| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 2, June 2018, pages 1-15
Influence of branched chain amino acids on insulin sensitivity and the mediator roles of short chain fatty acids and gut hormones: a review
Akram Abolbaghaeia, B. Dave Oomahb, Hamed Tavakolic, Farah Hosseinianc, d, *
aDepartment of Cellular and Molecular medicine, University of Ottawa, 2513-451 Smyth Rd., Ottawa, ON, Canada K1H 7N9
bRetired research scientist, Agriculture and Agri-Food Canada, Summerland, BC, Canada V0H 1Z0
cFood Science and Nutrition, Department of Chemistry, Carleton University 1125 Colonel By Drive, Ottawa, ON, Canada K1S 5B6
dInstitute of Biochemistry, Carleton University 1125 Colonel By Drive, Ottawa, ON, Canada K1S 5B6
*Corresponding author: Farah Hosseinian, Food Science and Nutrition, Department of Chemistry, Carleton University 1125 Colonel By Drive, Ottawa, ON, Canada K1S 5B6. E-mail:
DOI: 10.31665/JFB.2018.2136
Received: January 24, 2018
Revised received & accepted: March 29, 2018
| Abstract | ▴Top |
Circulating levels of branched chain amino acids (BCAAs) correlate strongly with type 2 diabetes (T2D). The correlation may be associated with insulin-resistance factors independent of glycemic markers currently used in the diagnosis and monitoring of diabetes. This can revolutionize the thought process and methodology not only in diabetes treatment, but also in its advance screening and prevention with BCAAs used as biomarkers and targets for treatment. Whether insulin resistance is the cause or result of BCAAs imbalances requires further investigation. Although the overall diet is important, the role of specific diets targeting the gut microbiome composition and hormone secretion affecting BCAA absorption and metabolism will be explored. Generic diet modifications apparently induce only negligible changes in the intrinsic genetic make-up of the gut and BCAA levels but influence specific modulation of the gut microbiome. This genetic make-up is indeed similar among T2D patients independent of numerous variables including obesity. Short-chain fatty acids (SCFAs), the primary end-products of non-digestible carbohydrates (NDC) fermentation, mediate metabolic imbalances through gut microbiota and gut hormone secretion. This review focuses on extensive evidence gathered using diverse methodologies on the strong parallel correlation between BCAA levels and insulin resistance. Furthermore, the role of specific diets particularly SCFAs as mediators of the stubbornly fixed intrinsic genetic make-up of gut microbiota will be scrutinized to delineate BCAA levels and insulin resistance in T2D.
Keywords: Branched chain amino acids (BCAAs); Gene Ontology; Diabetes; Enrichment analysis; Propionate; Short chain fatty acids (SCFAs); Gut microbiota
| 1. Introduction | ▴Top |
1.1. Branched chain amino acids and their relationship with type 2 diabetes (T2D)
The occurrence of T2D is increasing around the world and is now identified as one of the foremost threats to human health. The global prevalence of diabetes among adults has risen from 4.7% in 1980 to 8.5% in 2014 (WHO, 2017). T2D correlate strongly with comorbidities particularly cardiovascular and cerebrovascular diseases. Furthermore, T2D is a condition categorised by abnormalities in carbohydrate, lipid, and protein metabolism with hyperglycemia and dyslipidemia as the most distinctive features. The key underlying pathological abnormalities include insulin resistance (Chen et al., 2015). The incidence of T2D and insulin resistance may increase with high-protein diets that are linked to impaired glucose tolerance (Chen et al., 2015). More specifically, increased plasma levels of essential branched chain amino acids (BCAAs), namely leucine, isoleucine, and valine, and their impaired catabolism have been associated with T2D, and obesity; increased plasma BCAAs has been postulated to promote insulin resistance (Schooneman et al., 2016; Varemo and Nielsen, 2015). Increased plasma BCAA levels have also been evident in obese individuals (Varemo and Nielsen, 2015). However, the exact mechanism of action of increased BCAAs leading to insulin resistance has not yet been investigated in detail. Particularly the role of the gut, gut hormones, and microbiota has not been fully investigated in relation to BCAAs absorption from the lumen or their metabolism after absorption. Furthermore, the role of diet needs to be investigated, particularly the role of short chain fatty acids (SCFAs) effects on BCAA levels via the amount ingested or the diet’s action on the gut, gut hormones, and microbiota. This review aims at unravelling: i) the relationship between BCAA, insulin resistance, type 2 diabetes, gut hormones and microbiota; ii) the mechanisms involved in these relationships particularly the mTOR1 (mammalian target of rapamycin complex1) iii) the direct or indirect roles of diet particularly short chain fatty acids in metabolic imbalances; iv) the level of various gene expression involved in BCAAs regulation within the microbiota of diabetic patients using gene ontology (GO) and enrichment analysis.
1.2. Role of myocytes in insulin resistance and BCAA Metabolism
BCAAs acylation of transfer RNA for protein synthesis is catabolised by extrahepatic tissues (muscle, adipose, kidney, and brain) (Badoud et al., 2014; Herman et al., 2010; Mooijaart et al., 2011; Morris et al., 2012; Yoon, 2016). The catabolism process is unique for BCAAs; in the fasted state, it is primarily catabolised by peripheral tissues (particularly muscle) rather than liver and therefore, myocytes are a good starting place for BCAAs metabolism and insulin resistance (Vrieze et al., 2012).
Myocytes are responsible for almost 10% of insulin-stimulated clearance of blood glucose after a meal (Varemo and Nielsen, 2015). They are also one of the tissues most sensitive to insulin resistance and therefore display fragility in metabolic dysfunction in obesity, metabolic syndrome, and T2D (Vinderola et al., 2006; Vuong et al., 2010). Thus, myocyte genes were investigated to explore those most affected in T2D patients, including BCAAs role in dysregulating metabolism (Varemo, et al., 2015a).
These investigators used “Genome Scale Metabolic Models” also known as GEMS, a variety of mass-balanced metabolic reactions for large-scale network structure application. The vastus lateralis human muscle was dissected from 6 (3M+3F) individuals with T2D, mRNA isolated from myocytes for cell-type-specific RNA sequencing (RNA-Seq) that was then compared with antibody-based immunohistochemistry of myocytes from the “Human Protein Atlas”. This allowed creation of a specific GEM for skeletal myocyte, in addition to transcription data from a previous study using 153 T2D patients. iMyoctye 2419 emerged as a great model for studying metabolic diseases (Varemo, et al., 2015a). This model has 4,448 metabolites, 2,419 genes, and 5,590 reactions in 8 different compartments involved in its metabolism. Specific metabolites or metabolic reactions significantly affecting T2D patients were searched by further analyzing this model for gene reactions, gene analysis and its expression (Varemo et al., 2016). “Reporter metabolites” from the mitochondrion indicator for specific metabolic reactions were used to measure the activity of reactions (Varemo et al., 2016). Specific genes were also blocked to investigate their effects on these metabolites and myocyte function (Varemo and Nielsen, 2015). Oxidative phosphorylation, beta-oxidation, TCA cycle, glycolysis, BCAA, and omega-6 fatty acid, vitamin-E, and nucleotide metabolisms were downregulated/suppressed in myocyte of T2D patients (Varemo et al., 2016).
Some immune-related processes was upregulated especially those relevant to inflammation (Vrieze et al., 2012). It was claimed that BCAAs degradation involved dihydrolipoamide dehydrogenase (DLD) found in the mitochondrial matrix and part of the branched-chain alpha-keto acid dehydrogenase (BCKD) complex (Varemo et al., 2016). DLD enzyme is also part of the pyruvate dehydrogenase and glycine dehydrogenase and thus also has important roles in pyruvate and THF metabolisms (Varemo, et al., 2015b). This enzyme was downregulated in the muscle, indicating that all components of metabolism will consequently be affected and downregulated. However, these downregulations do not provide adequate insight to whether they are the cause or effects of T2D predisposition. It was also shown that lipoproteins especially lipoamide-containing proteins resulted in DLD downregulation (Varemo and Nielsen, 2015).
