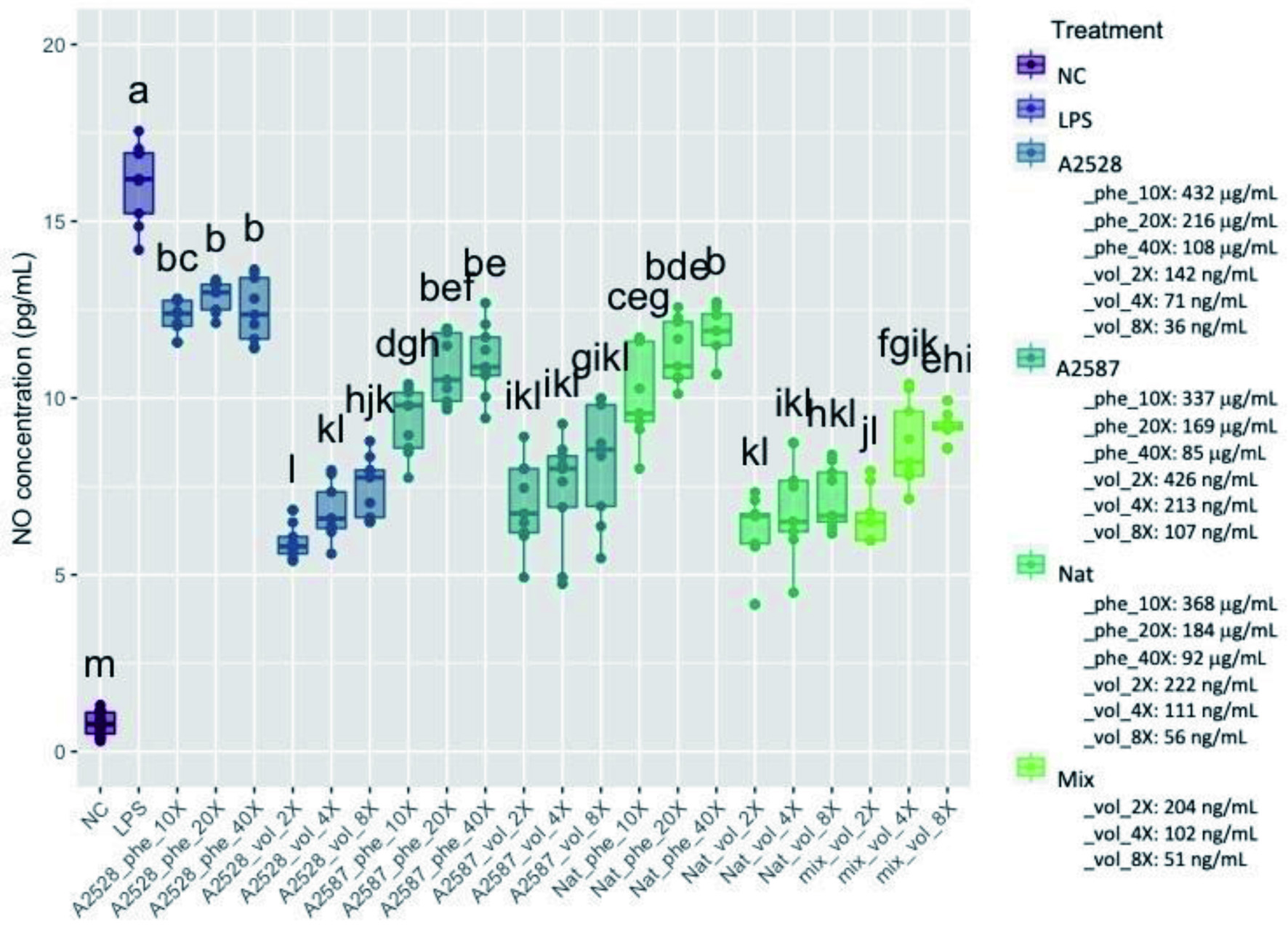
Figure 2. Average concentration of nitric oxide (NO, μmol/L) produced by RAW264.7 cells after a preventive treatment of one hr with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold, followed by LPS stimulation for 24 hr. The negative control (“NC”) corresponds to cells without treatment nor LPS stimulation. The positive control (“LPS”) corresponds to cells with LPS stimulation, but no treatment. The “Mix” is a lab-made solution of standard volatiles based on the average composition of the three blackberry volatile extracts. Conditions not connected by the same letter are significantly different (P<0.05).
