| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 13, March 2021, pages 93-101
In-vitro antidiabetic activities, chemical compositions, antioxidant activities, and toxicity of black tea polysaccharides as a potential source of dietary ingredients
Ebru Pelvana, Ayse Karadagb, Kubra Doganb, Soner Aksuc, Arzu Tasc, Kubra Akalınd, Özlem Atlı Eklioğlue, Cesarettin Alasalvara, *
aTÜBİTAK Marmara Research Center, Food Institute, P.O Box 21, 41470 Gebze-Kocaeli, Turkey
bDepartment of Food Engineering, Yıldız Technical University, 34210 Esenler-Istanbul, Turkey
cTÜBİTAK Marmara Research Center, Genetic Engineering and Biotechnology Institute, P.O Box 21, 41470 Gebze-Kocaeli, Turkey
dDepartment of Molecular Biology and Genetics, Institute of Science and Technology, Gebze Technical University, 41400 Gebze-Kocaeli, Turkey
eDepartment of Pharmaceutical Toxicology, Faculty of Pharmacy, Anadolu University, 26470 Tepebaşı-Eskişehir, Turkey
*Corresponding author: Cesarettin Alasalvar, TÜBİTAK Marmara Research Center, Food Institute, P.O Box 21, 41470 Gebze-Kocaeli, Turkey. E-mail: cesarettin.alasalvar@tubitak.gov.tr
DOI: 10.31665/JFB.2021.13263
Received: March 20, 2021
Revised received & accepted: March 31, 2021
| Abstract | ▴Top |
Type 2 diabetes (T2D) is one of the fast growing diet-related chronic diseases throughout the world. Tea contains several bioactive compounds, some of which render health benefits. Black tea polysaccharides (BTPS), extracted from low grade quality tea leaves, after processing, were examined for their in-vitro antidiabetic activities (α-glucosidase inhibitory and glucose uptake activities), chemical compositions (yield, monosaccharides, amino acids, and minerals), and antioxidant activities as well as toxicity (cytotoxicity and genotoxicity). In addition, 50% lethal dose (LD50) for BTPS was determined using an acute toxicity test to assess the safe use of it as a dietary ingredient. BTPS had strong α-glucosidase inhibitory activity with IC50 value of 3.4 µg/mL. This was much lower than that of the positive control, pharmaceutical glucosidase inhibitor acarbose with IC50 of 687.5 μg/mL. BTPS also increased glucose uptake into the adipocyte differentiated 3T3-L1 MBX cells. Neither cytotoxic nor mutagenic effects were found for BTPS. The LD50 of BTPS for acute toxicity demonstrated that it was safe to use. The present work suggests that BTPS can be used as an antidiabetic dietary ingredient without posing any potential health risk.
Keywords: Black tea polysaccharides; Antidiabetic activity; α-Glucosidase inhibitory activity; Glucose uptake activity; Toxicity
| 1. Introduction | ▴Top |
Diabetes mellitus has become one of the biggest global public health challenges in the 21st century with type 2 diabetes (T2D) being the most common form, accounting for 90–95% of all cases (Wang et al., 2016). In the early therapy for diabetes, one approach to decrease postprandial hyperglycemia is to retard absorption of glucose through inhibition of carbohydrate hydrolyzing enzymes (such as α-amylase and α-glucosidase) in the digestive tract (Chen et al., 2009). Due to the serious adverse effects associated with the oral synthetic hypoglycemic agents, exploration of novel, safe, and effective bioactive compounds with antidiabetic activity has become an important research area (Shori, 2015; Wang and Zhu, 2016).
A large number of studies have shown that soluble tea polysaccharides are the main hypoglycemic factor in tea (Fan et al., 2018; Akalın et al., 2019). It has been reported that glucose levels in the blood is reduced by black tea (Akalın et al., 2019) and green tea extracts (Tsuneki et al., 2004; Zhou et al., 2007). It is also believed that tea reduces dietary glucose intake by suppressing the activity of glucose transporters in the intestinal epithelium (Ramadan et al., 2009).
Compared to green tea, the enzymatic oxidation in black tea process changes its chemical composition in terms of polyphenols, soluble sugars, amino acids, organic acids, colourings, and volatile constituents, among others. Although there are a considerable number of studies on antidiabetic effects of green tea polysaccharides (GTPS) (Zhou et al., 2007; Cao, 2013; Cai et al., 2013; Wang et al., 2016; Karadag et al., 2018), studies on antidiabetic effects of black tea polysaccharides (BTPS) are scarce (Chen et al., 2009). Thus, the aim of the study was to assess the in-vitro antidiabetic activities, chemical compositions, antioxidant activities, and toxicity of BTPS as a potential source of dietary ingredients.
| 2. Materials and methods | ▴Top |
2.1. Samples and reagents
Black tea samples (low quality-grade 6) were procured from ÇAYKUR (State-Owned Tea Enterprise, Rize, Turkey). All chemical reagents were obtained from Sigma-Aldrich Co. Ltd. (Dorset, UK), unless otherwise stated.