1.3. BCAAs as potential markers for prediction of T2D vascular disease
Presently, glucose markers are used clinically to predict T2D or measure its severity (Badoud et al., 2014). However, recent studies suggest that insulin resistance is the root cause of T2D pathogenesis. Interestingly, BCAAs correlate and strongly associate with insulin resistance and thus can potentially be more sensitive and specific markers for T2D prediction and even subsequent vascular disease (Herman et al., 2010; Varemo et al., 2017). In this regard, The Framingham Offspring Study revealed that plasma-free BCAAs levels were significantly associated with future T2D diagnosis (Magnusson et al., 2013). This strong correlation of plasma BCAAs was conserved in 51 Japanese subjects (23M+28F) diagnosed with T2D according to cluster analysis where higher plasma BCAAs were observed in the higher insulin group (Figure 1) (Nakamura et al., 2014).
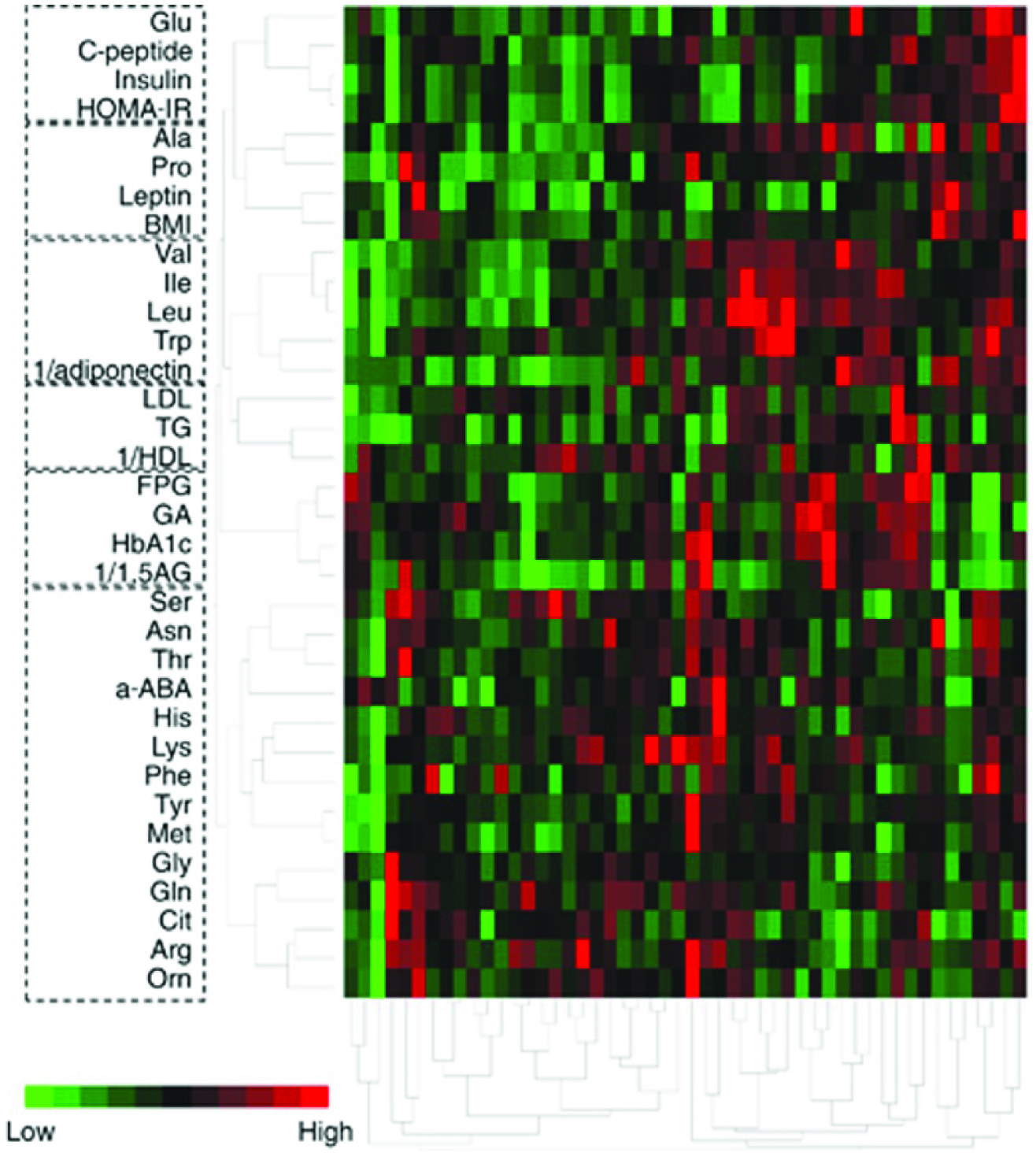 Click for large image | Figure 1. Cluster analysis of plasma amino acid with clinical variables relating to glucose, insulin resistance and lipid homeostasis in 51 type 2 diabetic patients using Ward’s method. |
Meanwhile, the amino acid profiles and levels did not really cluster (i.e., associated according to Pearson’s correlation coefficients) with either leptin or lipid variables such as LDL, HDL, or cholesterol or glucose-related variables such as fasting plasma glucose (FPG), HbA1C, glycated albumin (GA) or average glucose (AG) levels measured twice in each patient (Nakamura et al., 2014). Additionally, the glucose variables showed low correlations with insulin related variables; the concentrations were actually independent of one another in insulin-resistant T2D patients (Nakamura et al., 2014). Furthermore, other studies suggested that insulin-related variables are the predictors of future cerebrovascular or coronary heart disease; these variables correlated with BCAA plasma levels (Badoud et al., 2014; Morris et al., 2012; Yoon, 2016). Adiponectin is an adipose tissue-derived insulin sensitizer and plasma glucose modifier. Thus, adiponectin is hypothesized to be one of the key players in adjusting insulin sensitivity since it is depressed in both T2D and obese individuals (Varemo et al., 2015a; Yoon, 2016). Meanwhile, research comparing BCAAs to adiponectin or adipose-specific adipocytokines is very limited; Nakamura et al. (2014) was the first to compare BCAA profiles with adiponectin in humans. The AA profiles of mice with plasma amino acid imbalances and on high-fat diet actually normalized after adiponectin injection (Nakamura et al., 2014), but the reverse was not investigated. Adiponectin exerts anti-inflammatory and anti-atherogenic effects; its level is low in T2D patients and those with other metabolic syndromes, hypertension, cardiovascular disease, and even cancer (Vrieze et al., 2012). Therefore, glucose variables currently in clinical use for T2D diagnosis such as HbA1c, fasting plasma glucose, glycolated albumin, and average glucose measurements, may not be the best predictors for future coronary or cerebrovascular disease; insulin-related variables may instead be better (Nakamura et al., 2014). In principle, an imbalance in BCAA levels may be the root cause of distinction in insulin-related variables (Badoud et al., 2014; Herman et al., 2010; Morris et al., 2012). The catabolism process is unique for BCAAs; in the fasted state, it is primarily catabolised by peripheral tissues (particularly muscle) rather than liver and therefore, myocytes are a good starting place for BCAAs metabolism and insulin resistance (Vrieze et al., 2012). However, further substantial evidence may be required to measure BCAAs for the prediction and even monitoring of diabetes in the clinical world. Subsequently and in parallel, BCAA plasma levels and measurements can also become the major predictor for increased risk of coronary and cerebrovascular diseases. One way to examine this is to “normalize” the AA profiles of obese or T2D individuals compared to a closely defined placebo and observe any influence on insulin variables in a longitudinal study and overall disease progress (Herman et al., 2010; Shin et al., 2014; van Heemst, 2010; Yoon, 2016). This may then have the potential to serve as the “gold standard” for preventative measurements (Nakamura et al., 2014). Indeed, AA and BCAA imbalances may precede insulin or glucose imbalances and AA balancing may also lead to better metabolisms in adipose, liver, and muscle tissues and as a result make them more sensitive to insulin (Herman et al., 2010; Shin et al., 2014; van Heemst, 2010; Yoon, 2016).