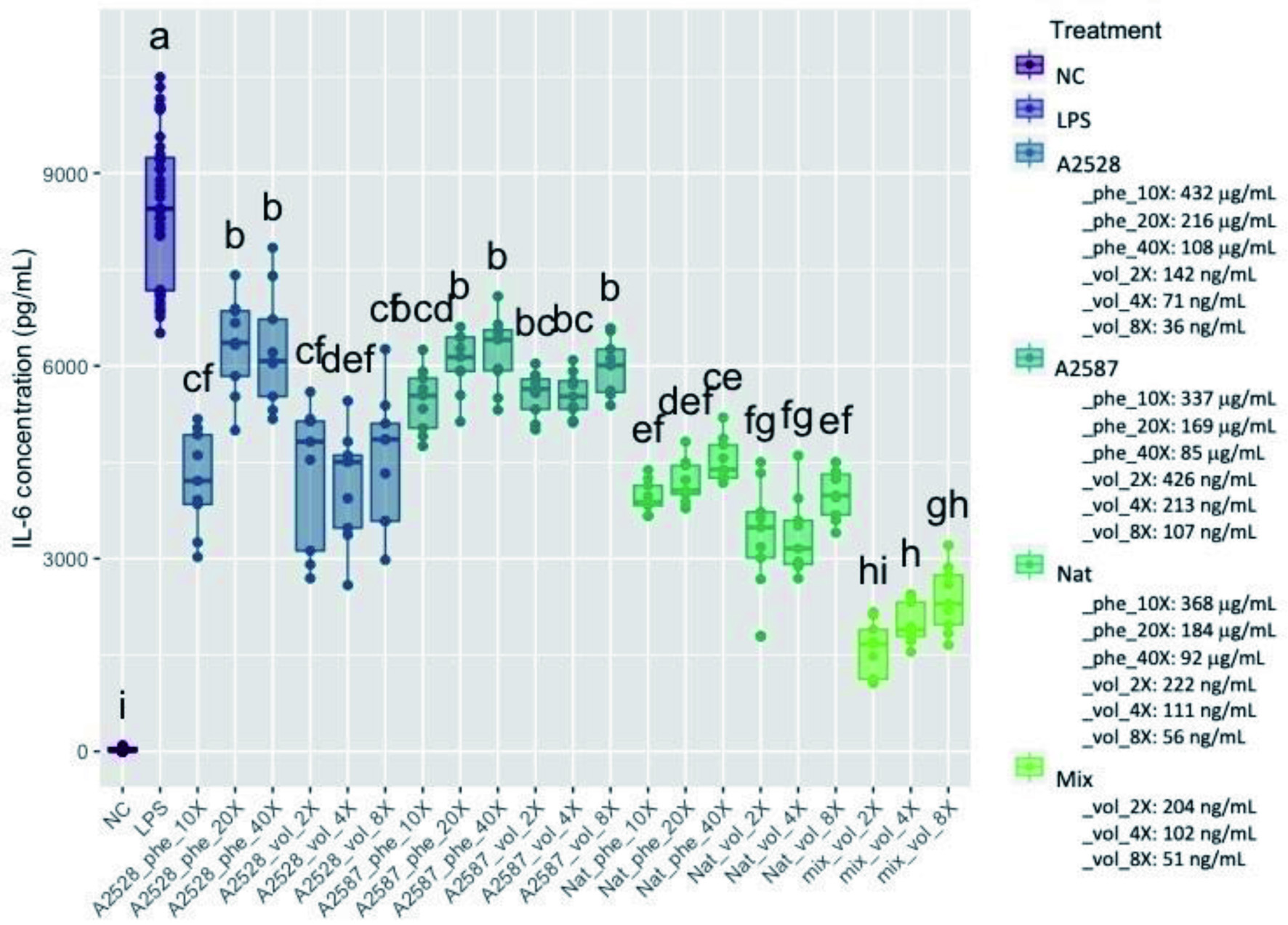
Figure 3. Average concentration of IL-6 (pg/ml) produced by RAW264.7 cells after a preventive treatment of one hr with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold, followed by LPS stimulation for 24 hr. The negative control (“NC”) corresponds to cells without treatment nor LPS stimulation. The positive control (“LPS”) corresponds to cells with LPS stimulation, but no treatment. The “Mix” is a lab-made solution of standard volatiles based on the average composition of the three blackberry volatile extracts. Conditions not connected by the same letter are significantly different (P<0.05).