2.2. Extraction of BTPS and determination of yield
The BTPS was extracted from black tea as described by Karadag et al. (2018), with slight modifications. Briefly, black tea was mixed with ethanol (1:5; w/v) and left overnight. After filtration, tea leaves were air-dried and submitted to hot-water extraction with a horizontal extractor (Niro Atomizer, AC-27, Soeborg, Denmark). The extract was then centrifuged at 5,870 ×g (Westfalia D-4740, Wesseling, Rheinland, Germany). The supernatant was subsequently mixed with four volumes of ethanol and kept at 4 °C overnight. Finally, the precipitates were obtained by centrifugation (Hettich, Rotina 420, Tuttlingen, Germany) at 15,000 ×g for 20 min at chill temperature (4 °C), and the pellet was washed with acetone and ethanol. The percentage yield of BTPS was calculated as follows:
2.3. Characterization of BTPS
2.3.1. Chemical compositions
Total polysaccharides (DuBois et al., 1956), uronic acid (Blumenkrantz and Asboe-Hansen, 1973), total phenolics (Horszwald and Andlauer, 2011), soluble and insoluble dietary fiber (AOAC, 1994), amino acids (Gheshlaghi et al., 2008), monosaccharides (Dai et al., 2010), mineral compositions (AOAC, 1999), and molecular weight (Mw) distribution (Cai et al., 2013) of BTPS were analysed accordingly. Values were expressed as mean ± standard deviation (n = 3).
2.3.2. Antioxidant activity
Antioxidant activities of BTPS were determined according to a previous publication (Karadag et al., 2018). Two test methods, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical cation activity and ferric reducing antioxidant potential (FRAP), were used to assess the antioxidant activities (Cheng et al., 2013). Each sample was dissolved in water and the results were expressed as milligrams of Trolox equivalents (TE) per 100 g of BTPS for ABTS assay and mg of FeSO4.7H2O equivalents per 100 g of BTPS for FRAP assay.
2.3.3. Spectroscopic analyses
Ultraviolet-visible (UV) spectra of BTPS was recorded with a spectrophotometer (Jasco V-650, Tokyo, Japan). Fourier transform infrared (FT-IR) spectra of BTPS was recorded with FT-IR spectrophotometer (Perkin Elmer Spectrum 400, Waltham, MA, USA). The finely powdered sample was pressed into the sample holder for FT-IR measurement in the frequency range of 4,000–650 cm−1.
2.4. Inhibition of α-glucosidase activity of BTPS
The effects of BTPS on α-glucosidase activity were determined according to the method given by Wei et al. (2010) with α-glucosidase from Saccharomyces cerevisiae (Sigma G5003, Sigma-Aldrich Co. Ltd., Dorset, UK). Acarbose (Sigma PHR1253) was used as a positive control. The α-glucosidase activity was determined by measuring the yellow-coloured nitrophenol released from p-nitrophenyl α-D-glucoside (pNPG) at 405 nm, and inhibitory activity was expressed as the percentage of the control sample (acarbose). Percentage inhibition was calculated as follows:
2.5. Glucose uptake of BTPS
Adipocyte differentiation was induced by treated 3T3-L1 MBX (ATCC® CRL-3242™) cells with 100 μL of differentiation cocktail per well containing 0.25 μM dexamethasone, 0.5 mM IBMX, 1 μg/mL insulin, and 2 μM rosiglitazone with 10% fetal calf serum and Dulbecco’s Modified Eagle’s Medium. Adipocyte cells were exposed with BTPS at 0.5 and 1 mg/mL doses of serum-free medium for 20 min. The glucose uptake test was carried out according to the Glucose Uptake Colorimetric Assay Kit (Sigma MAK083) protocol. A 100 μL of insulin solution with 1 μg/mL final concentration was used as a positive control. As a negative control, differentiated cells were stimulated with phosphate buffered saline in the absence of insulin. Glucose uptake levels were expressed as 2-deoxyglucose (2-DG, pmol/well).
2.6. Cytotoxicity of BTPS
NIH/3T3 mouse embryonic fibroblast (ATCC® CRL-1658™) cell line was used to investigate the cytotoxicity of BTPS against healthy cell lines. Briefly, NIH/3T3 cells were incubated according to the instructions of the supplier at 37 °C (at least 95% humidified atmosphere with 5% CO2). NIH/3T3 cells were seeded at 1 x 104 cells into each well of 96-well plates. After 24 h of incubation, the metabolites were added to the wells at 9 different concentrations range between 0.019 and 5 mg/mL with a dilution factor of 2 (0.019, 0.039, 0.078, 0.156, 0.313, 0.625, 1.25, 2.5, 5.0 mg/mL) in quadruplicates. The 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Xenometrix AG, Gewerbertrasse, Switzerland) was performed according to the manufacturer’s instructions, after 24 h incubation with the metabolites. The absorbance was determined after 2 h incubation at 480 nm with a reference wavelength of 680 nm using a microplate reader (BioTek, Winooski, VT, USA). Cell viability inhibition percentage was calculated for each concentration of BTPS according to the formula given below; obtained from the instructions of the supplier and IC50 (concentration that induced 50% enzyme inhibition) values were estimated by plotting a dose response curve of the inhibition % versus the concentration of test compound.