For balancing, other than solely investigating diet modification, one can also examine and analyze the gut microbiota and the unique personal microbiome genes (Nakamura et al., 2014). Is it possible that in fact these are the overlooked participants pulling the strings and dictating what gets absorbed? Perhaps these potential string holders work independent of diet modifications. On the other hand, they just might change the way they act only via extremely specific diet modifications. If so, then the verdict would lean toward gut microbiota bearing the weight of guilt for BCAA imbalances and consequent responsibility in insulin resistance, rather than general BCAA intake modification being primarily accountable. Then one can focus on trying to change the actions of these string holders which can be quite resistant and stubborn to change because of an intrinsic genetic make-up, unless very specific diets are introduced and subsequently their behavior is intensely scrutinized. Otherwise, they just might be stubbornly indifferent if the diet modification is very general and does not specifically target them.
1.4. BCAAs cause insulin resistance in the brain’s hypothalamus
BCAAs may be responsible for insulin resistance of hypothalamic cells in the brain rather than in peripheral tissues such as adipose, muscle, or liver cells; modulation may not emanate from the central level when resistance occurs in the peripheral tissues (Shin et al., 2014). Thus, it is an interesting proposal that the major reason for resistance in peripheral tissues is actually a consequence of insulin resistance by hypothalamic cells. Of course, insulin in addition to lowering blood glucose also effects growth and other metabolic and signaling functions (Badoud et al., 2014; Herman et al., 2010; Morris et al., 2012). The catabolism process is unique for BCAAs; in the fasted state, it is primarily catabolised by peripheral tissues (particularly muscle) rather than liver and therefore, myocytes are a good starting place for BCAAs metabolism and insulin resistance (Vrieze et al., 2012). Its neuronal signaling role has been reported, although the mechanisms are currently poorly understood (Herman et al., 2010; Nakamura et al., 2014; Yoon, 2016).
BCAAs decrease remarkably in gastric bypass patients (GBP) after surgery, but not with the control group receiving dietary interventions (Laferrere et al., 2011). In that study two groups were controlled to have the same amount of weight loss after the bypass or dietary intervention and parameters were measured again at that point in time. Both groups before the intervention had comparable or almost the same mean age, BMI, duration of diabetes, HbA1c, plasma glucose, fasting blood glucose (FBG), and the same or extremely similar pro-insulin, insulin, C-peptide, and glucagon levels. Additional close similarities between the two groups before the interventions were incretin levels (gut hormones named glucagon like peptide 1 (GLP-1), total lipid profile, uric acid, AST and ALT (markers for liver functionality), leptin, ghrelin, β-hydroxybutyrate, lactate, and AA levels which also included BCAAs (Laferrere et al., 2011). Most importantly with respect to our discussion, 45 acylcarnithines and 15 AAs were monitored using tandem mass spectrometry in both groups and their levels matched (Laferrere et al., 2011). Furthermore, all analysis was performed on samples taken from overnight-fasted subjects, minimizing the potential impact of recent ingestion of protein-containing meals on the AA profile.
The total amino acid levels and BCAAs decreased by 19.7 and 38.3%, respectively, in the GBP group vs 12.8 and 12.6%, respectively, in the diet intervention group (Laferrere et al., 2011). In addition, there was a marked increase in the BCAA catabolic enzymes, and branched-chain α-keto acid dehydrogenase (BCKD) levels in the GBPs. This indicates mechanisms other than weight loss and diet alone in BCAA metabolism because both groups lost the same amount of weight and samples which were taken from overnight upon fasting. In addition, the same gastric bypass surgery patients had better glycemic control than the equivalent diet-induced weight loss subjects; 50–80% of GBPs, diabetes remission (defined as normalization of HbA1c concentrations in the absence of medication) occurred within days, well before complete and even before any weight loss (Laferrere et al., 2011). The GBP discontinued their long-term diabetes medications within 1 month after their surgery while the diet intervention group needed to continue taking anti-diabetic medications (Laferrere et al., 2011). This suggests that BCAA metabolism and thus plasma levels and insulin sensitivity are strongly correlated because while one group had both immediate diabetes remission and low BCAA levels, the other group continued to have increased BCAA levels and diabetes. Furthermore, factors other than diet may modify BCAA plasma levels since no correction of the imbalance was observed in the diet intervention group.
BCAAs function has been studied on peripheral tissues such as liver, pancreas, muscle, and adipose (Herman et al., 2010; Morris et al., 2012; Yoon, 2016). However, BCAAs presumably affect the hypothalamus rather than peripheral tissues since hypothalamus dysfunction suffer metabolic imbalances (Shin et al., 2014). The study measured BCKDH expression, which is directly correlated with elevated BCAA levels and thus the level of their breakdown. Phosphorylation inhibits BCKDH and therefore phospho-specific antibodies were used to label the amount of phosphorylation and thus BCKDH activity in liver tissues where it is most abundant. Plasma BCAA levels were also measured (Shin et al., 2014). In the initial steps of the study, insulin was injected into the rodents’ peripheral vasculature to examine its effects on BCAA levels. The fasting rodents were dosed with somatostatin to prevent endogenous insulin secretion and control counter-regulatory hormones such as glucagon and delineate the effects of diet absorption from the gut (Shin et al., 2014). However, whether gut hormones still respond to insulin injections even in the absence of short-term ingestion of diet remains to be examined. For instance, there is entero-hepatic circulation between the gut and the liver in which the liver sends all sorts of products from pharmacological drugs to biliary acids into the gut lumen and subsequently these materials are reabsorbed via enterocytes into the hepatic portal vein (Herman et al., 2010; Morris et al., 2012; Yoon, 2016). The catabolism process is unique for BCAAs; in the fasted state, it is primarily catabolised by peripheral tissues (particularly muscle) rather than liver and therefore, myocytes are a good starting place for BCAAs metabolism and insulin resistance (Vrieze et al., 2012). Surely, the enterocytes and thus gut hormones would then have a chance to have a sample and signals from the blood and respond accordingly and the left-over will be sent down to the colonic microbiome. Nevertheless, the study tried to minimize the influences of diet and to isolate the actions of exogenous insulin. Insulin dose-dependently enhanced BCKDH activity (less phosphorylation) and complimentarily reduced BCAA plasma levels (Shin et al., 2014). Furthermore, insulin reduced the circulating BCAA levels via BCKDH induction, since transient hyperglycemia even at marked levels did not alter BCAA levels in the absence of a rise in insulin.
The effects of BCAAs were examined separately in the hypothalamus and peripheral tissues by injecting 2-deoxyglucose intracerebroventricaulary (I.C.V) into the ventricular system of the central nervous system (CNS) to induce glucopenia. Plasma BCAA levels increased 60–90 minutes after injection. Insulin injected by the same I.C.V method systematically reduced BCAA levels demonstrating that insulin affects peripheral BCAA levels via the hypothalamus (Shin et al., 2014). Thus, insulin controlled BCAA levels in both peripheral and central studies; therefore elevation in BCAA levels can be used in the future as a major predictor of potential insulin resistance (Herman et al., 2010; Morris et al., 2012). The catabolism process is unique for BCAAs; in the fasted state, it is primarily catabolised by peripheral tissues (particularly muscle) rather than liver and therefore, myocytes are a good starting place for BCAAs metabolism and insulin resistance (Vrieze et al., 2012). Furthermore, centrally acting anti-diabetic drugs can be another novel approach for treatment if more evidence suggests that a chain set of events starts centrally.