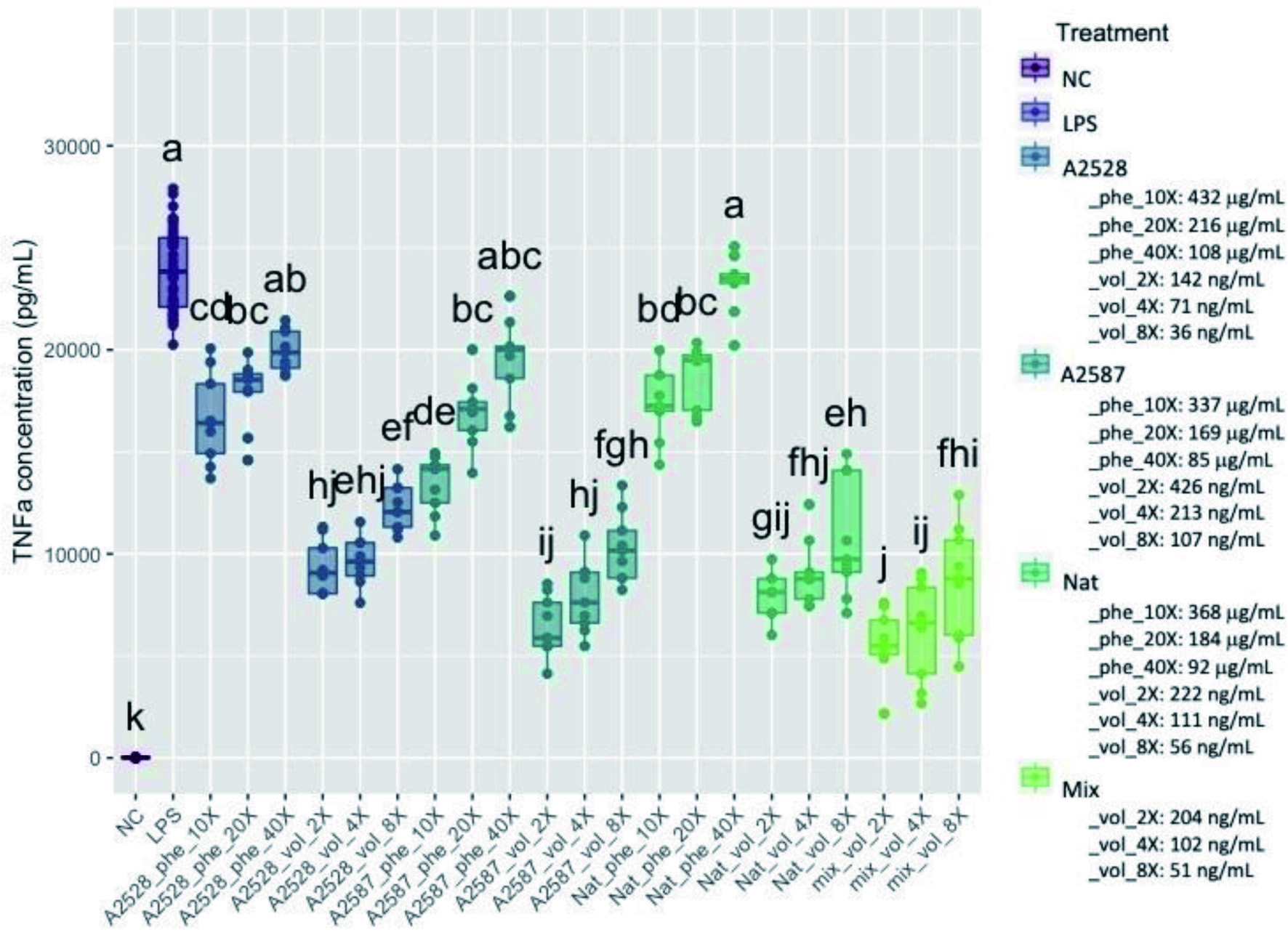
Figure 4. Average concentration of TNF-α (pg/ml) produced by RAW264.7 cells after a preventive treatment of one hr with phenolic extracts (“Phe”) diluted 10-, 20-, or 40-fold or volatile extracts (“Vol”) diluted 2-, 4-, or 8-fold, followed by LPS stimulation for 24 hr. The negative control (“NC”) corresponds to cells without treatment nor LPS stimulation. The positive control (“LPS”) corresponds to cells with LPS stimulation, but no treatment. The “Mix” is a lab-made solution of standard volatiles based on the average composition of the three blackberry volatile extracts. Conditions not connected by the same letter are significantly different (P<0.05).
