| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 16, December 2021, pages 25-33
The prevention and treatment of COVID-19 and related development during pandemic
Chin-Kun Wanga, Yuan-Ti Leeb, c, d, Chao-Bin Yehe, f, g, Chi-Ho Chang, h, *
aDepartment of Nutrition, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
bInstitute of Medicine, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
cSchool of Medicine, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
dDivision of Infectious Diseases, Department of Internal Medicine, Chung Shan Medical University Hospital, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
eDepartment of Emergency Medicine, School of Medicine, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
fDepartment of Emergency Medicine, Chung Shan Medical University Hospital, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
gDepartment of Medical Research, Chung Shan Medical University Hospital, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
hDepartment of Microbiology and Immunology, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC
*Corresponding author: Chi-Ho Chan, Department of Microbiology and Immunology, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City, Taiwan, ROC. Tel: +886 4 24730022 ext 12022; Fax: 886 4 24727178; E-mail: chiho@csmu.edu.tw
DOI: 10.31665/JFB.2021.16290
Received: September 16, 2021
Revised received & accepted: December 23, 2021
| Abstract | ▴Top |
When the outbreak of human novel coronavirus was first reported in Wuhan, China at the end of 2019, the epidemic spread rapidly around the world and finally became a pandemic in 2020. In order to seek effective drugs to treat the COVID-19 infected patients for emergent use and for the disease prevention, researchers examined numerous existed antiviral drugs that may have the potential for COVID-19 treatment. At the same time, antibody treatment and vaccines development were ongoing simultaneously. The aim of this review is to introduce antibody therapy, vaccine development and potential antiviral treatments on COVID-19 and to discuss the future perspective on the COVID-19 pandemic.
Keywords: SARS-CoV-2; COVID-19; Antibody therapy; Vaccine development; Antiviral drugs
| 1. Current status of Covid-19 | ▴Top |
The outbreak of novel coronavirus (2019-nCoV), was first reported in Wuhan, Hubei province, China since December, 2019 (Huang et al., 2020). The virus was renamed severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) based on phylogenetic analysis to differentiate it from the first outbreak caused by the SARS-CoV in 2003. At the same time the related disease of SARS-CoV-2 was renamed COVID-19 by World Health Organization (WHO). Due to its rapid spreading via international travelling of people and serious respiratory symptom in human population, WHO declared COVID-19 a public health emergency of international concern in 2019 (Huang et al., 2020; Wang et al., 2021a). Till now, more than two hundred million of confirmed cases and nearly four millions and six hundred thousand people died from COVID-19 infection. Meanwhile, several types of COVID-19 vaccines are produced for people to establish their immunity against SARS-CoV-2 globally. In fact, abundant of antiviral drugs and passive immunization therapy have been tried to treat COVID-19 patients, before the vaccines production, especially application on those severe COVID-19 patients. However, cases and number of death are still increasing on every day around the world. The most probably reason is due to the emerging of numerous of SARS-CoV-2 variants in different countries. Even some of the variants has been reported that they had the ability to break through patients’ immunity even after they were fully vaccination. In this review, we focused on the features of SARS-CoV-2 and its variants, vaccine development, antibody therapy and potential antiviral drugs on COVID-19.
| 2. Features of SARS-CoV-2 | ▴Top |
SARS-CoV-2 is an enveloped, single positive strand RNA virus, belongs to the family coronaviridae, genus betacoronavirus according to the update recommendation of the International Committee on Taxonomy of Viruses (ICTV) (Shchelkanov et al., 2020). Other viruses belong to this genus includes SARS-CoV, MERS-CoV, two human coronaviruses (OC43 and HKU-1) and murine hepatitis virus (MHV) (Fung and Liu, 2019; Malik, 2020; Velavan and Meyer, 2020).
The genome length of SARS-CoV-2 is about 30 kilobase. The organization of the genome includes two open reading frames (i.e. ORF1a and ORF1b) start from 5′ to 3′ in which encodes a series of non-structural proteins and enzymes. Next to the ORFs are those structural genes (i.e. S, E, M, and N) which encode spike (S), envelope protein (E), matrix protein (M), and nucleoprotein (N) (Bahrami and Ferns, 2020; Lu et al., 2020).