BCAA levels were elevated in knock out mice models deficient in central insulin receptors, but reduced in those deficient in peripheral receptors (Shin et al., 2014). The study also showed that high fat feeding as well as excessive nutrient intake has the same effects on CNS resistance to insulin and this was also shown in primates such as macaques and even in the worm C. elegans (small soil worm) in other studies (Deelen et al., 2013; Mooijaart et al., 2011; van Heemst, 2010).
1.5. BCAAs directly acting centrally before their effects are recognized peripherally
Circulating BCAAs are presumably sensed by the hypothalamus which then signals their catabolism in other organs/tissues (Morris et al., 2012; Yoon, 2016). Indeed central administration of leucine increases activation of rapamycin (mTOR) and S6K central receptors and reduces food intake in mice (Morris et al., 2012; Yoon, 2016). The proposed mechanism is that normally insulin decreases BCAA levels (Figure 2) reflecting good insulin activity. However, when systemic and consequent central BCAA levels pass a “red line” per se due to various causes such as amount of ingested BCAAs, imbalance in gut activity and absorption, or simply the intrinsic genetic make-up of an individual’s gut microbiota regardless of diet, then insulin becomes less effective reflected by rising BCAA levels (Morris et al., 2012; Yoon, 2016).
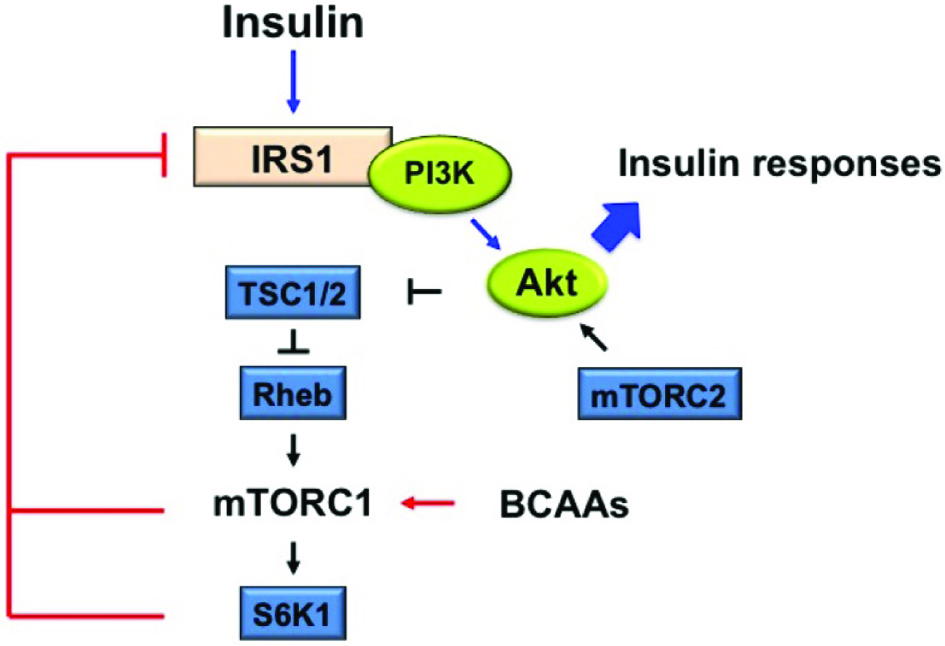 Click for large image | Figure 2. Proposed mechanism of branched-chain amino acids (BCAAs)-stimulated mammalian target of rapamycin complex1 (mTORC1) activation on insulin resistance (IR). |
On the other hand, elevated BCAAs can actually stimulate and accordingly desensitize hypothalamic mTOR (Shin et al., 2014) as observed with high fat diets and over-supplementation with amino acids (Laferrere et al., 2011). This desensitization then leads to lack of insulin sensitivity and hence hypothalamic insulin resistance. If this occurs with neurons, the concept is called synaptic plasticity where the desensitization is at neuronal synapses. This central resistance and lack of response and therefore lack of action, will then have trickling effects on numerous metabolic command centers lower in the hierarchy (Laferrere et al., 2011). The insulin resistance can then further increase BCAAs because insulin initiates their breakdown resulting in stimulation and desensitization of hypothalamic mTOR in an endless vicious loop (Shin et al., 2014). Furthermore, the out of control BCAA levels can now reach toxic levels that can have detrimental metabolic effects through other possible pathways (Shin et al., 2014). Thus, it is proposed that the remarkable glycemic and insulin resistance recovery observed in procedures such as gastric bypass is due to simultaneous BCAAs reduction and reversal of the over-activation of mTOR receptors especially centrally (Laferrere et al., 2011). Therefore, it remains to be seen if this reduction and subsequent reversal of over-activation can be achieved with other methods, perhaps by stimulating specific gut hormones or microbiota manipulation. Dramatic increase has been reported in the gut hormones glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP) and other hormones with similar actions known as incretins after gastric bypass surgery (Laferrere et al., 2011). These hormones, which are secreted during meals have major effects on metabolism particularly on insulin’s actions.
1.6. Acylcarnithines have the potential to play a role
Acylcarnithines (ACs) are generated during mitochondrial metabolism of fatty acids, AAs, and glucose; however, the mechanism of action is selective. For example, even-chain, medium- to long–chain AC species are generated almost exclusively from fatty acid oxidation, whereas C3 and C5 ACs come primarily from AA oxidation, particularly BCAA oxidation (Schooneman et al., 2013).
Recently, AC elevations have been observed not only in dysregulation of mitochondrial fatty acid oxidation, but also in the development of insulin resistance (Schooneman et al., 2013). When comparing gastric bypass patients with their closely controlled diet intervention counterparts, sum of all ACs actually increased after both interventions (Laferrere et al., 2011). However, C3 and C5 ACs decreased more prominently with GBP which can be seen complementary to BCAAs reduction (Laferrere et al., 2011). In parallel with BCAA levels before and after surgery, ACs metabolites reduction should be examined as the cause or result of diabetes remission. If ACs is the cause, then further research can observe whether gut hormones or microbiota metabolism of ACs during absorption also play a role.
1.7. Diet and prevention of oxidative stress
Oxidative stress, caused by reactive oxygen species (ROS), is responsible for modulating several pathological conditions in CVD, T2D, obesity and inflammation (Agil et al., 2016; Vinderola et al., 2006; Vuong et al., 2010). Consumption of food containing bioactives showed to decrease the risk of chronic diseases and these health benefits might be attributed to the antioxidant capacity of phytochemicals in foods (Dinu et al., 2017; Zhu et al., 2017). In-vitro studies have shown that bioactives in foods such as phenolics, dietary fibre and peptides have the ability to scavenge ROS (Agil et al., 2012; Chandrasekara et al., 2011; Gliwa et al., 2011; Yeo et al., 2015). Thus, it is crucial to find the relationship between the chemical structure of plant bioactives and their functionality in food and biological systems (Agil et al., 2013; Agil et al., 2016; Ainsley Reid et al., 2005; Ajibola et al., 2011; Al-Farsi et al., 2005; Chandrasekara et al., 2011; Gunenc et al., 2017). In plants, phenolics/polyphenols compounds can be divided into free, esterified and insoluble-bound forms. The bound phenolics are commonly linked/bonded to complex carbohydrates in the plant cell walls (Perez-Jimenez et al., 2009). For example, high contents of bound polyphenols present in fruits (70 to 80% of total phenolic content/TPC) suggest that polyphenol contents of plant foods have been underestimated and need re-assessments (Naczk et al., 2006; Perez-Jimenez et al., 2009). Additionally, several publications have shown that germination and fermentation are suitable methods to release polyphenolic compounds from the linked dietary fibre, enhancing the oxidative stability of foods (Gunenc et al., 2017; Ould Saadi et al., 2017). The total phenolic content and short chain fatty acids (SCFAs) (products of fermented dietary fibre) generation increased during fermentation (Gunenc et al., 2017; Ould Saadi et al., 2017).