The stock solutions of the metabolites were prepared in distilled water and further dilutions were made with fresh culture medium. All data were obtained from three independent experiments in quadruplicates.
2.7. AMES microplate format test of BTPS
AMES assay was performed to determine the mutagenicity of BTPS using AMES microplate format test 98/100 mutagenicity assay sample kit (Xenometrix AG), as described earlier (Altıntop et al., 2012, 2016). Salmonella typhimurium strains (TA98 and TA100) were used to mimic frameshift mutations and base-pair substitutions, respectively. First, the bacterial cultures were grown in an incubated shaker (SI-600, Jeio Tech, Daejon, Korea) overnight. The concentrations of the compound were selected according to the previous guidelines. The concentrations were 5.0, 2.5, 1.25, 0.625, 0.3125, 0.156 mg/mL in dimethyl sulfoxide (DMSO) (Chandrasekaran et al., 2011). The mutagenicity of the compounds were then determined with/without metabolic activation which was provided by Aroclor™-1254 induced male Sprague-Dawley rat liver microsomal enzyme (S9) mix (Xenometrix AG) with a final concentration of 4.5% (v/v). After that, 2-nitrofluorene (2-NF; 2 μg/mL) and 4-nitroquinoline N-oxide (4-NOQ; 0.1 μg/mL) were used as positive controls in the assays without of S9 mix. 2-Aminoanthracene (2-AA) was used as a positive control with S9 mix against TA 98 and TA100 (1 and 2.5 μg/mL, respectively). Solvent control was prepared as 4% DMSO. Finally, the number of positive wells in triplicates were counted and compared with the negative control. Fold induction over the negative control, fold induction over the baseline, and zero dose baseline were calculated as described earlier (Chandrasekaran et al., 2011; Altıntop et al., 2012; Flückiger-Isler and Kamber, 2012; Altıntop et al., 2016). All doses were tested according to Student’s t-test at p < 0.05. Mutagenity was determined according to the previous criteria (Flückiger-Isler and Kamber, 2012), which were as follows: if baseline was ≤ 3.0, then 2.0 and 3.0-fold increases: weak positive, ≥ 3.0-fold increases: positive; if baseline was > 3.0, 1.5 and 2.5-fold increases: weak positive, ≥ 2.5-fold increases: positive.
A mutagenic compound should show significant increases at least in two adjacent doses or a significant increase at the highest concentration (5 mg/mL). Compounds which did not have any of above properties were classified as negative.
2.8. Lethal dose 50 (LD50) of BTPS
The LD50 assay of BTPS was performed according to the “OECD 423-Acute Oral Toxicity (acute toxicity classification method)” standard test protocol. The LD50 value of BTPS was determined by applying the limit test protocol as described by Karadag et al. (2018).
2.9. Statistical analysis
Each determination was replicated three times. The value was expressed as the mean ± standard deviation. Differences were estimated by analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. Differences were considered to be significant at p ≤ 0.05. All statistical analysis were performed using the SPSS 20.0 version (SPSS Inc., Chicago, IL, USA)
| 3. Results and discussion | ▴Top |
3.1. Yield, chemical compositions, and antioxidant activities of BTPS
The yield of BTPS was 3.26% (w/w), which was similar to those previously found for different teas (Sun et al., 2013; Karadag et al., 2018). The contents of carbohydrate, uronic acid, total fiber, and total phenolics of BTPS are given in Table 1.
 Click to view | Table 1. Yield and compositional parameters of BTPS |
The relative mole percentages of monosaccharides, amino acids, and mineral compositions of BTPS are given in Table 2. The monosaccharide compositions of BTPS were mainly composed of galactose, arabinose, and glucose with the presence of some glucuronic and galacturonic acids. The latter two were acidic heteropolysaccharide. These results are similar to those in previous studies, showing that tea polysaccharides have arabinogalactan structures (Chen et al., 2009; Scoparo et al., 2016; Karadag et al., 2019).
 Click to view | Table 2. Monosaccharide, amino acid, and mineral compositions of BTPS |
BTPS was a protein-bounded polysaccharide and the major proportions of amino acids composition consisting of glutamic acid, aspartic acid, and glycine, with a total content of 2,498 mg/100 g. The amino acid compositions of black and green tea polysaccharides were similar to those reported by Scoparo et al. (2016) and consisted mainly of glycine, glutamic acid, and aspartic acid (Chen et al., 2005).
