| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 13, March 2021, pages 52-61
Polyphenols, vitamin C, and sugar in commercial apple beverages and their effects on antioxidant and anti-inflammatory activities in vitro
Claudine Loonga, Latasha Leoa, Wai Mun Lokea, b, *
aSchool of Chemical & Life Sciences, Nanyang Polytechnic, 180 Ang Mo Kio Ave 8, Singapore 569830
bInnovprof, 27 Orange Grove Road #05-02, Singapore 258356
*Corresponding author: Wai Mun Loke, Innovprof, 27 Orange Grove Road #05-02 Singapore 258356. Tel: +65 9437 8166; E-mail: wai.mun.loke@innovprof.com
DOI: 10.31665/JFB.2021.13259
Received: February 8, 2021
Revised received & accepted: March 29, 2021
| Abstract | ▴Top |
The concentrations of polyphenols, vitamin C, and sugars, as well as antioxidant and anti-inflammatory capacity of commercial apple beverage products in Singapore were investigated. The concentrations of vitamin C, total polyphenol content, and specific polyphenols, quercetin, and catechin of commercial apple beverages were determined using their respective high-performance liquid chromatography−ultraviolet detection method. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay and in vitro studies were conducted to examine the antioxidant and anti-inflammatory capacity. The apple beverages (n = 17) exhibited significant antioxidant activity as determined by DPPH radical scavenging assay, inhibition of cellular F2-isoprostanes and lipid hydroperoxide formation as well as anti-inflammatory capacity (inhibition of cellular leukotriene B4 formation and myeloperoxidase activity). These were found to be associated with vitamin C (10.35 ± 1.18 g/100g), total polyphenols (9.9 ± 1.2 mg GAE/100g), catechin (1.60 ± 0.10 mg/100g), and quercetin (0.46 ± 0.09 mg/100g) concentrations in the tested beverages. A separate simulation experiment demonstrated that antioxidant and anti-inflammatory capacity were augmented by increasing vitamin C, total and specific polyphenol concentrations.
Keywords: Apple beverage; Polyphenols; Vitamin C; Sugars; Antioxidant, anti-inflammatory
| 1. Introduction | ▴Top |
Apple beverage products are readily available in many commercial markets around the world. They are convenient to consume and considered to be made from apple. Apple and apple beverages offer similarities in numerous nutrient contents, such as sugar, vitamins, and minerals. Recently, apple beverage products are considered as sugar-sweetened beverages (SSB) by nutritionists and consumers owing to their sugar contents regardless of whether the sugars are added or originated naturally from the apples. The consumption of SSB has been found to associate with the development of metabolic diseases, such as obesity (Ahmad et al., 2020), metabolic syndrome (Ferreira-Pêgo et al., 2016), hypertension (Ahmad et al., 2020), diabetes mellitus (Malik and Hu, 2019), and cardiovascular diseases (Malik and Hu, 2019). While it is mainly accepted that the overconsumption of SSB may lead to adverse effects on health (Malik and Hu, 2019), the evidence on the consumption of apple beverages is a matter of debate (Pepin et al., 2019). It is well accepted that apple intake offers protection against human diseases (Bondonno et al., 2017), but there is no clear consensus about the health effects of consuming apple beverages. The relatively high sugar contents in apple beverages, commonly between 8 and 15% have become a health concern for discerned consumers. The fruit sugars, though natural, do not differ from added sugars physiologically, and may present unwanted calories in the human diet. Sugars, commonly sucrose, are also added as sweeteners into some of the apple beverages for desirable sensory attributes (Hutchings et al., 2019). Despite the similarity of apple beverages to the other SSB in terms of free sugar content, it remains unclear in the current literature whether they lead to the same metabolic consequences if consumed in equal doses. Unlike other SSB, apple beverage products can serve as a dietary source of various bioactive compounds such as apple polyphenols and vitamin C. The consumption of polyphenols and vitamin C are beneficial to human health by protecting against the development of chronic diseases (Granger and Eck, 2018; Durazzo et al., 2019). The vitamin C contained in apple beverage products originates naturally from the fruit, which is partially retained after juice extraction and subsequent processing. It can also be added as an antioxidant into apple beverage products. Polyphenols, like quercetin and catechin, are usually inherited from the fruit and are less likely to be added as ingredients into the commercial apple beverage products. Polyphenols and vitamin C have been reported to protect against numerous chronic diseases (Granger and Eck, 2018; Durazzo et al., 2019) via the antioxidant and anti-inflammatory mechanisms of action (Loke et al., 2009; Granger and Eck, 2018; Durazzo et al., 2019).
Currently, there are limited data on the concentrations of total and specific polyphenols, vitamin C, sugars present in the commercial apple beverages in Singapore. The effects exerted by total and specific polyphenols, vitamin C, and sugar present in these commercial beverages on the antioxidant and anti-inflammatory capacity are also less known. The study examined the concentrations of total and specific polyphenols, vitamin C, and sugar present in commercial apple beverage products. The same study also evaluated if their concentrations affect the antioxidant and anti-inflammatory capacity of the tested beverages. These data are valuable to nutritionists and food scientists to evaluate the nutraceutical values of consumer-ready fruit-derived SSB, such as commercial apple beverage products.
| 2. Materials and methods | ▴Top |
2.1. Chemicals and materials
F2-isoprostanes-d4, F2-isoprostanes, leukotriene B4 (LTB4), leukotriene B4-d4, and arachidonic acid (AA) were purchased from Cayman Chemical (Ann Arbor, MI, USA). Glucose, dextran 500, sodium carbonate, Folin–Ciocalteu’s reagent, gallic acid, 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), = vitamin C, Tris (2-carboxyethyl)-phosphine hydrochloride (TCEP-HCl), trichloroacetic acid (TCA), decylamine, quercetin, catechin, phorbol 12-myristate 13-acetate (PMA), calcium ionophore, trypan blue, phosphate-buffered saline (PBS), pyridine, toluene, isooctane, hydrogens peroxide (50% by volume), guaiacol, xylenol orange, ammonium ferrous sulfate, vitamin C, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), 2,3,4,5,6-pentafluorophenylbromide, and bis(trimethylsilyl) trifluoroacetamide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile, ethyl acetate, methanol, ethanol, and sulfuric acid were purchased from Tedia (Fairfield, OH, USA). Ficoll-paque was purchased from GE Healthcare (Uppsala, Sweden).