| 2. Role of short-chain fatty acids in BCAA metabolism and reduction of insulin resistance | ▴Top |
2.1. Characteristics of short-chain fatty acids (SCFAs)
Upon ingestion of food, gut microbiota in the large intestine digest the undigested or unabsorbed residual food from the small intestine. Short-chain fatty acids are formed in the large intestine as a result of this process and microbiota’s actions on residual foods that end up in the colon (den Besten et al., 2013; Koh et al., 2016). SCFAs have less than six carbons, present in straight and branched-chain conformation. The major SCFAs (90–95% of the total SCFAs in the colon) produced from microbial fermentation are acetic acid (C2, 60%), propionic acid (C3, 25%), and butyric acid (C4, 15%). Nonetheless, microbes can changeover to energetically less favorable sources for growth such as amino acids from dietary or endogenous proteins, or dietary fats (Cummings et al., 1991; den Besten et al., 2013; Wall et al., 2009), when fermentable fibers are in short supply leading to less formation of fatty acids (Koh et al., 2016; Russell et al., 2011).
2.2. Effects of diet on gut microbiota composition and SCFAs production
Diet has an enormous impact on gut microbiota composition and activity. Specifically the profiles of SCFAs and BSCFAs synthesized can deeply affect human health and metabolism (Brussow et al., 2014; Louis et al., 2014; Rios-Covian et al., 2016a), and thus an imbalance in their composition may have a role in an imbalance in metabolism and hence metabolic disorders. Table 1, shows the impact of diet on SCFAs produced by the gut microbiota by various epidemiological studies (Badoud et al., 2014; Hang et al., 2014; Louis et al., 2014; Salonen et al., 2014). SCFAs are the main end products from these fermentations. The healthy populations showed a higher amount of fecal SCFAs (Sgobio et al., 2010; Ou et al., 2013) while overweight individuals showed lower SCFAs levels (Rahat-Rozenbloom et al., 2014; Fernandes et al., 2014) (Table 1). On the other hand, patients with celiac disease showed lower SCFAs levels than the treated patients (Caminero et al., 2012). Some microbes in the gut are capable to use both lactate and acetate to synthesize butyrate which prevents accumulation of lactate and stabilizes the intestinal environment (Koh, et al., 2016). The Length of the gut can affect the concentration of SCFAs. The highest level observed in the cecum and proximal colon whereas it drops toward the distal colon (Cummings, et al., 1991). The favoured energy source for colonocytes is found to be Butyrate and is locally consumed. However, the other absorbed SCFAs drain into the portal vein. Since metabolization of Propionate occurs in the liver, it is only present at low concentration in the periphery, leaving acetate as the most abundant SCFAs in peripheral circulation (Cummings, et al., 1991). Table 2: shows the impact of diet on SCFAs produced by the gut microbiota in intervention studies. The high SCFAs levels are reflected by dietary intakes of high fiber-low fat ratios and a high fiber-meat ratio (Hang et al., 2014; Louis et al., 2014; Rios-Covian et al., 2016b; Salonen et al., 2014) and their high amounts have demonstrated benefits while their low amounts have shown the opposite as in the case of colorectal adenocarcinoma patients with reduced fecal butyrate levels (Chen et al., 2013).
 Click to view | Table 1. Impact of diet on SCFAs produced by the gut microbiota by various epidemiological studies conducted since 2010 |
 Click to view | Table 2. Impact of diet on SCFAs produced by the gut microbiota in intervention studies conducted since 2010 |
Although the main source of SCFAs are carbohydrates, amino acids such as leucine, valine and isoleucine extracted from protein breakdown are also able to convert into isobutyrate, isovalerate, and 2-methyl butyrate and form branched-chain fatty acids (BSCFAs) (Koh et al., 2016; Smith et al., 1997). BSCFAs contribute a small amount (5%) to the total SCFAs production (Koh et al., 2016; Newgard et al., 2009). SCFAs are reduced in the colon upon ingestion of diets rich in protein or fat, but can be restored with ingestion of dietary fiber (Koh et al., 2016; Sanchez et al., 2009). Metabolic routes and pathways for SCFAs biosynthesis are illustrated in Figure 3, where the bacterial species used to inspect and investigate the pathways were based on several studies of cultured isolates of dominant species and metagenomic analyses.
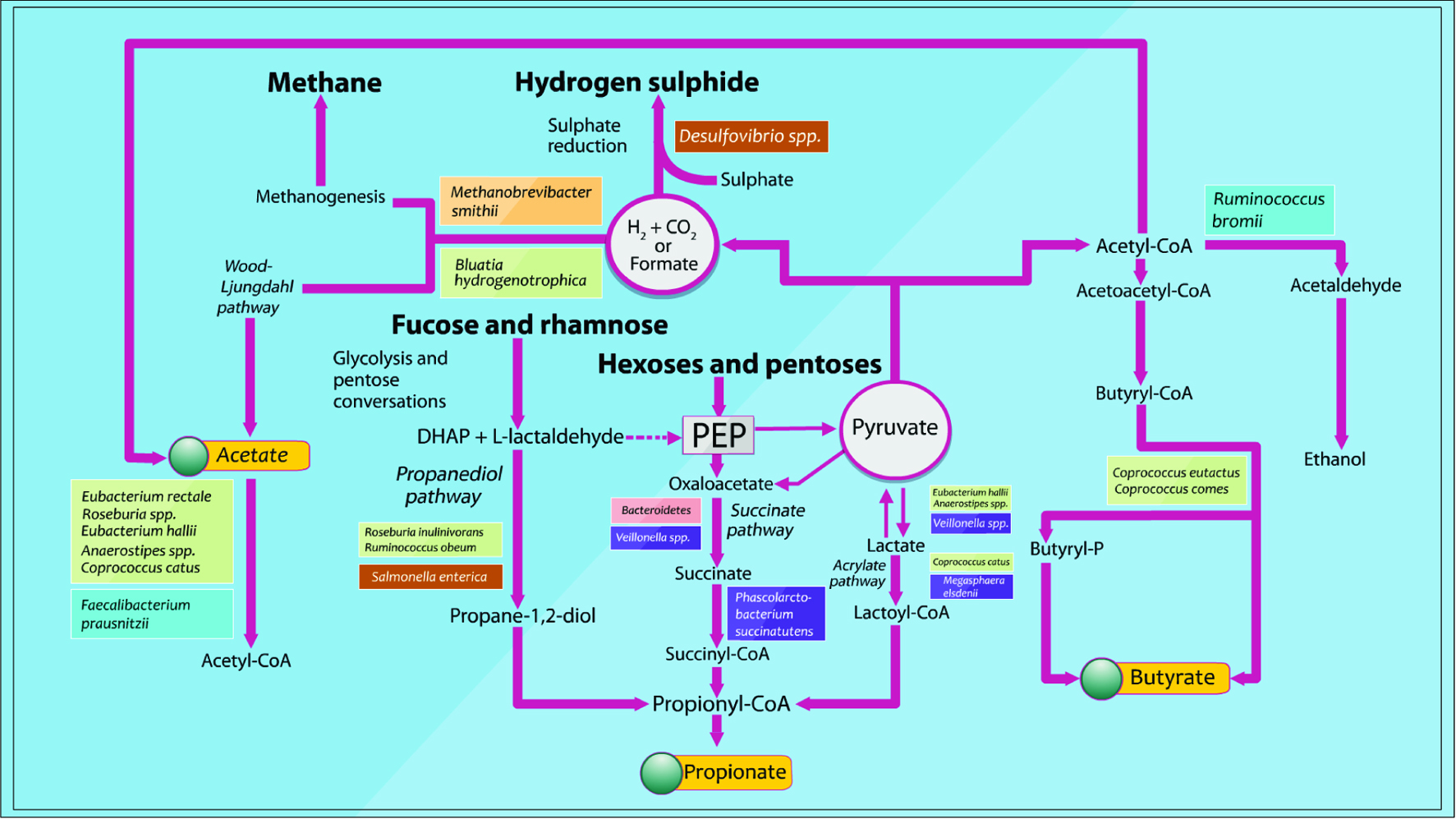 Click for large image | Figure 3. Pathways identified for the biosynthesis of major microbial metabolites and SCFAs from carbohydrate fermentation and bacterial cross-feeding (Adapted from Louis et al., 2014). |
Acetate of one of the SCFAs is produced from pyruvate by many gut bacteria via acetyl-CoA or via the Wood-Ljungdahl pathway. Propionate, another major SCFAs, is produced from succinate conversion to methylmalonyl-CoA via the succinate pathway (Koh, et al., 2016). Eventually, butyrate, the last major SCFAs, is made from the condensation of two molecules of acetyl-CoA and subsequent reduction to butyryl-CoA, which can be converted to butyrate by phosphotransbutyrylase and butyrate kinase (Koh, et al., 2016; Louis, et al., 2014).