Among the studied minerals, BTPS was rich in potassium, phosphorus, magnesium, and manganese. The mineral composition of tea may also be affected by variety, geographical origin, harvest time and year as well as climate and composition of the soil (Street et al., 2007).
The radical scavenging ability and reducing potentials of BTPS were determined by both ABTS and FRAP assays, respectively (Table 1). Wang et al. (2013a) revealed that tea polyphenols are the major antioxidant in the crude tea polysaccharide extract. The beneficial effect of antioxidants has been reported in animal models of diabetes and in diabetic patients (Rizvi et al., 2005; Babu et al., 2006; Bhattacharya et al., 2013). The plasma antioxidant potential showed a significant decrease (73%) in diabetic rats. Black tea extract supplementation, rich in polyphenols, was effective in ameliorating diabetes associated with oxidative stress parameters (Kumar and Rizvi, 2015). Therefore, the presence of polyphenol in BTPS would contribute to its potential use as an antidiabetic ingredient.
3.2. Molecular weight distributions and spectroscopic analyses of BTPS
The Mw distributions and the peak pattern of BTPS are illustrated in Figure 1a. Mw (weight-average molecular weight) value was calculated by the regression equation [log(Mw) = −0.081x3 + 2.215x2 − 20.965x + 72.956; R2 = 0.998 obtained by dextran standards and retention time]. BTPS contained three peaks and predominant peak (94.15%) was eluted at 10.27 min with a Mw of 1.864x103 Da and its polydispersity was 1.23. The minor fractions eluted at a retention time of 9.84 (0.80%) and 10.87 (5.06%) had Mw of 14.27 × 103 and 174 Da, respectively. This was lower than the value of 3.8–37.2 kDa found previously, and it also indicated that the Mw of BTPS was lower than oolong and green tea polysaccharides (Chen et al., 2009). The changes in the Mw of tea polysaccharides were observed with different degrees of fermentation by Wang et al. (2012). Therefore, the lower Mw determined in the present study may not only be related to the origin of tea leaves, but also fermentation conditions of Turkish black tea.
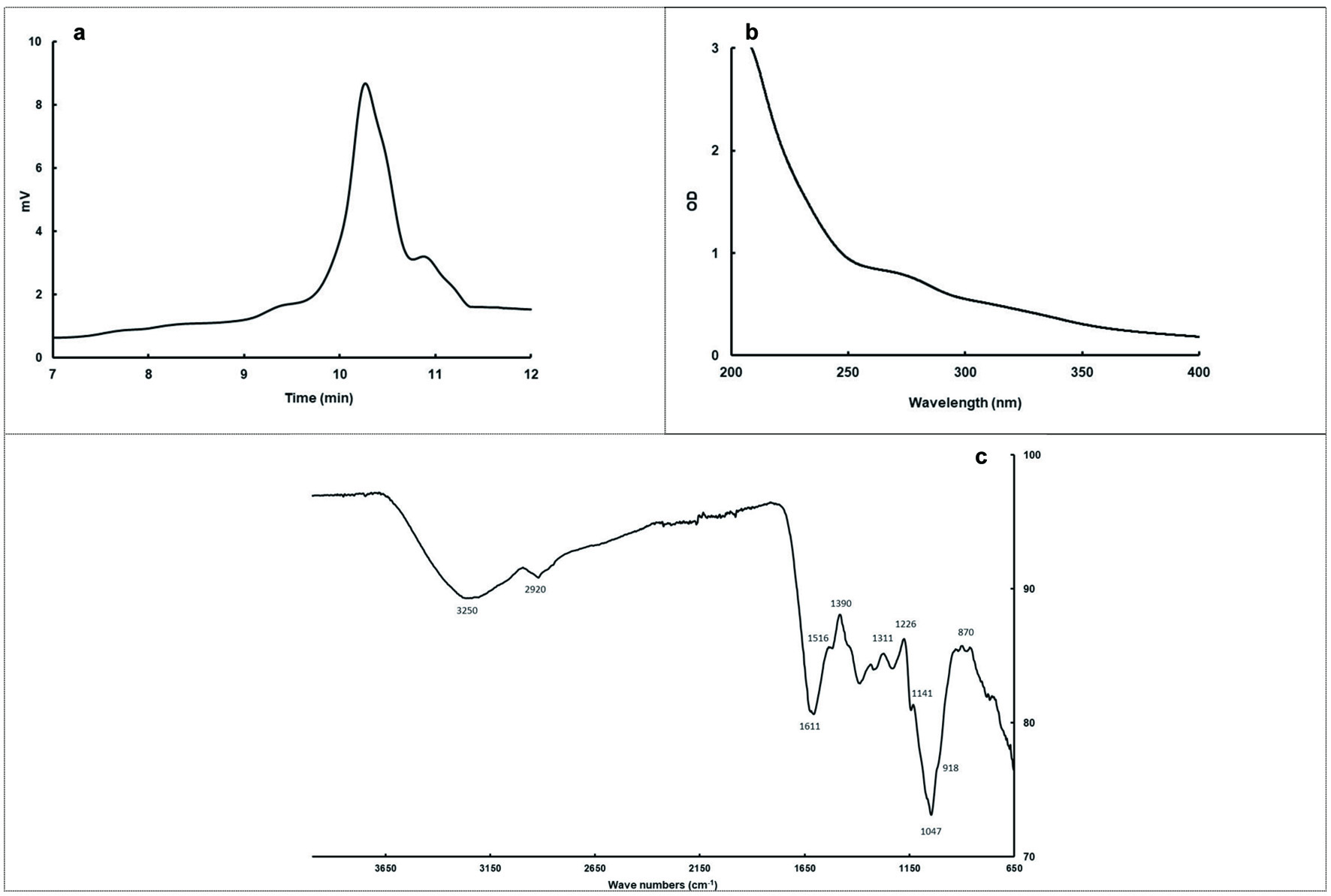 Click for large image | Figure 1. Gel permeation chromatogram (a), UV Spectra (b), and FT-IR spectra (c) of BTPS. |
The UV spectrum of the BTPS is shown in Figure 1b. BTPS clearly emerged with a stronger absorption peak at 200–250 nm, which indicates that the samples might contain an unsaturated carbonyl, carboxyl group related the polysaccharide structure. The shoulder-shape absorption peak was observed in the 260-280 nm region, with the inflection at 270 nm which is mostly related to the presence of protein portion (Wang et al., 2013b).