Tables
Table 1. Volatile composition (ng/mL) of low temperature vacuum distillates obtained from three blackberry genotypes
| Compound | Quantified as | RI1 | Genotype |
|---|
| A2528T | A2587T | Natchez |
|---|
| 1RI = retention index. 2Means (N=3 ± Std.Dev.). 3Means within rows with different letters are significantly different (p < 0.05). |
| 1-Butanol | 1-Butanol | 642 | ND | 1.53 ±0 .622a3 | 6.53 ± 4.16a |
| 2-Methylbutan-1-ol | 1-Butanol, 2-methyl- | 723 | 0.71 ± 0.12a | ND | 0.92 ± 0.28a |
| Trans-3-hexen-1-ol | Trans-3-hexen-1-ol | 846 | 1.79 ± 0.13a | 0.31 ± 0.08b | 0.79 ± 0.13c |
| Cis-3-hexen-1-ol | Cis-3-hexen-1-ol | 851 | 8.90 ± 0.94a | 5.73 ± 0.88b | 9.07 ± 1.25a |
| 2-hexen-1-ol, | 1-Hexanol | 861 | 16.89 ± 1.73a | 1.85 ± 0.36b | 2.94 ± 1.08b |
| 1-Hexanol | 1-Hexanol | 863 | 26.36 ± 1.34a | 5.56 ± 0.57b | 34.68 ± 4.83c |
| 2-Heptanol | 2-Heptanol | 895 | 8.92 ± 1.32b | 190.43±28.11a | 1.68 ± 0.30b |
| 1-Heptanol | 1-Heptanol | 968 | 1.19 ± 0.49a | 1.25 ± 0.29a | 0.83 ± 0.22a |
| 1-Octen-3-ol | 1-Octen-3-ol | 978 | 0.94 ± 0.18a | 1.36 ± 0.69a | 0.76 ± 0.05a |
| 1-Pentanol, 3-ethyl-4-methyl- | 1-Hexanol | 1,019 | 0.21 ± 0.09 | ND | ND |
| 2-Ethyl-1-hexanol | 1-Hexanol | 1,028 | 3.74 ± 0.55a | 3.17 ± 1.31a | 3.96 ± 0.64a |
| 2- Phenylethyl alcohol | Phenylethyl alcohol | 1,117 | ND | 44.21 ± 2.78a | 23.47 ± 3.74b |
| 1-Nonanol | 1-Nonanol | 1,169 | 0.82 ± 0.24b | 2.56 ± 1.01a | 1.09 ± 0.17b |
| 6-Camphenol | α-Terpineol | 1,177 | 2.49 ± 0.93b | ND | 40.42 ± 3.75a |
| 1-Decanol | 1-Decanol | 1,271 | 0.36 ± 0.19b | 1.45 ± 0.52a | 0.69 ± 0.08b |
| p-Mentha-1-en-9-ol | Perillic alcohol | 1,305 | ND | 32.72 ± 2.98a | 10.71 ± 1.36b |
| 1-Undecanol | 1-Decanol | 1,371 | 0.05 ± 0.09 | ND | ND |
| Dodecanol | 1-Decanol | 1,474 | 0.67 ± 0.30b | 1.65 ± 0.13a | 0.28 ± 0.15b |
| Total alcohols | | | 74.04 ± 7.71b | 293.79 ± 40.33a | 138.84 ± 18.45b |
| 2-Butenal (E) | Butanal, 3-methyl- | 629 | 0.37 ± 0.33 | ND | ND |
| 2-Butenal (Z) | Butanal, 3-methyl- | 647 | 9.58 ± 2.01 | ND | ND |
| Pentanal | Pentanal | 677 | 4.32 ± 2.43a | 3.36 ± 1.60a | 1.46 ± 0.27a |
| 2-Ethyl-2-butenal | Butanal, 3-methyl- | 727 | 1.06 ± 0.20a | ND | 2.34 ± 0.53b |
| 2-Pentenal | Pentanal | 744 | 1.30 ± 0.60a | 0.31 ± 0.27b | 0.33 ± 0.29b |
| 3-Methyl-2-butenal | Butanal, 3-methyl- | 776 | 0.63 ± 0.64a | 1.13 ± 0.38a | 0.82 ± 0.19a |