Briefly, the SARS-CoV-2 enter the host cell through endocytosis by binding host angiotensin-converting enzyme 2 (ACE-2) receptor with the S protein on the envelope of the virus. After cleavage of the S1-S2 domain of the S protein by TMPRSS2, a cysteine protease, the virus enters into the cytoplasm of the host cell by fuses its envelope to the cell membrane and forms an endosome. After uncoating, the viral genome is released into the cytoplasm of the host cell, and the genome is translated into replicase polyprotein, PP1a, PP1ab and RNA-dependent RNA polymerase (RdRp). Through genome replication and a series of protein translation, the progeny virus is released by exocytosis and continue to infect other host cells or spread from respiratory tract to surroundings (Trougakos et al., 2021; V’kovski et al., 2021).
Just like other RNA virus, one of characteristic of SARS-CoV-2 is its RNA-dependent RNA polymerase lacking the proofreading during genomic RNA replication. After the virus infects the host cells, the genomes of the progeny viruses are not identical with each other and that of the parent virus. All the progeny viruses form a subpopulation of viruses that is called quasispecies (Gao et al., 2021; Jary et al., 2020). In addition, after virus infection, host’s body will produce specific antibodies to neutralize the viruses via adaptive immune response. Despite of eliminate the virus, host immune response always prompt the virus to produce more variants under the selective pressure (Eskier et al., 2020; Presti et al., 2020). As a result, variants of SARS-CoV-2 continuously emerges from the patients around the world. All these variants have different phenotypes such as increasing of their transmissibility, increasing of disease severity and mortality in patients and decreasing the sensitivity from neutralizing effect of antibody after vaccination. For this reason, WHO designated these types of variants as the variants of concern (VOC) (Duong, 2021). In addition, there are other variants have certain mutations found in particular genes, i.e. S protein or other regions of the virus genome and they have possibility to affect public health in future. WHO also designated them as the variants of interest (VOI) (Chakraborty et al., 2021a; Konings et al., 2021). For most update information, a new SARS-CoV-2 variant, named Omicron was announced on November 26, 2021 by WHO. Surprisingly, the speed of this variant is faster than other known circulated variants (He et al., 2021). The characteristics of Omicron might include increase of infectivity, vaccine breakthrough, antibody resistance and drug resistance (Chen et al., 2021) (Table 1).
 Click to view | Table 1. Variants of SARS-CoV-2 |
| 3. Vaccine development | ▴Top |
Nowadays, there are more than 300 vaccine candidates has being developed according to the information of WHO since the beginning of the COVID-19 pandemic. On September, 2021, totally 117 vaccines are being test in different phases on clinical trials while 185 vaccines are in subclinical development (Shrotri et al., 2021). The vaccine candidates are still increasing. Among them, there are 18 vaccines were approved for emergency use authorization (EUA) by at least one regulatory authority 2021 (Ndwandwe and Wiysonge, 2021). Among these vaccines, Oxford/AstraZeneca ChAdOx1-S (England), BioNTech/Pfizer BNT162b2 (German), Moderna mRNA-1273 (USA), and MVC-COV1901 (Taiwan) are allowed to use in Taiwan.
Ten platforms are adopted for COVID-19 vaccines developments currently. It can be divided into two categories, the classical platforms and novel platforms. The classical platforms include protein subunit (PS) (Heath et al., 2021; Ryzhikov et al., 2021; Hsieh et al., 2021), inactivated whole virus (IV) (Ariamanesh et al., 2021; Organization, 2021; Palacios et al., 2020), virus-like particle (Burgos-Salcedo, 2021; Park et al., 2020), and live attenuated virus (Wang et al., 2021a; Qian et al., 2021). The novel platforms include non-replicating virus vector (NRVV) (Cao et al., 2021; Choo and Teo, 2021; Knoll and Wonodi, 2021; Sadoff et al., 2020), DNA (Nishikawa et al., 2021; Tebas et al., 2021), mRNA (Chagla, 2021; Teo, 2021), replicating virus vector (Misra et al., 2021), replicating virus vector plus antigen presenting cell (Haidere et al., 2021), non-replicating virus vector plus antigen presenting cell (van Riel and de Wit, 2020; Zhang et al., 2020). Four out of 10 platforms (i.e. PS, NRVV, RNA and IV) are being used to produce the COVID-19 vaccines massively and supplying globally for the prevention and even termination of COVID-19 pandemic (Heinz and Stiasny, 2021).
Table 2 summarized the platforms on vaccine development and the types of vaccines for current used for the prevention of COVID-19.