2.3. Different actions of SCFAs – Effects on metabolism and inflammation reduction
Histones are proteins wrapped around the DNA. When a histone is acetylated, the chromatin is activated by unwinding and becomes more available for transcription. Histone acetyltransferases (HATs) are one of the main agents responsible for this acetylation and thus “turning on” the gene. On the other hand, if one takes away the acetylation, then gene becomes less active and less available for transcription machinery and thus will be “turned off.” The enzymes responsible for deacetylation and thus “turning off” the gene, are histone deacetylases (HDACs).
As a rule of thumb, in order to enhance gene transcription, one either has to induce HATs and thus induce acetylation or inhibit HDACs and inhibit deacetylation. Indeed, there are cancer chemotherapy drugs that are HDAC inhibitors which enhance gene transcription and “turning on” genes (Maa et al., 2010). One might ask “the genes of cancer cells are already overexpressed and why would one want to express them even more”? Studies have shown that in this specific case, the chemotherapeutic drugs which are HDAC deacetylases and thus gene activators actually turn on and enhance transcription of tumor suppressor genes such as p53 (Ropero et al., 2007). Astonishingly, butyrate which is a SCFAs acts as an HDAC inhibitor in cancerous cells and thus activates their tumor suppressor genes without actually affecting the growth of healthy colonic epithelial cells in rodents (Ropero et al., 2007). Furthermore, high butyrate consumption has protective and anti-inflammatory effects on normal colonic stem or progenitor cells (Kaiko et al., 2016) as again colorectal adenocarcinoma patients have characteristically low fecal butyrate levels (Chen et al., 2013). Additionally, these epidemiological data have been supported by dietary intervention investigations with different human population thus taking genetic variance into account.
SCFAs use similar mechanisms as HDAC inhibitors or “gene activators” to improve the imbalances in T2D. As seen in Figure 4, SCFAs activate and not only enhance genetic expression of pancreatic beta cells that increases insulin levels (Patil et al., 2012a). They also enhance enteroendocrine L-cells gene expression in the gut resulting in increased gut hormone level 1 (GLP-1). Glucagon is also a hormone produced by the pancreas to increase blood glucose levels and it is the main antagonist to insulin with both inversely related with one another. However, increase in gut glucagon like peptide 1 actually increased insulin levels. Although the exact mechanism is unknown (Patil et al., 2012a), this can simply be because of negative feedback actions of GLP-1 on pancreatic glucagon secreting cells thus reducing glucagon secretion resulting in a corresponding increase in insulin secretion.
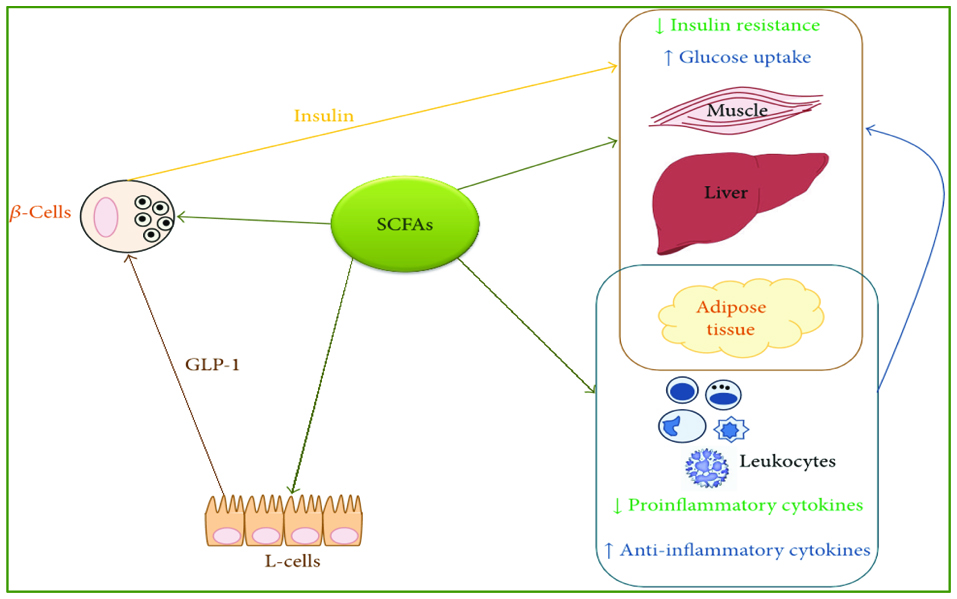 Click for large image | Figure 4. A graphic depiction of SFCAs as HDAC inhibitors and thus genetic inducers of Enteroendocrine L-cells, pancreatic beta-cells, and anti-inflammatory cytokines resulting in increased insulin levels and anti-inflammatory states (With permission; from Patil et al., 2012a). |
In addition to their role in insulin secretion, SCFAs also improve the genetic enhancement of anti-inflammatory cytokines rather than those of pro-inflammatory cytokines (Mariadason et al., 1997; Peng et al., 2009). These actions can overlap and account for their role in metabolic rebalancing. Perhaps SCFAs use the same mechanism of gene enhancements to increase the sensitivity of insulin receptors on muscle, adipose, or liver tissue indirectly via gut hormones, a notion that needs further investigation.
2.4. SCFAs ligands for some orphan G-protein-coupled receptors (GPCRs)
The human genome holds roughly 800 GPCRs. Recently, a cluster of four GPCR genes was recognized in close proximity to the CD22 gene on chromosome 19q13.1, named GPR40 to GPR43 (Brown et al., 2003; Le Poul et al., 2003; Nilsson et al., 2003). These were orphan receptors because although the receptor was discovered, no ligand was found to associate with them. GPCRs was later found to have free fatty acids as their ligand and thus termed free fatty acid receptors (FFARs) (den Besten et al., 2013; Koh et al., 2016). Therefore, with the work of three individual research groups GPR43 and GPR41 were “de-orphanized” and were renamed FFAR2 and FFAR3, respectively (Brown et al., 2003; Le Poul et al., 2003; Nilsson et al., 2003), having SCFAs as their ligands. Future research can follow the steps that come after the activation of these receptors and determine whether a link can be found to increased insulin sensitivity in the main tissues involved in glucose balance and metabolism.