| Hexanal | Hexanal | 798 | 3.46 ± 0.43a | 4.20 ± 1.23a | 4.08 ± 0.68a |
| Heptanal | Heptanal | 902 | 0.05 ± 0.01a | 0.09 ± 0.03a | 0.06 ± 0.02a |
| Benzaldehyde | Benzaldehyde | 965 | 2.20 ± 0.51b | 1.57 ± 0.03b | 4.85 ± 1.13a |
| Octanal | Octanal | 1,003 | 1.64 ± 0.35b | 4.30 ± 2.04a | 1.31 ± 0.50b |
| Nonanal | Nonanal | 1,104 | 4.48 ± 4.18a | 8.48 ± 3.74a | 2.26 ± 0.47a |
| Decanal | Decanal | 1,206 | 2.85 ± 1.02a | 9.08 ± 5.78a | 7.02 ± 1.30a |
| 2-Decenal | 2-Decenal | 1,265 | 0.71 ± 0.41b | 1.35 ± 0.75b | 2.55 ± 0.43a |
| Perilla aldehyde | 2-Decenal | 1,289 | ND | 0.67 ± 0.10 | ND |
| Undecanal | Undecenal | 1,308 | 0.29 ± 0.16a | 0.87 ± 0.59a | ND |
| Dodecanal | Decanal | 1,411 | 0.34 ± 0.21ab | 0.63 ± 0.24a | 0.14 ± 0.09b |
| Total aldehydes | | | 33.85 ± 13.80a | 36.04 ± 16.78a | 27.22 ± 5.89a |
| α,p-Dimethylstyrene | α,p-Dimethylstyrene | 1,095 | 9.80 ± 5.23a | 45.74 ± 6.70b | 27.83 ± 3.63c |
| Total aromatic hydrocarbons | | | 9.80 ± 5.23c | 45.74 ± 6.70a | 27.83 ± 3.63b |
| Ethyl acetate | Ethyl acetate | 593 | 76.29 ± 11.89a | 50.07 ± 38.26a | 67.25 ± 13.08a |
| Methyl propionate | Methyl butanoate | 604 | ND | 0.23 ± 0.21 | ND |
| Ethyl propanoate | Methyl butanoate | 699 | 0.26 ± 0.18a | 0.25 ± 0.12a | ND |
| Methyl butanoate | Methyl butanoate | 711 | 0.30 ± 0.19a | 0.25 ± 0.09a | ND |
| Methyl 3-methylbutanoate | Methyl butanoate | 770 | 0.19 ± 0.21 | ND | ND |
| Ethyl butanoate | Ethyl butanoate | 794 | 1.70 ± 0.35 | ND | ND |
| Butyl acetate | Butyl acetate | 815 | 0.84 ± 0.27 | ND | ND |
| Methyl hexanoate | Methyl hexanoate | 921 | 1.38 ± 1.01a | 0.33 ± 0.25a | 0.57 ± 0.18a |
| Hexyl formate | Methyl butanoate | 929 | 0.27 ± 0.11 | ND | ND |
| Ethyl 3-hydroxybutyrate | Methyl butanoate | 939 | ND | ND | 1.05 ± 0.17 |
| Ethyl hexanoate | Ethyl hexanoate | 998 | 2.36 ± 1.44a | 1.56 ± 0.49a | 2.47 ± 0.36a |
| Methyl octanoate | Methyl octanoate | 1,122 | 0.44 ± 0.21a | 0.36 ± 0.20a | 0.22 ± 0.11a |
| Total esters | | | 84.05 ± 15.85a | 53.06 ± 39.61a | 71.56 ± 13.89a |
| 2-Heptanone | 2-Heptanone | 890 | 0.88 ± 0.35ab | 0.84 ± 0.04a | 1.37 ± 0.24b |
| 5-Hepten-2-one, 6-methyl- | 5-Hepten-2-one, 6-methyl- | 987 | 0.50 ± 0.11a | 0.52 ± 0.14a | 0.23 ± 0.03b |
| Acetophenone | Acetophenone | 1,076 | ND | 0.73 ± 0.68a | 0.71 ± 1.23a |