The FT-IR spectroscopy (Figure 1c) can be used for approximate identification of polysaccharides and proteins when combined with chemical analyses. Peaks at 1,000–1,200, 2,800–3,200, and 3,200–3,600 cm−1 were characteristics for polysaccharides, the broadly intense peak at 3,250 cm−1 represents the stretching vibrations of O-H, while the weak band at 2,920 cm−1 is attributed to stretching of asymmetric CH2. The presence of protein component was demonstrated by the occurrence of two bands of decreasing intensity: amide I towards 1,611 cm−1 and amide II towards 1,516 cm−1 with N-H and C-N links. The peaks between 1,400 and 1,300 cm−1 were the characteristic of C-O stretching and C-H or OH bending, and the peak of 1,390 cm−1 might be attributed to the stretching of OH of phenolics in the structure. The broadband at 1,047 cm−1 suggests the presence of C-O-H side groups and C-O-C glycosidic band vibration and should be due to the characteristic absorption bands of pyran glycosides. The peaks around 920 and 880 cm−1 corresponds to skeletal modes of pyranose rings, which is characteristic of β-glycosidic type linkages between the sugar units (Chen et al., 2009; Zhao et al., 2014).
3.3. Inhibitory effects of BTPS on α-glucosidase activity
The α-glucosidase inhibitors are currently of interest owing to their promising therapeutic potential for treatment diabetes as oral hypoglycaemic agents. The α-glucosidase inhibitors act on the brush border of intestinal mucosa to inhibit the post-meal blood glucose increase and to decrease fasting blood glucose to some extent by inhibiting the hydrolysis of oligosaccharides and disaccharides to monosaccharide and their intestinal absorption (Wei et al., 2010). Acarbose is an α-glucosidase inhibitor used for treatment of T2D, but it is also responsible for many side effects such as abdominal distention, flatulence, diarrhea, and meteorism. Therefore, many efforts have been made to identify novel, safe and effective polysaccharides with antidiabetic activity.
As shown in Figure 2a, BTPS obtained from black tea, had a dose-dependent effect on α-glucosidase inhibitory activity, and inhibition varied from 4.35 to 93.17% with a concentration increase from 1.25 to 6.25 µg/mL. IC50 value of BTPS was found to be 3.40 µg/mL. As a positive control, the IC50 of acarbose was 1.06 mM (687.5 µg/mL). BTPS obtained in the present study showed a higher α-glucosidase inhibitory activity compared to that of a study by Chen et al. (2009), where BTPS showed 91 and 14.3% inhibition at a concentration of 200 and 25 µg/mL, respectively. These differences may be due to the facts that the coarse tea, its processing conditions, and the preparation and extraction methods of samples are different. It was revealed that α-glucosidase inhibitory activity of tea polysaccharides depends on fermentation time. Wang et al. (2012) found that IC50 value was 20 µg/mL for heavily fermented oolong teas, whereas it was >500 µg/mL for lightly fermented teas.