Apple polyphenols were extracted from a combined mixture of apple beverages (100 g from each of the seventeen commercial apple beverages) using a previously published method. Briefly, the combined apple beverage (100 g) was extracted by sonicating with aqueous ethanol (50:50, v/v, 2 × 50 mL) at 40 °C for 30 min (Matsuyoshi ultrasonic cleaner KS-120N, Japan). The aqueous ethanolic extractant was combined and concentrated via evaporation at 40 °C (Ika rotary evaporator RV10, Germany). The final apple polyphenol concentrate was standardized to 5 g gallic acid equivalents (GAE)/100g total polyphenol content using a modified Folin-Ciocalteu assay (Loke et al., 2016). The standardized apple polyphenol concentrate was used to prepare the experimental mixture.
2.2. Nutrient profiling
All the apple beverage products found on the shelves and chillers of ten randomly selected local supermarkets were included in this study. They were subdivided into apple juice and apple drinks. Apple juice is defined as the unfermented liquid extracted from sound, ripe, fresh apple, with or without sugar, dextrose, invert sugar, liquid glucose, permitted coloring matter, chemical preservatives, and ascorbic acid (Singapore Food Agency, 2019). Apple drinks are beverages for consumption without dilution and may contain apple juice (Singapore Food Agency, 2019). The nutrient contents (including the sugar content) declared on the nutrition information panel were recorded by trained research personnel.
2.3. Total polyphenol, catechins, quercetin, and vitamin C measurements
The amounts of polyphenols in the commercial apple beverages were determined using a modified Folin-Ciocalteu assay (Loke et al., 2016). Briefly, the apple beverage (100 g) was extracted by sonicating with aqueous methanol (80%, 2 × 50 mL) at 25 °C for 30 min (Matsuyoshi ultrasonic cleaner KS-120N, Japan). The extract was assayed for its total polyphenol, quercetin, and catechin contents. The total polyphenol content in each test sample was quantitated against standard gallic acid solutions and was expressed as mg GAE/100g beverage.
The amounts of total catechin and quercetin were measured using high performance liquid chromatography coupled with a diode array detector (HPLC-DAD) (Loke et al, 2017). Briefly, the extract was hydrolyzed in methanolic potassium hydroxide (3 mol/dm3) under nitrogen gas at 60 °C for 3 hours. The supernatant was filtered through a 0.45 µm cellulose acetate syringe filter before HPLC injection. The filtered supernatant (20 µL) was chromatographed isocratically on a reversed-phase C18 column (Merck Purospher® Star RP-18, 5 µm particle size, 8 A pore size, 100 × 4.60 mm) at a flow rate of 1.0 mL/min using a Waters HPLC series 2695 with mobile phase methanol, acetonitrile, and water (60:20:20, v/v/v). Quercetin and catechin were measured at 262 nm and 276 nm, respectively, and determined by comparing the quercetin and catechin integrated peak areas with respective, predetermined calibration curves.
The vitamin C content of the resulting mixture was determined by a modified HPLC – ultraviolet detection (HPLC-UVD) method. (Fontannaz et al., 2006). Briefly, the apple beverage (10 g) and TCEP-HCl (250 µg/mL, 40 mL) were added into a 100 mL volumetric flask before topping up to the mark with 1% TCA. The resulting solution was shaken for about 1 min and filter. The filtered supernatant (20 µL) was chromatographed isocratically on a reversed-phase C18 column (Merck Purospher® Star RP-18, 5 µm particle size, 8 A pore size, 100 × 4.60 mm) at a flow rate of 1.0 mL/min using a Waters HPLC series 2695. The mobile phase was prepared as follow: decylamine (1.6 g), acetonitrile (80 mL), sodium acetate solution (0.25 mol/L, 100 mL), and distilled water (820 mL) were introduced into a 1,000 mL flask; then the pH of the solution was adjusted to 5.4 with phosphoric acid 85% and 50 mg TCEP-HCl. Vitamin C was measured at 265 nm and determined by comparing the vitamin C integrated peak area with a predetermined calibration curve.
2.4. Antioxidant and anti-inflammatory capacity
The radical scavenging capacity of the apple beverage was determined using the DPPH radical scavenging assay (Miliauskas et al., 2004). The radical scavenging results were expressed in mg vitamin C-equivalents/100g.