 Click to view | Table 2. Platforms of vaccine development on COVID-19* |
| 4. Antibody therapy | ▴Top |
When Emil von Behring and Shibasaburo Kitasato discovered that the serum of animals immune to diphtheria or tetanus possessed anti-toxic activity, i.e. antibody in 1890s, it became a useful method to treat so many infectious diseases (Ripoll et al., 2021). Paul Ehrlich first used passive immunotherapy by developed antiserum to treat diphtheria. With the continuous development of immunology, passive immunotherapeutic included hyperimmune immunoglobulin from immunized animals and convalescent plasma from recovery patients. Especially during sudden outbreak of particular viral diseases such as SARS, MERS and avian influenza, etc., when there were lacking of both anti-viral drugs and vaccines in a short time. Passive immune therapy by using neutralization antiserum from recovered patients became a useful strategy for emergency use and lifesaving (Gupta et al., 2020). Especially in the severe cases of COVID-19 patients, convalescent serum and monoclonal antibody are the two main strategies in antibody therapy.
4.1. Convalescent plasma against SARS-CoV-2/COVID-19
Convalescent plasma can be used to treat COVID-19 patients. The source of convalescent plasma is from the donors who has recovered from COVID-19 infection. However, some requirements must be eligible for the donors. For example, the donor must has no symptoms of COVID-19 for at least 14 days and has enough high titer of neutralization antibodies (Ripoll et al., 2021). A multi-center randomized controlled trial was held to evaluate the efficacy, safety and dose response of convalescent plasma transfusion in severe COVID-19 patients and built up a standardized protocol for the treatment (Chowdhury et al., 2020). Not only convalescent plasma is used for the treatment of the COVID-19 patients, but can also be used for the prophylaxis of normal individual (Montelongo-Jauregui et al., 2020). For example, administration of convalescent plasma in COVID-19 high risk group will prompt to prevent infection while in infected patients will reduce symptom and mortality (Sheervalilou et al., 2021; Wang et al., 2021b).
There are still some limitations and risks on using convalescent plasma. First, not all the COVID-19 patients had high enough titer of neutralization antibody in the serum. Second, the plasma of the patients may contain unknown pathogens that have risk to infect recipients. Third, antibody may cause antibody dependent enhancement (ADE) or activate the complement reaction or inflammatory on patients (de Alwis et al., 2020; Fleming and Raabe, 2020; Lutz, 2012; Yager, 2020).
4.2. Monoclonal antibody (MAb) against SARS-CoV-2/COVID-19
For seeking specific MAbs for the treatment of COVID-19 patients. Different strategies to develop MAb are employed. Ho’s group studied the antibody response of COVID-19 patients and they showed that the neutralization antibody titers were higher in severe cases (Wang et al., 2020). For seeking MAbs from the patients, they sorted the memory B cells from patients’ peripheral blood lymphocytes. They isolated sixty-one MAbs from five patients successfully. Nine of the MAbs had the potency to inhibit virus infectivity. The antigenic specificity of these MAbs was receptor binding domain or N-terminal domain of S protein and have good neutralization ability on SARS-CoV-2 (Liu et al., 2020a).
In order to develop a universal vaccine and MAb against SARS-CoV-2 and the variants, Liao’s group used the highly conserved region of S protein sequences and designed a monoglycosylated S protein (Smg). They used this Smg to immunize mice and found that the antibodies could neutralize UK and South Africa variants. In addition, MAb produced by this Smg protein could also neutralize wild type and variants of SARS-CoV-2 (Liao et al., 2021).
The most advantage of MAb is very specific to target the virus. However, when the virus product variants, the efficacy of the MAb will decrease rapidly. In addition, MAb is not recommended for all cases of COVID-19 patients. For instant, only patients with mild to moderate COVID-19 patients who might progress to severe COVID-19 cases would recommended to receive MAb therapy. These patients usually had underlined diseases, overweight or cancer (Kim et al., 2021; Puing et al., 2021).
| 5. Anti-virus drug development | ▴Top |
During the pandemic of COVID-19, there are no FDA-approved antiviral drugs available for treatment of the patients, especially for the emergency use in the serious cases. Therefore, the most rapid therapeutic method is trying to use the already existed antiviral drugs that may have potential to apply to COVID-19 patients. We are going to describe the properties of these current antiviral drugs and their potential effect on COVID-19 therapy.
5.1. Camostat mesilate (CM)
CM was a cysteine protease inhibitor that has been developed since 1980s in Japan. CM was a drug to treat the acute symptom of chronic pancreatitis in patients in the past. Former studies indicated that CM had the potential against SARS-CoV-1, MERS-CoV and Ebola virus on mouse model (Zhou et al., 2015). For SARS-CoV-2, CM blocked TMPRSS2, a serine protease on the host’s cell membrane and blocked the entry of the virus into the cytoplasm (Breining et al., 2021; Maggio and Corsini, 2020; Uno, 2020).