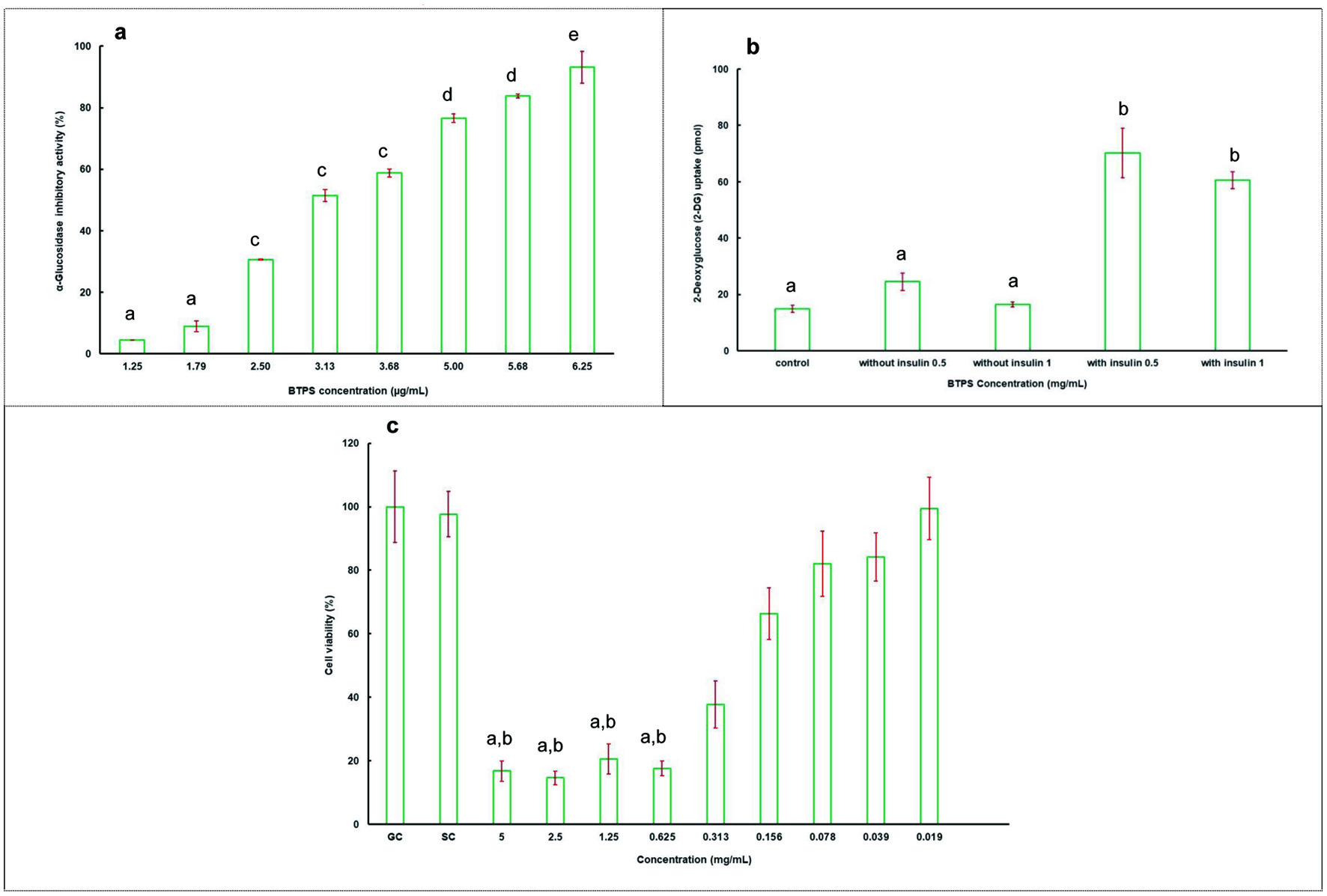 Click for large image | Figure 2. The α-glucosidase inhibitory activity of BTPS (a), 2-deoxyglucose (2-DG) uptake in adipocytes in the absence (0 nM) and in the presence (1 µg/mL) of insulin at indicated concentrations of BTPS extract (0.5 and 1 mg/mL) (b), and percentage viability of BTPS (c). Abbreviations: GC, growth control; SC, solvent control. Data are expressed as the mean ± SD (n = 3). Means ± SD followed by different letters are significantly different (p < 0.05) for Figures 2a and b. For Figure 2c, a significant difference from GC (p < 0.001) and b significant difference from SC (p < 0.001). |
3.4. Glucose uptake activity of BTPS
Insulin signals the uptake of glucose from blood to produce energy in liver, fat, muscle, and other body cells (Du et al., 2012). This ensures that the glucose level in the blood remains at a normal level. In T2D, this balance is disturbed and a high glucose level is observed in the blood because of the impaired insulin action (Del Prato et al., 2002). Therefore, the results of glucose uptake studies provide an important information in terms of effectiveness of the remedy.