The cellular antioxidant capacity was determined by measuring the inhibition of F2-isoprostanes and lipid hydroperoxides (LPO) productions from freshly isolated human neutrophils. The cellular anti-inflammatory capacity of the apple beverage was determined by measuring the inhibition of LTB4 production and MPO activity of freshly isolated human neutrophils. Human neutrophils were isolated from the neutrophil/erythrocyte pellet of fresh human whole blood after Ficoll-Paque gradient centrifugation and dextran sedimentation of red blood cells (Tsen et al., 2016). The whole human blood was obtained in kind from the study researchers and as suchdid not require dethics approval. The freshly isolated neutrophils were resuspended in PBS at a concentration of 5 × 106 cells/mL. Cell viability was assessed using trypan blue exclusion and was typically >98%. The freshly isolated neutrophils (5 × 106 cells/mL in PBS, 1 mL) were incubated with the aqueous methanolic extract of the beverage (1 g/mL, 100 µL), AA (final concentration, 10 mmol/L) at 37 °C for 5 min before stimulation. The neutrophils were incubated with PMA (final concentration, 200 nmol/L) at 37 °C for 15 minutes to stimulate the F2-isoprostanes production. For LPO productions, the neutrophils were stimulated with AAPH (final concentration, 5 mmol/L) at 37 °C for 15 min. The neutrophils were incubated with calcium ionophore (final concentration, 200 nmol/L) at 37 °C for 15 min to stimulate the production of LTB4 in vitro (Loke et al., 2008). Positive control experiments were performed by incubating neutrophils with AA (final concentration, 10 mmol/L) before activating with either PMA (final concentration, 200 nmol/L), AAPH (final concentration, 5 mmol/L), or calcium ionophore (final concentration, 200 nmol/L). Negative control experiments were carried out by incubating neutrophils with AA (final concentration, 10 mmol/L). The supernatant from the cell suspension was collected and stored at −80 °C before F2-isoprostanes, LPO, and LTB4 analyses. F2-isoprostanes and LTB4 were quantified using stable isotope-labeled gas chromatography-mass spectrometry (Mori et al., 1999; Loke et al., 2008b). The formation of LPO was quantitated using the ferrous oxidation-xylenol orange assay (Nourooz-Zadeh et al., 1994). To examine the effects of the apple beverage on the functional MPO activity, the aqueous methanolic extract of the beverage (1 g/mL, 100 µL) was added into the freshly isolated human neutrophils (1 × 106 cells/mL in PBS, 1 mL). The mixture was incubated for 5 min at 37 °C before the neutrophils were resuspended in fresh PBS and lyzed by sonication. Untreated neutrophils (1 × 106 cells/mL in PBS, 1 mL) were used as positive controls. Functional MPO activity was determined by measuring its catalytic action on the oxidation of guaiacol in the presence of hydrogen peroxide (Klebanoff et al., 1984). The apple beverages were compared after segregation into low (lower than 10 g/100g) and high (equal or greater than 10 g/100g) sugar, low (lower than 20 mg/100g) and high (equal or greater than 20 mg/100g) vitamin C, low (lower than 10 mg GAE/100g) and high (equal or greater than 10 mg GAE/100g) total polyphenols, low (lower than 1.2 mg/100g) and high (equal or greater than 1.2 mg/100g) catechin, and low (lower than 0.5 mg/100g) and high (equal or greater than 0.5 mg/100g) quercetin concentrations.
2.5. Effects of total polyphenols, catechin, quercetin,, vitamin C and fructose concentrations on the antioxidant and anti-inflammatory capacity
The following experimental beverage mixtures were freshly prepared:
- Beverage mixture, Apple Beverage with Low Polyphenol Contents (AL) contained apple polyphenols (10 mg GAE/100g), catechin (1.2 mg/100g), quercetin (0.5 mg/100g), and vitamin C (20 mg/100g) in deionized water;
- Beverage mixture, Apple Beverage with Sugar and Low Polyphenol Contents (ALS) contained apple polyphenols (10 mg GAE/100g), catechin (1.2 mg/100g), quercetin (0.5 mg/100g), vitamin C (20 mg/100g), and fructose (10 g/100g) in deionized water;
- Beverage mixture, Apple Beverage with High Polyphenol Contents (AH) contained apple polyphenols (40 mg GAE/100g), catechin (5 mg/100g), and quercetin (2 mg/100g), and vitamin C (80 mg/100g) in deionized water;
- Beverage mixture, Apple Beverage with Sugar and High Polyphenol Contents (AHS) contained apple polyphenols (40 mg GAE/100g), catechin (5 mg/100g), quercetin (2 mg/100g), vitamin C (80 mg/100g), and fructose (10 g/100g) in deionized water;
- Apple Beverage (AB) was one of the studied commercial apple beverage measured to contain apple polyphenols (9.5 mg GAE/100g), catechin (1.35 mg/100g), quercetin (0.42 mg/100g), vitamin C (20.5 mg/100g), and fructose (10.2 g/100g);
- Beverage, Apple Beverage with Heightened Polyphenol Contents (ABH) contained AB and added apple polyphenols (30.5 mg GAE/100g), catechin (3.65 mg/100g), quercetin (1.58 mg/100g), and vitamin C (59.5 mg/100g).
The mixtures AL and ALS were prepared to simulate the vitamin C, total polyphenols, catechin, quercetin concentrations without and with fructose in the commercial apple beverages. The mixtures AH and AHS simulated the heightened vitamin C, total polyphenols, catechin, quercetin concentrations (four folds greater than the AL and ALS) without and with fructose in the proposed apple beverage. AB was one of the studied apple beverage whose vitamin C, apple polyphenol, catechin, and quercetin contents matched the simulated ALS. ABH was prepared from AB to match the vitamin C, apple polyphenol, catechin, and quercetin concentrations to those of AHS.
The DPPH radical scavenging capacity, inhibitions of cellular F2-isoprostanes, LPO, LTB4 production, and MPO activity of each of the freshly prepared aqueous mixture were determined as previously described. Triplicate experiments were performed.
2.6. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 26.0 (USA). Data were presented as mean ± standard deviation (SD). Differences between two and more than two groups were compared using two-sample independent t-tests and ANOVA with Bonferroni post-hoc test, respectively. A significant difference was observed when p < 0.05.
| 3. Results | ▴Top |
3.1. Nutritional profiling, total polyphenol content, and specific polyphenol content
Seventeen apple beverages (twelve juices and five drinks) were included in the study. The nutritional profiles, total polyphenol, total catechin, and total quercetin contents of the apple beverages–juices and drinks were presented in Table 1.