| Total ketones | | | 1.38 ± 0.46a | 2.09 ± 0.86a | 2.31 ± 1.50a |
| β-Myrcene | β-Myrcene | 992 | 4.25 ± 0.92a | 14.21 ± 1.01b | 6.82 ± 0.52c |
| D-Limonene | D-Limonene | 1,036 | 2.05 ± 0.82a | 10.50 ± 1.87b | 6.96 ± 1.67c |
| p-Cymene | p-Cymene | 1,040 | 0.22 ± 0.10a | 2.60 ± 0.41b | 1.04 ± 0.003c |
| m-Cymene | p-Cymene | 1,045 | ND | ND | ND |
| β-Ocimene | β-Myrcene | 1,050 | 0.56 ± 0.15b | 2.74 ± 0.54a | 1.13 ± 0.17b |
| Dihydro myrcenol | Myrtenol | 1,072 | 0.52 ± 0.11a | 0.47 ± 0.35a | 0.14 ± 0.07a |
| Linalool oxide | Linalool | 1,078 | 0.48 ± 0.11 | ND | ND |
| Linalool | Linalool | 1,100 | 5.09 ± 1.30b | 11.59 ± 0.97a | 4.47 ± 0.29b |
| 6-Camphenone | Acetophenone | 1,130 | 0.47 ± 0.07a | ND | 2.36 ± 0.11b |
| Cis-p-mentha-2,8-dien-1-ol | 1-Nonanol | 1,142 | ND | 0.56 ± 0.20 | ND |
| Verbenol | Terpinen-4-ol | 1,158 | ND | 0.88 ± 0.16a | 2.53 ± 1.16a |
| Isopulegol | Isopulegol | 1,164 | 0.37 ± 0.12 | ND | ND |
| α-Phellandrene-8-ol | α-Terpineol | 1,175 | ND | 29.54 ± 6.83 | ND |
| Borneol | Borneol | 1,187 | 0.96 ± 0.26a | 4.03 ± 0.50b | 7.45 ± 0.79c |
| Terpinen-4-ol | Terpinen-4-ol | 1,188 | 4.91 ± 2.67a | 52.89 ± 4.20b | 35.44 ± 4.86c |
| p-Cymene-8-ol | Terpinen-4-ol | 1,191 | 5.78 ± 3.21a | 32.37 ± 5.65b | 19.75 ± 4.94c |
| α-Terpineol | α-Terpineol | 1,200 | 24.55 ± 13.61b | 68.60 ± 13.80a | 36.21 ± 4.45b |
| Myrtenol | Myrtenol | 1,208 | 1.67 ± 0.64b | 18.58 ± 6.97a | 1.88 ± 0.46b |
| Nerol | Nerol | 1,224 | 5.80 ± 2.31a | 7.20 ± 1.02a | 5.12 ± 0.92a |
| Verbenone | D-Carvone | 1,228 | 2.54 ± 1.11b | 35.07 ± 5.02a | 7.67 ± 1.37b |
| Citronellol | Citronellol | 1,233 | 0.42 ± 0.28a | 6.32 ± 0.86b | 4.81 ± 0.78c |
| D-Carvone | D-Carvone | 1,256 | ND | 51.87 ± 8.33 | ND |
| Geraniol | Geraniol | 1,258 | 5.59 ± 1.36b | 5.02 ± 0.67b | 16.19 ± 3.86a |
| Geranial | 2-Decenal | 1,274 | 0.53 ± 0.31b | 1.97 ± 0.42a | 0.88 ± 0.22b |
| Perillic alcohol | Perillic alcohol | 1,299 | 1.87 ± 1.22a | 14.38 ± 1.03b | 7.30 ± 1.47c |
| β-Damascenone | β-Damascenone | 1,398 | 11.08 ± 6.85a | 21.85 ± 1.85a | ND |
| Total monoterpenes | | | 79.72 ± 36.52c | 393.23 ± 61.23a | 168.15 ± 27.58b |
| β-Ionone | β-Ionone | 1,502 | 0.42 ± 0.16a | 0.26 ± 0.01ab | 0.10 ± 0.01b |
| Total Noisoprenoids | | | 0.42 ± 0.16b | 0.26 ± 0.01a | 0.10 ± 0.01b |
| Epicubenol | Perillic alcohol | 1,657 | ND | 19.73 ± 3.45a | 5.23 ± 1.56b |