In the present study, in-vitro antidiabetic activity of BTPS was also evaluated using 2-DG uptakes in 3T3-L1 adipocytes. Compared to the control group, 3T3-L1 MBX cells treated with BTPS at two different concentrations (0.5 and 1 mg/mL) increased glucose uptake into the cells (Figure 2b), and no significant differences (p > 0.05) were observed in glucose uptake values with change in the concentration of BTPS. Our results suggest that BTPS may possess insulin-mimetic properties. Insulin stimulation of glucose uptake by adipose tissue is critical in lowering postprandial blood glucose level. Abnormal regulation of this process is one of the significant factors in the development of T2D. The inhibition effect of epigallocatechin-3-O-gallate (EGCG) was shown on the dexamethasone-induced insulin resistance. Additionally, EGCG improves insulin-stimulated glucose uptake in rat L6 cells. This effects were associated with the glucose transporter type 4 (GLUT-4) membrane protein (Zhang et al., 2010). The expression of genes related to glucose uptake and insulin-signalling pathway can be regulated by green tea polyphenol extract in rats fed with a fructose-rich diet (Cao et al., 2007). In this study, dose dependent gene expression differences were reported in several genes classified in glucose transporter family and insulin signalling pathway. These reported findings are in good agreement with our results.
3.5. Cytotoxicity of BTPS
The tea extracts which might be used for food additives or a treatment agent should be evaluated in terms of their toxicological aspects. Therefore, the cytotoxicity of BTPS was investigated using the NIH/3T3 mouse embryonic fibroblast cells. The IC50 values of BTPS was 0.615 mg/mL (Figure 2c). According to the results of XTT assay, the tested compounds were found to be non-cytotoxic at their active concentrations, hence demonstrating their therapeutic value.
It is an expected that tea polysaccharides do not show cytotoxic effects. Chen et al. (2018) investigated the cytotoxicity of Fuzhuan brick tea polysaccharides (FBTPs) at concentrations ranging from 25 to 400 μg/mL in human hepatic epithelial (L-02) cells. They found that cell viability remained between 80 and 120% and suggested that none of FBTPs exhibited cytotoxic effect (Chen et al., 2018). Indeed, biosafety of polysaccharides from natural resources has confirmed in several studies (Nie and Xie, 2011).
3.6. AMES microplate format test of BTPS
According to the results, Salmonella typhimurium strains, TA98 and TA100, showed more than 25 positive wells against the positive controls known as, 2-AA (with S9), 4-NOQ and 2-NF (without S9). The negative control wells were found to be less than 8 against TA98 and TA100 with and without S9. Therefore, our tests reached the validity criteria based on the instructions of the manufacturer. BTPS showed a baseline of 3.82 and 4.18 against TA98 with and without S9, respectively (Table 3). The number of revertants did not reach 1.5–2.5-fold over the baseline so the compound was accepted as non-genotoxic. The same compound showed a baseline of 5.55 and 4.89 against TA100 with and without S9, respectively. Any of the doses tested did not reach 1.5–2.5 fold increases (Figure 3). Therefore, BTPS was accepted as non-genotoxic against TA100 with and without metabolic activation. This compound, which has biological activites, should undergo further investigations as a therapeutic ingredient due to its non-genotoxic potential.