 Click to view | Table 1. Nutritional profile, total and specific polyphenol contents of apple beverages (N1) |
The apple beverages offered significant amounts of energy, almost all contributed by their sugar content, and vitamin C. They contained significantly greater sugar concentrations than the healthier 5% limit for sugar-sweetened beverages set by the Singapore Health Promotion. Similar observations were made after stratification between apple juices and drinks. The apple juices provided significantly larger amounts of sugar and energy than apple drinks. Fat and protein were nearly absent from the apple beverages. The drinks contained significantly higher vitamin C concentrations than the tested juices (Table 1). The juices and drinks did not differ in the concentrations of the other macronutrients and micronutrients.
The apple beverages offered significant amounts of polyphenols with the apple juice containing significantly higher polyphenol concentrations than the drinks (Table 1). Significant higher concentrations of total catechin and quercetin were present in the apple juices than in the apple drinks (Table 1).
3.2. Antioxidant and anti-inflammatory capacity of commercial apple beverages
The beverages were found to exhibit antioxidant and anti-inflammatory activities. The apple drinks demonstrated significantly stronger DPPH radical scavenging capacity and inhibitions of cellular F2-isoprostanes and LPO productions than the juices (Table 2). Contrary, the apple juices inhibited cellular production of LTB4 and MPO activity to significantly greater extents than the apple drinks (Table 2).
 Click to view | Table 2. Antioxidant and anti-inflammatory capacity of apple beverages (N1) |
3.3. Effects of total polyphenols, quercetin, catechin, vitamin C, and sugar contents of apple beverages on the antioxidant and anti-inflammatory capacity