5.2. Hydroxychloroquine/chloroquine
Chloroquine or a less toxic derivative, hydroxychloroquine is an anti-malaria drug. Chloroquine or hydroxychloroquine also have anti-inflammatory and immuno-modulation effects and have broadly used for rheumatic diseases such as lupus erythematosus (Sinha and Balayla, 2020). Several studies indicated that CQ and HCQ have anti-viral activity on SARS-CoV-2 in vitro experiments (Liu et al., 2020a; Pastick et al., 2020). The mechanism associated on inhibition of endosome fusion (Principi and Esposito, 2020). However, a systemic review and meta-analysis by searching 12 observational and 3 randomized trials for the effect of CQ and HCQ. They concluded that CQ and HCQ did not improve the clinical outcomes of COVID-19 patients (Elavarasi et al., 2020).
5.3. Arbidol (Umifenovir)
Arbidol, or the other name, umifenovir was developed in Russia. It had a broad spectrum antiviral drug and it currently used in Russia and China. Arbidol possessed anti-viral activities against a broad spectrum of DNA and RNA viruses, such as influenza viruses, respiratory syncytial virus, parainfluenza virus, SARS-CoV-1, adenovirus, poliovirus, coxsackievirus, rhinovirus, Zika virus, and hepatitis B and C viruses. The action of arbidol was inhibition of the fusion process between the virus and the host’s cell membrane (Nojomi et al., 2020). For COVID-19, arbidol was shown to inhibit the release of the virus from the host cells (Zheng et al., 2020).
5.4. Lopinavir/ritonavir
Ritonavir and lopinavir, an improved version of ritonavir, were HIV protease inhibitor. Coformulated Lopinavir/Ritonavir was applied for a part of combination therapy on AIDS patients (Cvetkovic and Goa, 2003). It was a proposed drug for treatment of COVID-19 via binding the 3C-like protease of SARS-CoV-2 (Nutho et al., 2020). However, several clinical researches showed that lopinavir/ritonavir was not associated with reduction of clinical outcomes of COVID-19 patients (Horby et al., 2020; Stower, 2020). In contrast, a meta-analysis in 2020 reported that lopinavir/ritonavir still improve the outcomes on COVID-19 patients (Verdugo-Paiva et al., 2020).
5.5. Favipiravir
Favipiravir was developed for the treatment on influenza virus initially. It is a prodrug which is converted to active favipiravir ribofuranosyl-5B-triphosphate (favipiravir-RTP) via intracellular phosphoribosylation. Based on its structure a nucleoside analog. Favipiravir acts as a competitor on viral RNA replication and inhibit viral RNA-dependent RNA polymerase (Ghasemnejad-Berenji and Pashapour, 2021; Joshi et al., 2021). There are several clinical trials are held in different countries on COVID-19 treatment. However, systematic reviews indicated that favipiravir had certain adverse effects such as hyperuricaemia, teratogenicity and QTc prolongation, etc. The safety of favipiravir would still require more studies to evaluate its safety especially to the COVID-19 patients (Pilkington et al., 2020; Udwadia et al., 2021).
5.6. Remdesivir
Remdesivir is an adenosine analog prodrug. It blocks the viral RNA replication by competing normal nucleotides to cooperate into the newly synthesized viral genomic RNA (Jorgensen et al., 2020). In animal studies, pretreatment of remdesivir on rhesus monkeys could protect them from MERS-CoV infection. It also protected African green monkeys from Nipah virus infection and rhesus monkeys dying from Ebola virus (Aleissa et al., 2020; Malin et al., 2020). During the pandemic, remdesivir was one of the potential drugs administered to COVID-19 patients on clinical trials (Beigel et al., 2020; Young et al., 2021).
5.7. Molnupiravir
Molnupiravir is a newly oral antiviral drugs also initially developed for influenza viruses. It had antiviral effect on multiple RNA viruses such as various coronaviruses, respiratory viruses, togaviruses and Ebola virus (Painter et al., 2021a). Its action acts as a ribonucleoside analog and itself will be incorporated into viral RNA, induced SARS-CoV-2 to form mutagenesis and caused blockade of viral genome RNA replication (Gordon et al., 2021; Kabinger et al., 2021). It was demonstrated that molnupiravir reduced viral load on SARS-CoV-2 infected animal experiments including Syrian hamster model, infected ferrets model and humanized mouse model. Molnupiravir have demonstrated good tolerability and dose-proportional pharmacokinetics in phase 1 clinical trial (Painter et al., 2021b). Molnupiravir is also used for evaluation for the treatment of COVID-19 in phase 2 clinical trial (Abdelnabi et al., 2021).