 Click to view | Table 3. The result of AMES microplate format genotoxicity test of BTPS |
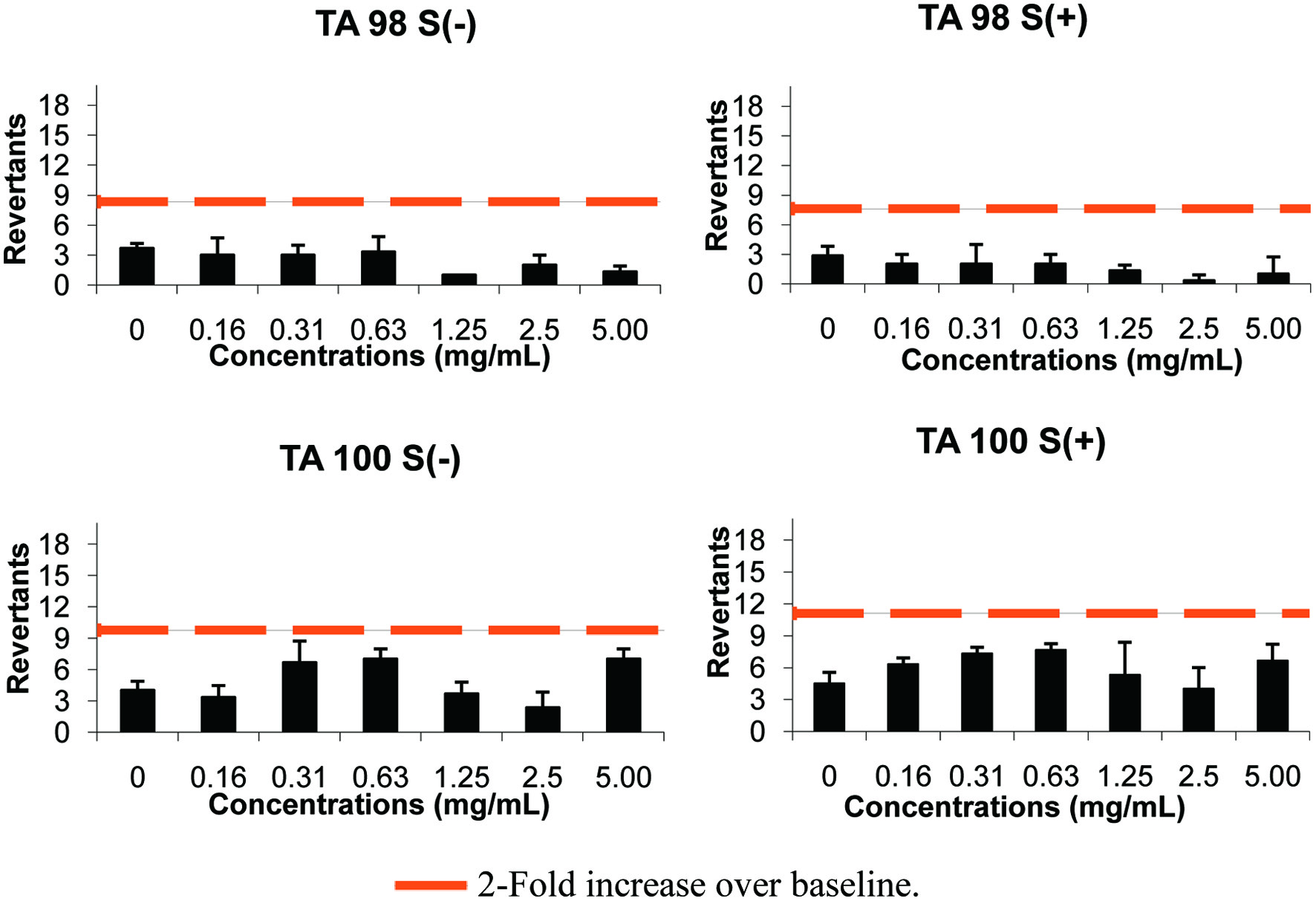 Click for large image | Figure 3. The results of AMES microplate format test of BTPS in TA 98 and TA 100 strains. |
In contrast to genotoxicity, anti-genotoxic effects of polysacharides was reported by plasmid DNA strand break assay. The inhibition of supercoiled plasmid DNA strand breakage was used to investigate the DNA protective properties of crude polysaccharides extracted from Schinus terebinthifolius (PSTF) and Schinus molle (PSMF) fruits. The results of this study showed that PSTF and PSMF at 25 and 50 μg/mL concantrations significantly reduced the percentage of DNA damage (Feriani et al., 2020). These results indicate that the tested polysaccharides may have anti-genotoxic effect.
3.7. The lethal dose 50 (LD50)
According to the limit test protocol, BTPS was administrated to mice by gavage 5.000 mg/kg dose level. For the following 5 days, BTPS administered animals were monitored for mortality and clinical signs. None of the BTPS administrated mice died during the trial and abnormal clinical findings were not observed during the entire experiment. At the end of the experiment, gross pathology and histopathological examination was showed no abnormality in the test and control animals. According to these results and the survival of all tested mice, BTPS was categorized as “GHS 5 or unclassified” according to the OECD 423-Acute Oral Toxicity (acute toxicity classification method) test method.
| 4. Conclusions | ▴Top |
The in-vitro antidiabetic potential of BTPS was evaluated by both α-glucosidase inhibitory activity and glucose uptake activity. The α-glucosidase is a key enzyme that plays a crucial role in the digestion of carbohydrates. Its inhibitors can decrease the release of D-glucose from carbohydrates and retards intestinal glucose absorption. These actions of α-glucosidase inhibitors can help reducing plasma glucose levels and the regulation of glucose metabolism. Hence, α-glucosidase inhibitors are accepted as hypoglycemic agents due to their promising therapeutic potential for treatment of diabetes. The uptake of glucose from blood provides vital energy to cells and maintains the balance of blood glucose level. Therefore, the molecules that have an effect on the mechanism of glucose uptake also serve to establish a normal blood glucose level and prevent T2D. Our results confirmed glucose uptake and α-glucosidase inhibitory activities of BTPS. Additionally, any toxicological effects that may cause a serious health risk was not recorded for investigated BTPS. Therefore, BTPS should be considered as a potent antidiabetic candidate for developing functional foods and/or dietary supplements. This compound, which has biological activities, should undergo further investigations as a therapeutic ingredient due to its non-genotoxic potential.
Acknowledgments
This study is a part of a project funded by TÜBİTAK (under 1003 programme-project no 105O037). We are grateful to ÇAYKUR Tea Enterprise.
Conflict of interest
The authors declare that they have no conflicts of interest.
| References | ▴Top |