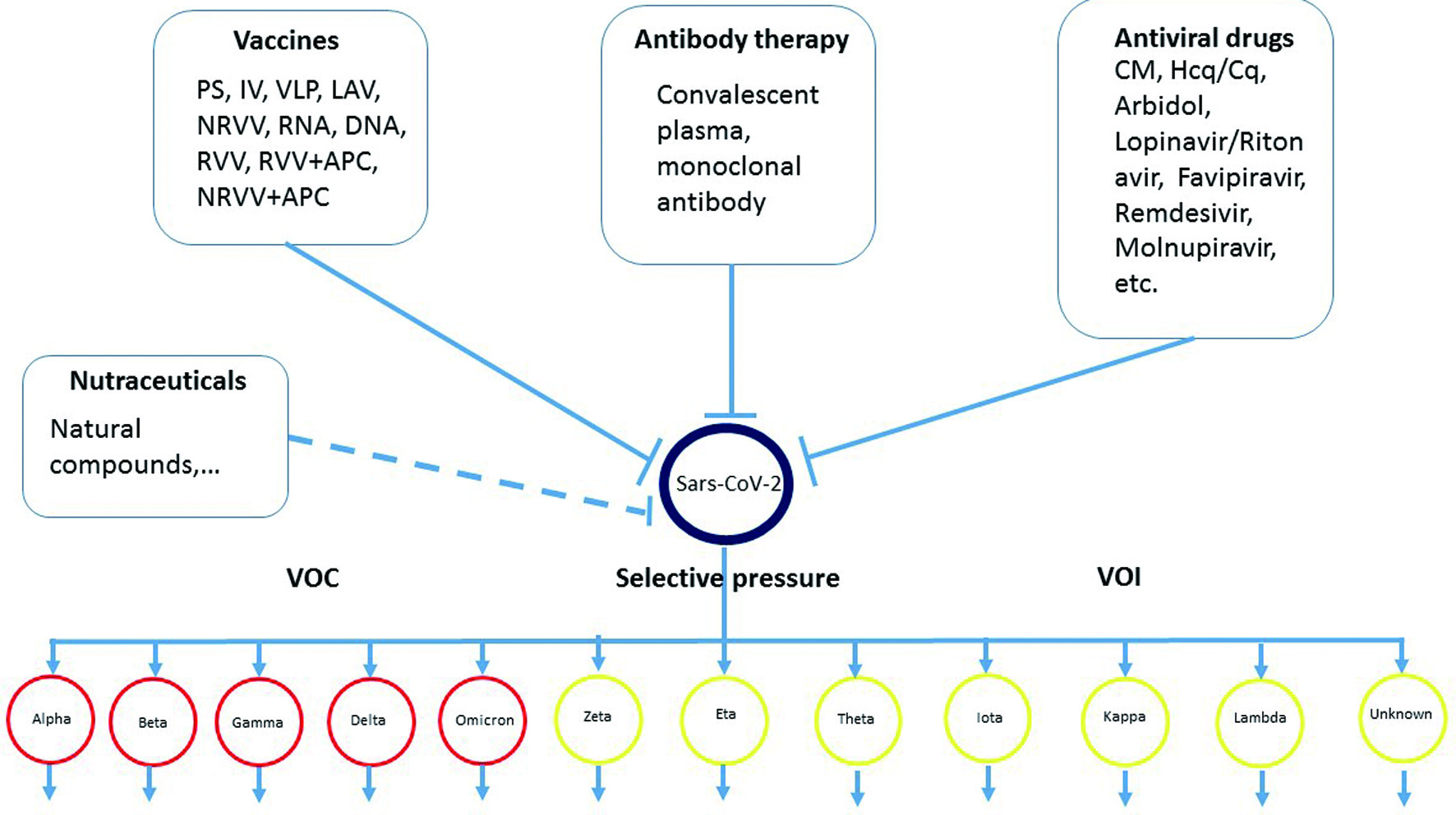
Figure 1. Overview the main axis of this review, summarizes the main strategies for the treatment and prevention on COVID-19 infection, and the evolution of the SARS-CoV-2.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 16, December 2021, pages 25-33
The prevention and treatment of COVID-19 and related development during pandemic
Figure

Tables
| Lineage | Place of emergence | Date of emergence | Phenotypes |
|---|---|---|---|
| Reference sequence | |||
| Wuhan-Hu-1 | China | Dec 2019 | Pneumonia (Ciotti et al., 2019) |
| Variants of concern (VOC) | |||
| Alpha | UK | Sep 2020 | Increase transmissibility, severity and mortality (Aleem et al., 2021) |
| Beta | South Africa | Aug 2020 | Increase transmissibility, reduce virus neutralization ability of antibody therapy |
| Gamma | Brazil | Jul 2020 | Reduce virus neutralization ability of antibody therapy |
| Delta | India | Dec 2020 | More transmissible than Alpha variant, vaccine is less effective |
| Omicron | South Africa | Nov 2021 | More transmissible than other variants, vaccine is less effective |
| Variants of interest (VOI) | |||
| Epsilon | USA | Sep 2020 | Increase transmissibility, reduce virus neutralization ability of antibody therapy (Arav et al., 2021) |
| Zeta | Brazil | Oct 2020 | Potential reduction in neutralization by antibody treatments and vaccine sera (Panzera et al., 2021) |
| Eta | Worldwide | Dec 2020 | Potential reduction in neutralization by antibody treatments and vaccine sera (Ozer et al., 2021; Pereira et al., 2021) |
| Theta | Philippines | Jan 2021 | Potential reduction in neutralization by antibody treatments and vaccine sera (Ferraz et al., 2021) |
| Iota | USA | Nov 2020 | Lower susceptibility to the combination bamlanivimab-etesevimab monoclonal antibody treatment (Mahase, 2021a) |
| Kappa | India | Oct 2020 | (Chakraborty et al., 2021b; Kumar et al., 2021) |
| Lambda | Peru | Dec 2020 | Increase transmissibility, evasion from neutralizing antibodies, resistance to antiviral immunity (Kimura et al., 2021; Romero et al., 2021) |
| Type of platform | Example of candidate vaccines | Number of doses required | Route of administration† |
|---|---|---|---|