5.8. Paxlovid
Paxlovid was the second newly oral antiviral drugs against COVID-19. The components of paxlovid contains mainly PF-07321332 (nirmatrelvir), and a combination of low dose of ritonavir. The action of PF-07321332 acts as a 3-C like protease inhibitor against SARS-CoV-2. (Mahase, 2021b). More importantly, Paxlovid has been proved to be effective on different SAR-CoV variants (Ullrich et al., 2021). The phase 3 clinical trial of paxlovid is still ongoing.
5.9. Combination therapy
Besides using a single antiviral drug for COVID-19 therapy, combination of different antiviral drugs have been reported to treat COVID-19 patients. Combination of remdesivir and ivermectin, an antiparasitic drug was shown synergic antiviral effect against murine hepatitis virus, a surrogate model of SARS-CoV-2 (Tan et al., 2021). The combination of hydroxychloroquine plus azithromycin indicated that the treatment could reduce in-hospital morbidity, mortality, clinical outcome and duration of hospitalization (Davido et al., 2020). However, systemic review and meta-analysis concluded that the combination of hydroxychloroquine plus azithromycin did not improve the clinical status of the patients (Cavalcanti et al., 2020). A cohort study even showed that hydroxychloroquine combined with azithromycin would increase risk of mortality when compared with single used of hydroxychloroquine (Sbidian et al., 2020). It required more studies to warrant their antiviral effect on COVID-19 patients. Besides combination of different antiviral drugs for the therapy of COVID-19, antiviral drug combined with immunomodulatory drugs have been reported. One example is the early administration of lopinavir/ritonavir and interferon has shown to shorten the virus shedding in COVID-19 patients (Zuo et al., 2020). Similar treatment by using interferon beta-1a with lopinavir-ritonavir could reduce risk of death on patients (Baghaei et al., 2021). A combination of favipiravir and an interleukin-6 receptor blocker (tocilizumab) showed significantly improve pulmonary inflammation and reduce mortality (Zhao et al., 2021). Combination of traditional Chinese medicine (TCM) with antiviral drug has also been reported. Lianhuaqingwen, a TCM formula and arbidol was reported to accelerate recovery rate and improved prognosis of moderate COVID-19 patients (Fang et al., 2020).
5.10. Nutraceuticals
In spite of antiviral drugs discovery and development, numerous potential anti-covid-19 natural bioactive compounds were massively revised in recent publications. These compounds originated from herbal plants, food supplement and nutraceuticals. In Wong’s recent publication, a collection of functional food/nutraceuticals (i.e. probiotics, fish oil, curcumin, etc.) and micronutrients (i.e. vitamins, minerals, etc.) might have the potential to suppress COVID-19 infection by improvement of immunity and anti-inflammation (Lordan et al., 2021; Wong et al., 2021). Noticeably, some plant protease inhibitors of several food products might have the potential to suppress COVID-19 replication (Savant et al., 2021). However, clinical trials were essential to prove the authentic effect of these bioactive compounds from food and related supplements in future.
Table 3 summarized the potential antiviral drugs on COVID-19 treatment and Figure 1 represents the overview of the main axis of this review, highlighting the main strategies for the treatment and prevention on COVID-19 infection, and the evolution of the SARS-CoV-2.