Total polyphenol, quercetin, and catechin concentrations did not significantly influence the DPPH radical scavenging activity and inhibition of cellular F2-isoprostane and LPO productions (Figures 1–3). Similar observations remained after stratifying by beverage types – juices vs. drinks (Figures 1–3). The DPPH radical scavenging activity, inhibition of cellular F2-isoprostane, and LPO productions were significantly higher in the presence of high vitamin C compared to low vitamin C concentrations (Figure 4). The inhibitions of cellular LTB4 and MPO productions were significantly greater in the presence of high compared to low total polyphenol, catechin, and quercetin concentrations (Figures 1–3). The vitamin C concentrations did not affect the cellular LTB4 and MPO productions (Figure 4). The observations were unchanged after stratifying into juices and drinks (Figures 1–4). The presence of low and high sugar concentrations did not affect the antioxidant and anti-inflammatory capacity of the apple beverages (data not shown).
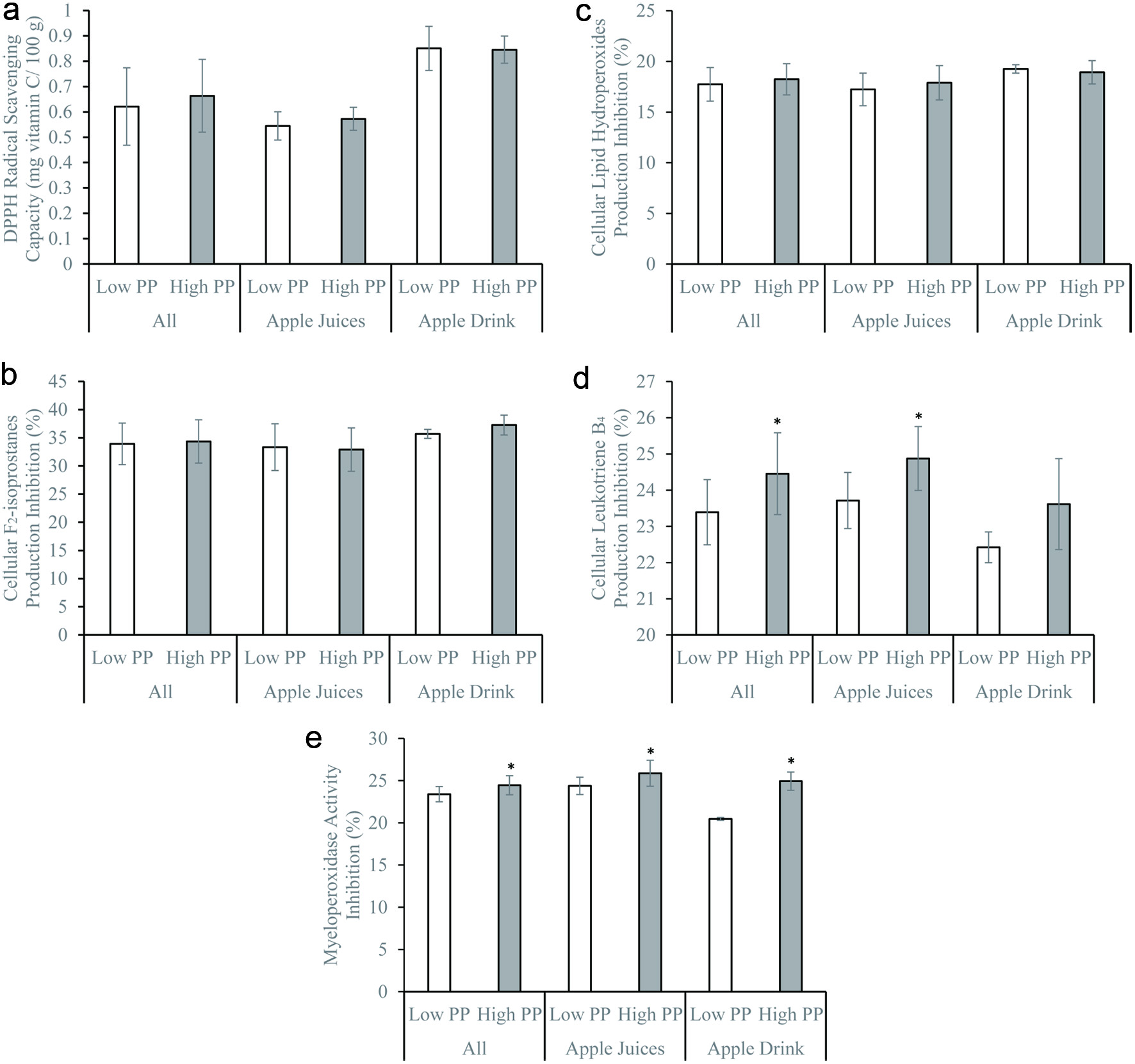 Click for large image | Figure 1. (a) 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity, (b) inhibitions of formation of F2-isoprostanes, (c) lipid hydroperoxides, (d) leukotriene B4, and (e) myeloperoxidase activity by freshly isolated human neutrophils in vitro of apple beverages – juices and drinks with low (lower than 10 mg GAE/100g; apple beverages n = 8, juices n = 6, drinks n = 2) and high (equal or greater than 10 mg GAE/100g; apple beverages n = 9, juices n = 6, drinks n = 3) total polyphenol contents. *p < 0.05 using two-sample independent t-test. |
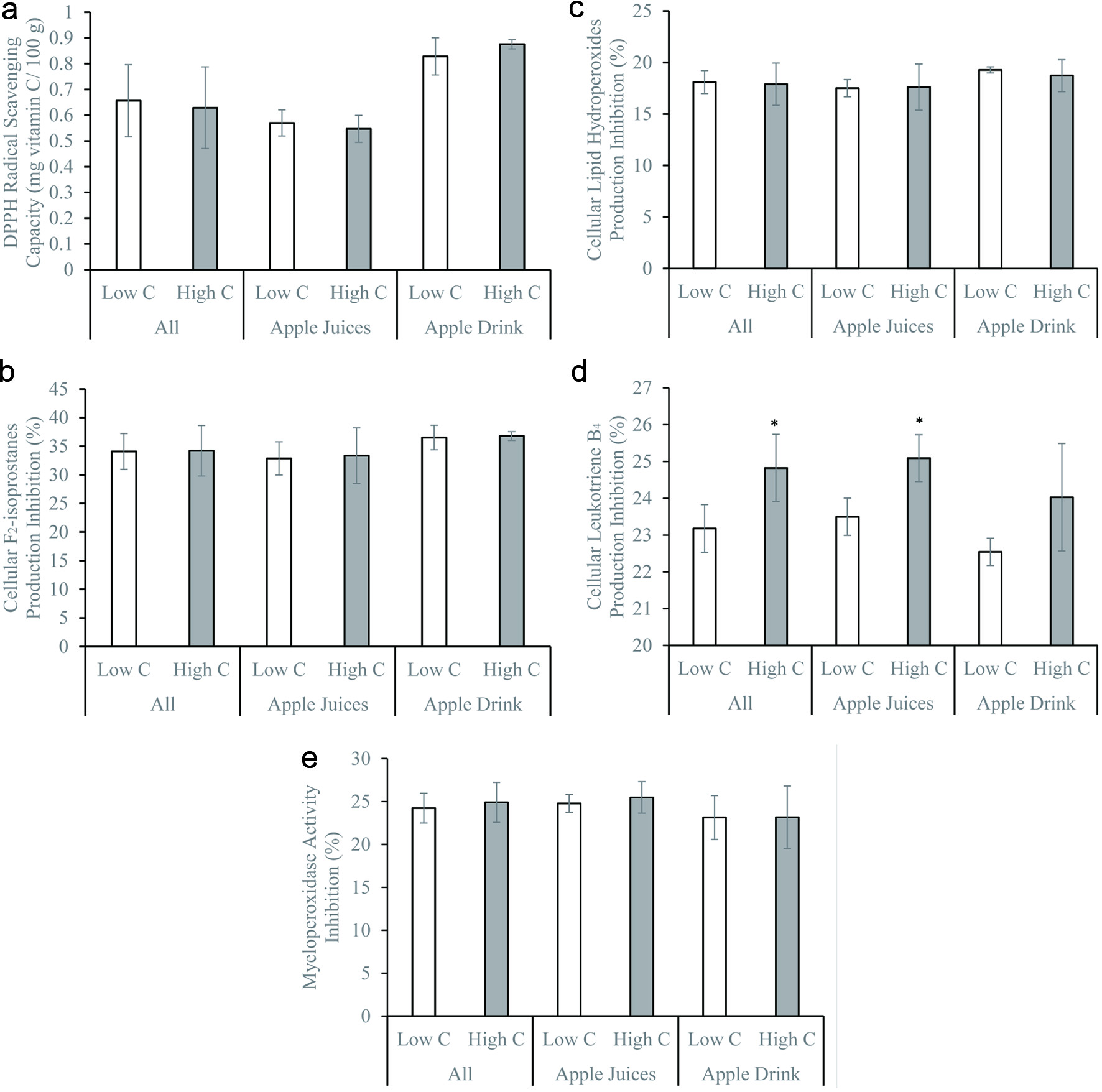 Click for large image | Figure 2. (a) 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity, (b) inhibitions of formation of F2-isoprostanes, (c) lipid hydroperoxides, (d) leukotriene B4, and (e) myeloperoxidase activity by freshly isolated human neutrophils in vitro of apple beverages – juices and drinks with low (lower than 1.2 mg/100g; apple beverages n = 9, juices n = 6, drinks n = 3) and high (equal or greater than 1.2 mg/100g; apple beverages n = 8, juices n = 6, drinks n = 2) total catechin contents. *p < 0.05 using two-sample independent t-test. |