| α-Cadinol | Perillic alcohol | 1,669 | ND | 7.78 ± 3.78a | 2.39 ± 0.83a |
| Total sesquiterpenes | | | 0.42 ± 0.16b | 27.77 ± 7.24a | 7.72 ± 2.40b |
| Total volatiles | | | 283.25 ± 45.39c | 851.73 ± 49.23a | 443.62 ± 34.98b |
Table 2. Standard volatiles used to prepare the simulated blackberry volatile mixture
| Compound | Concentration (ng/mL) | Compound | Concentration (ng/mL) |
|---|
| 2-Heptanol | 67.01 | Hexanal | 3.91 |
| Ethyl acetate | 64.54 | Citronellol | 3.85 |
| α-Terpineol | 43.12 | Pentanal | 3.04 |
| Terpinen-4-ol | 31.08 | Benzaldehyde | 2.87 |
| α, p-Dimethylstyrene | 27.79 | 1-Butanol, 3-methyl- | 2.59 |
| 1-Octanol | 22.30 | Octanal | 2.42 |
| 1-Hexanol | 22.20 | Ethyl hexanoate | 2.13 |
| Geraniol | 8.93 | Toluene | 1.68 |
| β-Myrcene | 8.43 | 2-Decenal | 1.54 |
| 3-Hexen-1-ol | 7.90 | 1-Nonanol | 1.49 |
| Perillic alcohol | 7.85 | Cymene | 1.29 |
| Myrtenol | 7.38 | 1-Heptanol | 1.09 |
| Linalool | 7.05 | 2-Heptanone | 1.03 |
| Thymol | 6.59 | 1-Octen-3-ol | 1.02 |
| D-Limonene | 6.50 | 3-Hexen-1-ol | 0.97 |
| Decanal | 6.32 | 1-Decanol | 0.83 |
| 2-Hexenal | 6.25 | 5-Hepten-2-one, 6-methyl- | 0.42 |
| Nerol | 6.04 | Methyl octanoate | 0.34 |
| Nonanal | 5.07 | β-Ionone | 0.26 |
| α-Terpinene | 5.00 | 2-Octenal | 0.22 |
| 1-Pentanol | 4.32 | Heptanal | 0.07 |
| Borneol | 4.15 | Total concentration | 407 |
Table 3. Composition and antioxidant activity of blackberry phenolic extracts
| Compound | Genotype |
|---|
| A2528T | A2587T | Natchez |
|---|
| 1Total anthocyanins and total flavonols were measured by HPLC-PDA and expressed as cyanidin 3-glucoside equivalents (C3GE) and rutin equivalents (RE), respectively. 2Total phenolics were measured using the Folin Ciocalteu assay and are expressed as gallic acid equivalents (GAE). 3Antioxidant activity was measured by the DPPH method and expressed as Trolox equivalents (TE). 4Results are expressed as mean ± SD (n = 3). 5Means within rows with different letters are significantly different (p < 0.05). |
| Total anthocyanins1 (µg C3GE/mL) | 1,484 ± 96b4,5 | 2,078 ± 28a | 2,263 ± 144a |
| Total flavonols1 (µg RE/mL) | 163 ± 13a | 105 ± 16b | 129 ± 13ab |
| Total phenolics2 (µg GAE/mL) | 4,315 ± 121a | 3,369 ± 92b | 3,680 ± 171c |
| Antioxidant activity3 (µmol TE/mL) | 30 ± 1a | 24 ± 1b | 25 ± 2b |