 Click to view | Table 3. : Potential antiviral drugs on COVID-19 treatment |
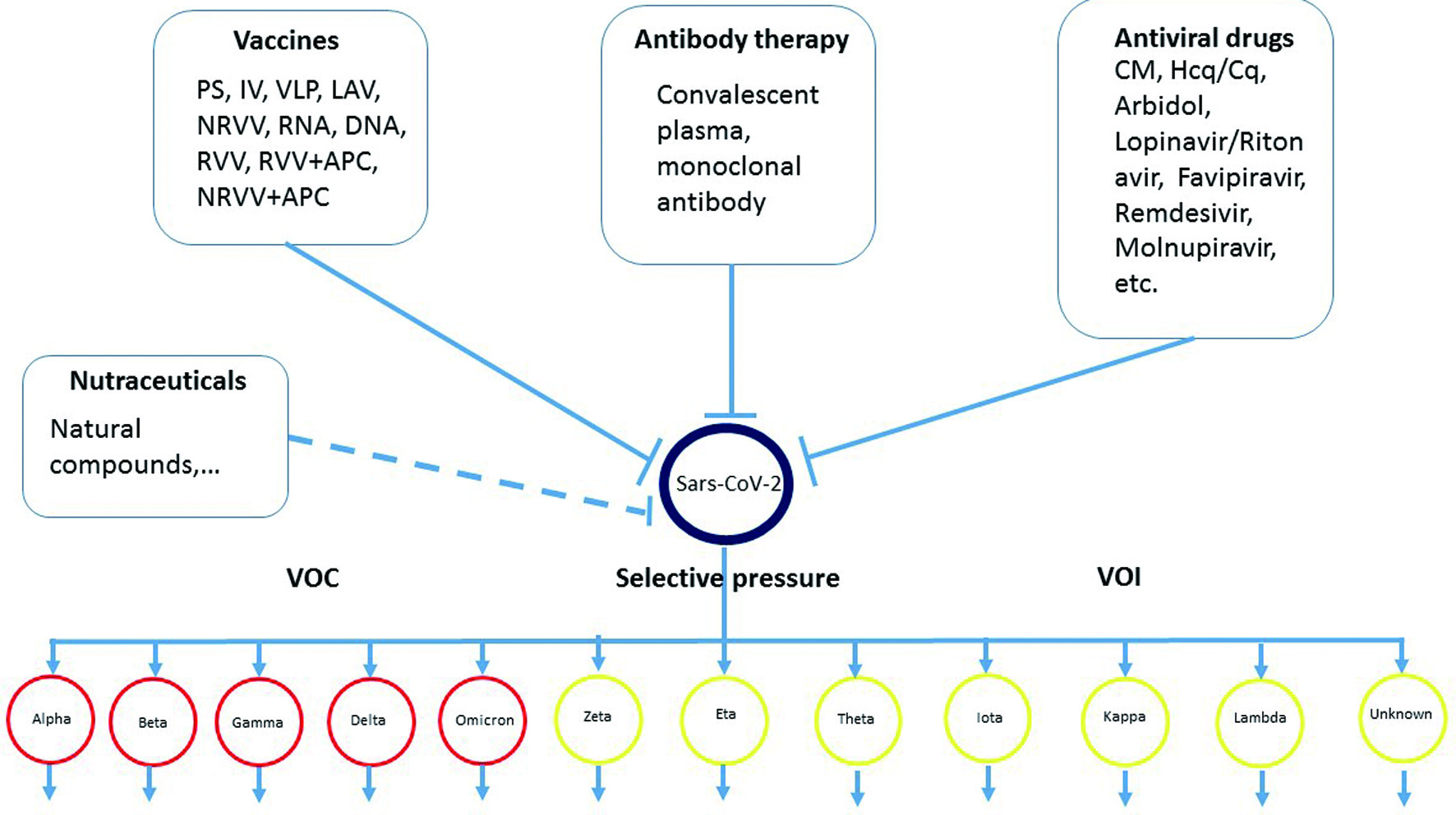 Click for large image | Figure 1. Overview the main axis of this review, summarizes the main strategies for the treatment and prevention on COVID-19 infection, and the evolution of the SARS-CoV-2. |
| 6. Conclusion and future perspective | ▴Top |
In order to develop effective vaccines and antiviral drugs simultaneously, more effort must be use to realize the SARS-CoV-2 including its replication, virus-host interaction, pathogenesis based on fundamental research. However, not a single strategy can eradicate the COVID-19 pandemic completely. For the treatment of patients, combination therapy of antiviral drug and cytokine therapy may be a rational approach in order to improve the prognosis and also reduce the mortality. For prevention, global vaccination to increase herd immunity is a right direction. To date, there are more than two hundred vaccines are on-going for subclinical trial and clinical trials on different phases. Some of the vaccines has been approved based on emergency use authorization (EUA) by WHO. However, examples of immunological breakthrough of the virus on completely vaccinated individual has been reported. In order to avoid repeating vaccination due to the virus variants emerging, it is reasonable to develop a universal vaccine. Discovery of new anti-viral drugs against SARS-CoV-2 is also essential at the same time. While the coverage of the vaccination and immune status is continuing to increase in human being, the virus is also evolving into more and more variants. Therefore, learning how to living with the virus may be the best ending.
| References | ▴Top |