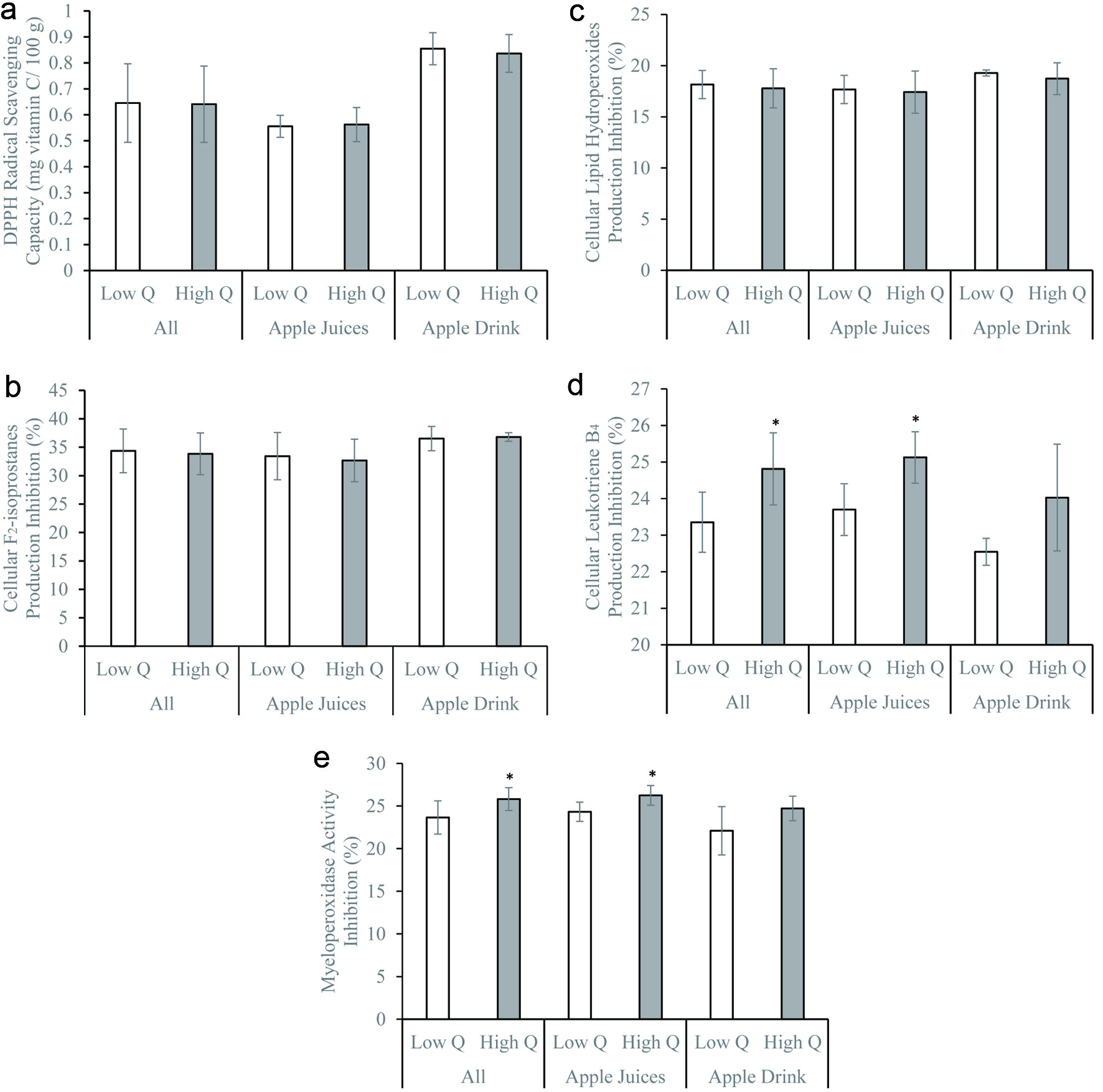 Click for large image | Figure 3. (a) 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity, (b) inhibitions of formation of F2-isoprostanes, (c) lipid hydroperoxides, (d) leukotriene B4, and (e) myeloperoxidase activity by freshly isolated human neutrophils in vitro of apple beverages – juices and drinks with low (lower than 0.5 mg/100g; apple beverages n = 10, juices n = 7, drinks n = 3) and high (equal or greater than 0.5 mg/100g; apple beverages n = 7, juices n = 5, drinks n = 2) total quercetin contents. *p < 0.05 using two-sample independent t-test. |
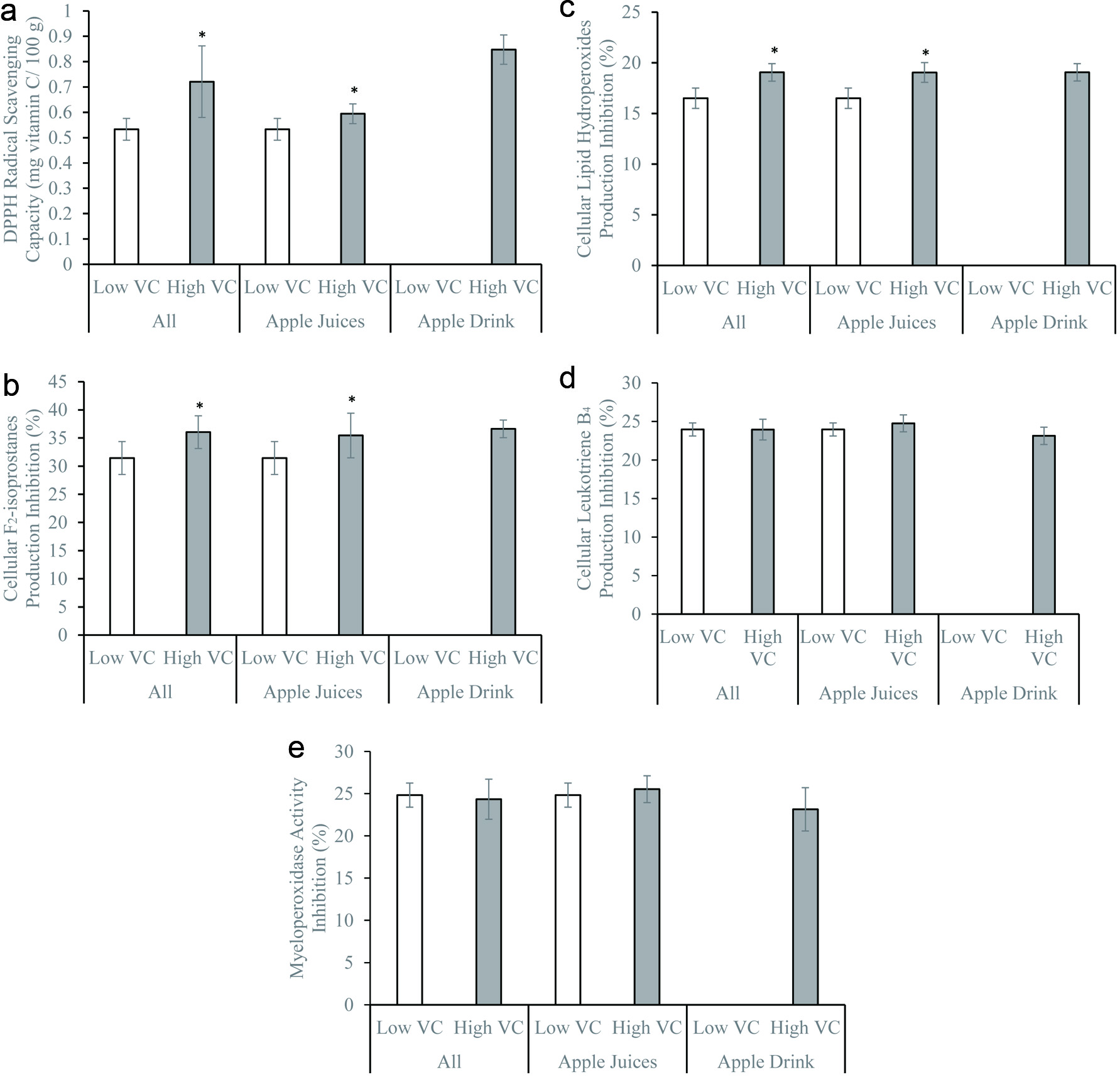 Click for large image | Figure 4. (a) 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity, (b) inhibitions of formation of F2-isoprostanes, (c) lipid hydroperoxides, (d) leukotriene B4, and (e) myeloperoxidase activity by freshly isolated human neutrophils in vitro of apple beverages – juices and drinks with low (lower than 20 mg/100g; apple beverages n = 7, juices n = 7, drinks n = 0) and high (equal or greater than 20 mg/100g; apple beverages n = 10, juices n = 5, drinks n = 5) vitamin C. *p < 0.05 using two-sample independent t-test. |
3.4. Fructose and increased total polyphenol, quercetin, and catechin contents on antioxidant and anti-inflammatory capacity
The presence of fructose did not influence the DPPH radical scavenging activity, inhibitions of cellular productions of F2-isoprostanes, LPO, and LTB4, and cellular MPO activity of the vitamin C, apple polyphenols, quercetin, and catechin mixtures (Table 3). The mixture containing higher vitamin C, apple polyphenols, quercetin, and catechin concentrations significantly elevated DPPH radical scavenging activity, cellular F2-isoprostanes, LPO, LTB4 production inhibitions, and cellular MPO activity when compared to the one with lower concentrations (Table 3).
 Click to view | Table 3. Antioxidant and anti-inflammatory capacity of simulated apple beverages |
| 4. Discussion | ▴Top |