| 3. Microbiome—Another potential for SCFAs | ▴Top |
We used genetic data to compare biological functions of the gut microbiota of diabetic patients with common genes relevant to propionate (a SCFA) expression via enrichment analysis (Figure 5) (The Database for Annotation, Visualization and Integrated Discovery, DAVID: http://david.abcc.ncifcrf.gov). The created graphs comparing the group of genes and their biological processes was obtained using REVIGO method - a java program application called Glassfish 3 (Supek et al., 2011). This is a novel method to identify related genes by measuring the similarity of their global annotation profiles (Supek et al., 2011). Based on their hypothesis, if two gene share similar annotation profiles then they should be functionally related (Supek et al., 2011). Figure 6, illustrates this relationship with the highly similar genes or biological functions linked by edges in the graph, where the width of lines indicates the degree of similarity (Supek et al., 2011). After the analysis of the specific microbiota species and their specific genetic make-up using this novel methodology of data collection, it was found that the gut microbiota of diabetic patients had many similarities in biological processes (Supek et al., 2011) and is in agreement with findings in the literature (Nakamura et al., 2014; Patil et al., 2012a; Vrieze et al., 2012; Laferrere et al., 2011). The comparison using p value significance showed that the gut microbiota of diabetic patients had highly similar biological processes mainly in cytolysis, gamma delta T-cell activation, viral processes, response to epidermal growth, and caveolin-mediated endocytosis and regulation of Golgi inheritance [http://david.abcc.ncifcrf.gov].
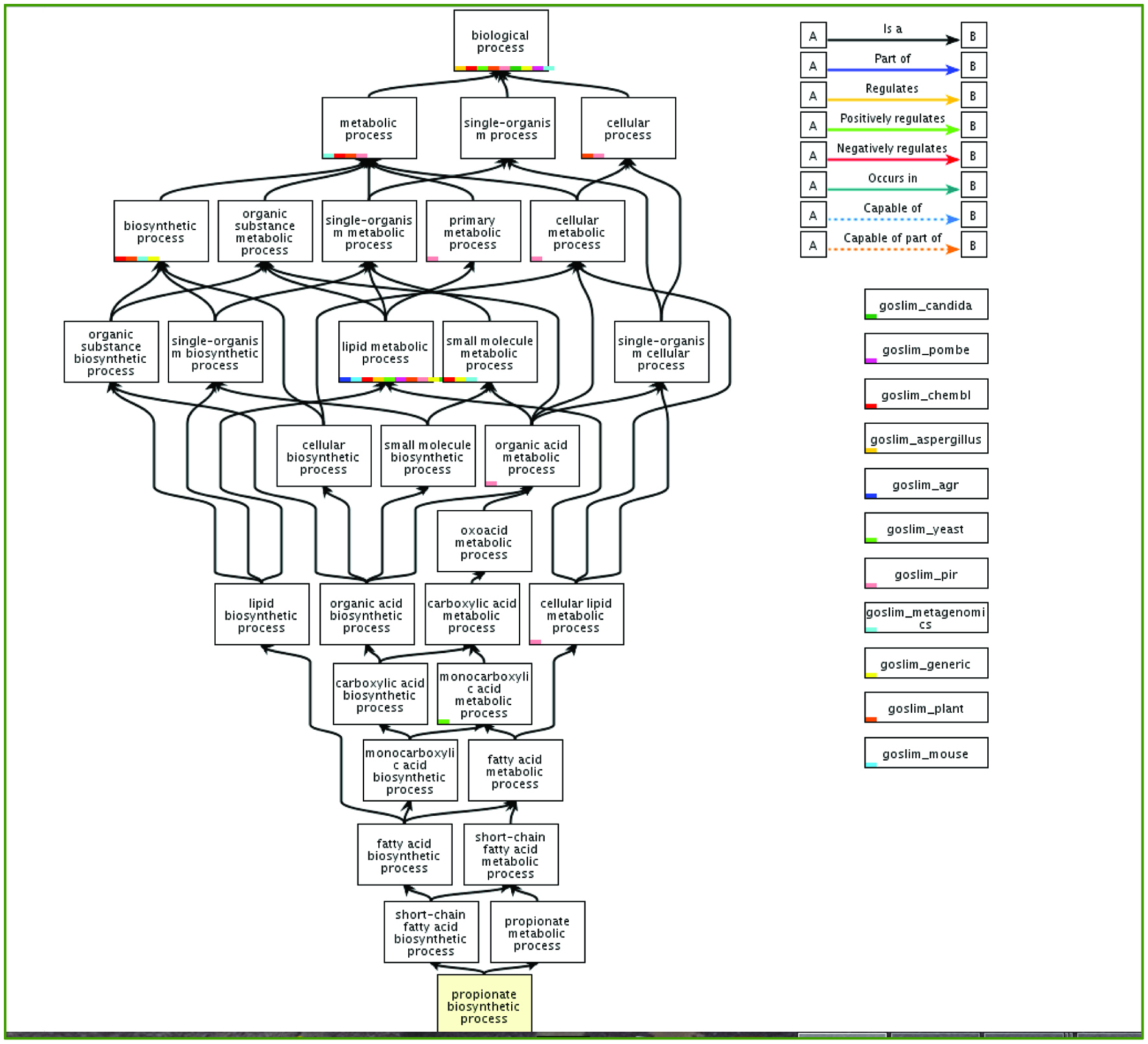 Click for large image | Figure 5. Metabolic and biosynthetic pathway of propionate and its interaction with various components in gut microbiota in humans. (With permission; http://www.geneontology.org/). |
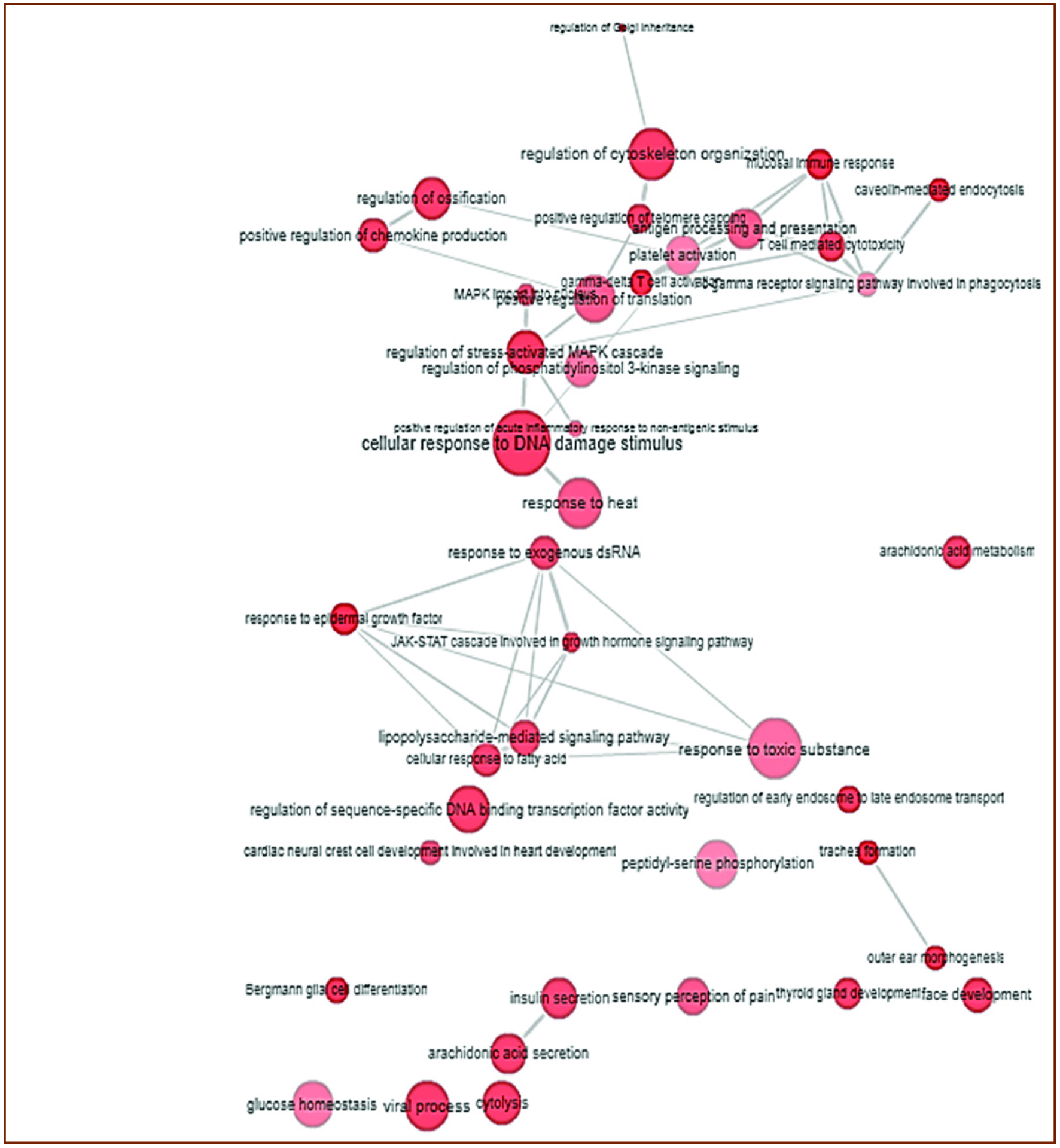 Click for large image | Figure 6. Interactive graph showing the relationship between biological process (Go term) and group of genes regulating the propionate levels in gut microbiota of diabetes (With permission; http://www.geneontology.org/). |
| 4. Discussion | ▴Top |
Insulin resistance and BCAA levels are strongly correlated as evident from numerous studies (Badoud et al., 2014; Herman et al., 2010; Morris et al., 2012; Yoon, 2016). For example, the correlation between BCAA levels and insulin resistance in T2D patients was examined in a longitudinal study involving 429 Chinese adults, 213 of whom had increased risk factors for diabetes while 216 were healthy subjects (Chen et al., 2016). The two groups were followed for 10 years and showed strong correlation between BCAA levels and insulin resistance (Chen et al., 2016). In a different cohort study involving 685 non-diabetic US participants of Caucasian, African American and Hispanic descent, plasma BCAA levels was inversely related to insulin sensitivity and metabolic clearance and positively associated with fasting insulin (Lee et al., 2016). The association of increased T2D risk with high BCAA consumption was also demonstrated in a meta-analysis with 16,097 incidents of T2D during 32 years of follow-up even after stratification for diabetes risk factors, such as obesity (Zheng et al., 2016).
These studies along with several other large studies have validated the association between BCAAs with insulin resistance and T2D, particularly since BCAA biosynthetic pathway is deregulated in insulin-resistant participants (Piening et al., 2018). Meanwhile, it is still crucial to further investigate which of the variables is the initiator when it comes to influencing the other and whether it is insulin resistance leading to increased BCAAs or is it the increased BCAA levels leading to insulin resistance. If it is indeed the BCAA affecting insulin resistance, this can create new avenues for the treatment, preventative prediction, and monitoring of T2D.
While some dim light has been projected on the mechanism between BCAA imbalances and insulin resistance, the mechanisms and the causes are still mostly hidden in the dark. Some have shown that this is simply due to each individual’s genetic make-up, perhaps irrespective of other variables. Genetic predisposition of BCAAs imbalances have been investigated and shown to be interrelated with T2D. For example, in a Mendelian randomized study 1,992 cases of T2D were compared to 4,319 non cases (Lotta et al., 2016) and the study identified 5 common genomes in T2D patients that were associated with increased BCAA levels. Furthermore, high BCAA level was strongly associated with high T2D risk and insulin resistance (Lotta et al., 2016). Insulin resistance causally affected each individual BCAA and inflammation in a recent genome wide association study of 24,925 individuals with data on 58 measured metabolic outcomes (Wang et al., 2017).