| *Part of information was adopted from WHO web site: Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. †IM: intramuscular, ID: intradermal, IV: intravenuous, SC: subcutaneous, IN: intranasal. | |||
| Inactivated whole virus (IV) | Sinovac, Beijing/Sinopharm BBIBP-CorV, Sinovac CoronaVac | 2 | IM |
| Protein subunit (PS) | Novavax NVX-CoV2373, Vector Institute EpiVacCorona, MVC-COV1901 | 2 | IM |
| Virus-like particle (VLP) | Medicago CoVLP | 2 | IM |
| Live attenuated virus (LAV) | Covi-Vac, MV-014-212 | 1–3 | IN |
| Non-replicating virus vector (NRVV) | Oxford/AstraZeneca ChAdOx1-S, Cansino Ad5-nCoV, Gamaleya Gam-COVID-Vac/Sputnik V, Janssen Ad26.COV2.S | 1–2 | IM |
| DNA | Inovio INO-4800, AnGes AG0302-COVID19 | 2 | ID/IM |
| mRNA | BioNTech/Pfizer BNT162b2, Moderna mRNA-1273 | 2 | IM |
| Replicating virus vector (RVV) | AdCLD-CoV-19 | 1 | IM |
| Replicating virus vector plus antigen presenting cell (RVV+APC) | Dendritic cell vaccine AV-COVID-19 | 1 | IM |
| Non-replicating virus vector plus antigen presenting cell (NRVV+APC) | LV-SMENP-DC | 1 | SC & IV |
| Name of drugs | Origin use | Mode of Action on SARS-CoV-2 | Current situation on COVID-19* |
|---|---|---|---|
| *Part of the information is adopted from https://viralzone.expasy.org/9078. | |||
| Camostat mesilate | Relieve symptom of chronic pancreatitis | Blockade of TMPRSS2 | ND |
| Hydroxychloroquine/chloroquine | Anti-malaria drug | Inhibition of endosome fusion | No benefit in clinical trials |
| Arbidol (Umifenovir) | Broad spectrum antiviral drugs | Inhibition of virus releasing | ND |
| Lopinavir/Ritonavir | Anti-retroviral drug | Inhibit 3C-like protease | No benefit in clinical trials |
| Favipiravir | Anti-influenza virus drug | Nucleoside analog, inhibit viral RNA-dependent RNA polymerase | No evidence of SARS-CoV-2 inactivation |
| Remdesivir | Viral polymerase inhibitor | Nucleoside analog, inhibit viral RNA-dependent RNA polymerase | Approved, but weakly effective in clinical trials |
| Molnupiravir | Anti-influenza virus drug | Nucleoside analog, inhibit viral RNA-dependent RNA polymerase | Under investigation, approved |
| Paxlovid | Anti-SARS-CoV-2 | Inhibit 3C-like protease | Under investigation |