Oxidative stress and inflammation have been implicated as mechanisms involved in the pathogenesis of cardiovascular and chronic metabolic diseases (Dhalla et al., 2000; Furman et al., 2019). F2-isoprostanes are stable prostaglandin-like isomers formed in situ in cell membranes by free radical-induced peroxidation of arachidonic acid (Morrow et al., 1990). It has been established as a stable in vivo marker of oxidative damage (Basu, 2008). LPO are products of lipid peroxidation and have been regarded as an in vivo marker of lipid peroxidation (Proudfoot et al., 2009). The results demonstrated that apple polyphenols, quercetin, catechin, and vitamin C exert significant total DPPH radical scavenging activity and inhibited the cellular productions of F2-isoprostanes and LPO. Vitamin C exerted significantly stronger antioxidant effects than the apple polyphenols, quercetin, and catechin at concentrations usually present in the apple beverage products. Radical-initiated reactions form the main mechanisms for the formation of F2-isoprostanes and LPO (Morrow et al., 1990; Proudfoot et al., 2009), explaining similar associations between the DPPH free radical scavenging and cellular antioxidant results. LTB4 is a potent chemoattractant for neutrophils, monocytes, and eosinophils (Hersberger, 2010), and is usually involved in inflammatory diseases such as rheumatoid arthritis (Chen et al., 2006) and atherosclerosis (Libby, 2002). MPO, a heme-containing enzyme found in neutrophils, monocytes, and macrophages, catalyzes the conversion of hydrogen peroxide and chloride ions to hypochlorous acid (Zhang et al., 2002). Thus, MPO has been suggested as a physiological catalyst for in vivo LDL modification in studies using monocytes and neutrophils isolated from humans (Zhang et al., 2002). The results showed that apple polyphenols, quercetin, catechin, and vitamin C exhibited significant anti-inflammatory activity via the inhibition of cellular LTB4 production and MPO activity. Apple polyphenols, like quercetin and epicatechin, had previously been shown to inhibit LTB4 production and MPO activity in vitro (Loke et al., 2008a) and in vivo (Loke et al., 2010). Vitamin C exhibited a significantly lesser LTB4 and MPO inhibition compared to the apple polyphenols, quercetin, and catechin. These results suggest that the bioactive constituents, such as apple polyphenols, catechin, quercetin, and vitamin C, in apple beverages exert differential health benefits.
Evidence on the health benefits associated with apple juice consumption has been encouraging. In an unblinded, randomized, crossover intervention study involving healthy men and women, apple juice consumption over six weeks increased ex vivo copper-mediated low-density lipoprotein oxidation lag time compared to baseline, and reduced conjugated diene formation (Hyson et al., 2000). Another blinded, randomized, and crossover intervention study showed that the consumption of vitamin C-rich (60 mg/L) apple juice, but not polyphenol-rich (993 mg catechin equivalent/L), over four weeks elevated plasma antioxidant capacity in healthy men and women (Soriano-Maldonado et al., 2014). It is widely hypothesized that apple polyphenols and vitamin C were responsible for the reported bio activity. These results also suggest that apple polyphenols and vitamin C in apple juice exert differential bioactivity, and their bioactivity requires them to be present at higher than the normal concentrations. Pharmacological studies have suggested that the bioactive molecules may need to be ingested at higher doses for increased bioavailability and effective biological activity. Ingestion of pure quercetin and (−)-epicatechin (200 mg) augmented nitric oxide products and reduced endothelin-1 acutely in healthy men (Loke et al., 2008c). Quercetin and theaflavin (350 mg/d equivalent to human dose) attenuated atherosclerosis in ApoE −/− knockout mice by alleviating inflammation, improving nitric oxide bioavailability, and inducing heme oxygenase-1 (Loke et al., 2010). The reported doses used in those pharmacological studies were much higher than those normally present in food and beverages. Our results demonstrated that apple polyphenols, catechin, quercetin, and vitamin C in apple beverages exerted a concentration-dependent antioxidant and anti-inflammatory effect. In other words, increasing the concentrations of these antioxidant and anti-inflammatory molecules in the beverages can potentially improve the health-benefiting efficacy, and thereby nutraceutical value of these functional beverages.
The results indicated that apple beverages contained significant amounts of sugar and energy. Most apple beverages, regardless of the juices or drinks, exceeded the healthy levels of sugar (below 5%) as suggested by Singapore Health Promotion Board, and were classified as the less healthy “C” band (5 to 10% sugar content) and “D” band (11 to 15% sugar content) of the SSB (Goh, 2020). Sugars present in beverages are gaining focus in the field of food and health sciences as SSB consumption has been found to associated with metabolic diseases (Ahmad et al., 2020; Ferreira-Pêgo et al., 2016; Malik and Hu, 2019). Fructose is the most prevalent sugar in apple and was therefore assumed to be the predominant sugar present in apple beverages. The sugar contents of the apple drinks may be deliberately managed by the manufacturers to keep the amounts of sugar in their products within specified lower levels while maintaining acceptable sensory attributes. This may explain why commercial apple drinks were found to contain significantly lower amounts of sugar than apple juices. The beverage industry in Singapore had pledged to reduce the sugar content of their products to less than 12% by 2020 (Lai, 2017). This move is aligned with the Singapore government policy to reduce sugar intake by Singapore residents (Singapore Health Promotion Board, 2020a). The sugar contents of the apple beverages can be further reduced to healthier levels by replacing the added sugar completely or partially with alternative low- or non-caloric sweeteners, like stevia and erythritol (Malik, 2019). The challenges of costs and sensory retention, however, remain. Reducing the innate sugar content of apple juice seems to be a bigger challenge. The presence of sugar or fructose did not affect the antioxidant and anti-inflammatory capacities of the apple beverages, as shown by the experiments with the simulated apple beverages. Sugar in commercial apple beverages may pose health concerns, but the bioactive apple constituents are still able to exert their innate biological activities.
Vitamins have been regarded as the main nutrient driver of fruit consumption (Mielgo-Ayuso et al., 2017). Vitamin C was known by consumers to be omnipresent in fruits and their beverages. The results verified the consumer belief and showed that the consumption of one cup (250–300 mL) of apple beverage daily should suffice to fulfill the Recommended Daily Allowance of vitamin C (75–90 mg) (Singapore Health Promotion Board, 2020b). In addition to its nutritional value, vitamin C exhibited antioxidant activity that may help reduce oxidative stress and damage in the human body (Granger and Eck, 2018). Polyphenols have been shown to contribute to the nutraceutical property of fruits and vegetables. As demonstrated in our results, fruit beverages, like apple beverages may serve as a dietary source of polyphenols and vitamin C. This is particularly important when consumers are not ingesting sufficient fruits and vegetables in their usual diet. Fortification of polyphenols and vitamin C may potentially elevate the nutraceutical value of these apple beverages. Vitamin C is commonly added as an antioxidant into beverage products. The addition of polyphenols, however, is less common. The manufacturing processes of apple beverages allow better control and customization of the nutrient contents, such as sugars, vitamins, minerals, and unique phytochemicals, like polyphenols that may offer additional nutraceutical benefits. However, such practices pose a challenge for food technologists to upkeep the consumers’ sensory acceptance of the final beverage product.
| 5. Conclusion | ▴Top |
Albeit of the diminishing effects of food processing on the polyphenol and vitamin C contents (Shahidi, 2020), the commercial apple beverages sold in Singapore were found to contain significant amounts of apple polyphenols, quercetin, catechin, and vitamin C. The results demonstrated that polyphenols and vitamin C at the concentrations present in the commercial apple beverages were capable of providing significant biological activities via antioxidant and anti-inflammatory mechanisms. Elevating the concentrations of polyphenols and vitamin C in these commercial beverages may likely increase their antioxidant and anti-inflammatory capacity. The presence of fructose at concentrations normally present in these beverages did not influence the antioxidant and anti-inflammatory capacity. Apple beverages may be consumed as sources of bioactive compounds, rather than just SSB. Food scientists may want to increase the polyphenol and vitamin C contents of apple beverages to further improve their nutraceutical value.
Acknowledgments
None to declare.
Conceptualization, CL, WML; methodology, LL, WML; formal analysis, CL, WML; writing—original draft preparation, CL, WML writing—review and editing, CL, LL, WML. All authors have read and agreed to the published version of the manuscript.
| References | ▴Top |