On the other hand, is it probable that the intrinsic genetic make-up particularly that of the gut microbiota be influenced by environmental factors such as diet and in particular, special substances in the diet and subsequently influence BCAA levels and consequent insulin resistance. The stomach is the first site where proteins are broken down. Is it possible that some proteins or lipoproteins that now bypass the stomach after stomach bypass surgery and escape breakdown, can now influence the gut cells’ transcription or hormone production? Can similar products with potential to have similar influences be found in nature or synthesized in the lab? Is it possible that although previous studies which controlled the short-term ingestion of diet by having T2D patients fast and then measuring their blood content including BCAAs and glucose overnight, overlooked the middle phenomenon which is the influence of the gut, gut hormones, and microbiota on intrinsic metabolism especially upon overlooking enterahepatic circulation during the fast? Is it possible that it is rather the gut hormones and microbiota’s genes and products dictating the amount and type that gets absorbed from the intestinal lumen thereby modulating the behavior of enterocytes and their cytoplasmic mechanism through receptor mediated signal transduction via secondary mediators? Perhaps the gut hormones or microbiome products regulate the quantity of BCAA that gets through (via tight junctions) or into the enterocytes regardless of the diet. The microbiome products probably can directly induce enzymatic actions on BCAAs within the lumen, in the same way exocrine pancreatic enzymes or bacterially produced lactase work, almost independent of enterocytes. On the other hand, it can be gut hormones or microbiome acting indirectly on BCAA metabolism through enterocytes. The mechanism can be acting not only as gatekeepers of what gets in the cells from the lumen and out of the cells into the blood, but also on the tight junctions located in between the cells. The gut hormones or microbiome as commanders may lock in with enterocyte receptors and communicate how much of for instance BCAAs need to be broken down within enterocytes before they reach the hepatic portal vein. Additionally, the gut hormones might be exerting their effects not only on enterocytes via enterohepatic circulation, but also on the organs of metabolism such as liver, muscle, or adipose tissue via systemic circulation. Within the systemic circulation there can be further “cross-talk” with other intermediator proteins that convey the message of gut hormones on their behalf, to the organs of metabolism.
Then is the bigger question of whether the diet can influence the level of gut hormones and the composition of microbiota. More light needs to be shed on the concept that diet and in particular specific diets such as SFCAs can change the genetic expression of microbiome and thus the type and amount of their released products which could then influence pathways inside enterocytes including those for BCAA absorption and metabolism.
The fermentation of dietary fibers leads to increased SCFAs production via various biochemical pathways. These pathways and the distribution of SCFAs and their receptors are illustrated in Figure 7. The SCFAs enter the cells through diffusion or SLC5A8-mediated transport and act as an energy source or an HDAC inhibitor in the distal gut (Koh et al., 2016). Luminal acetate or propionate via binding on GPR41 and GPR43 of enterocytes releases PYY and GLP-1 by the enterocytes into the bloodstream, which then affect satiation and intestinal transit thereby influencing BCAA intake and absorption respectively (Koh et al., 2016). Furthermore, propionate is converted into glucose by intestinal gluconeogenesis leading to satiation and decreased hepatic glucose production (Koh et al., 2016) which may be beneficial in T2D patients. Butyrate on the other hand exerts anti-inflammatory effects by crossing into the extracellular space and acting on GPR109A receptors in the immune cells of the lamina propria (Koh et al., 2016). Acting as an HDAC inhibitor, butyrate induces and activates the transcription machinery of these immune cells to produce anti-inflammatory cytokines (Koh et al., 2016) which can further have beneficial metabolic effects via protective mechanisms. SCFAs also act on other sites in the gut such as the enteric nervous system (ENS) which controls motility and secretory activity (Koh et al., 2016) and resultant BCAA absorption. Small amounts of SCFAs, mostly acetate and propionate can potentially reach the circulation and directly influence tissues and organs of metabolism such as adipose, muscle, and liver cells (Koh et al., 2016) with potential to directly impact intracellular BCAA metabolism.
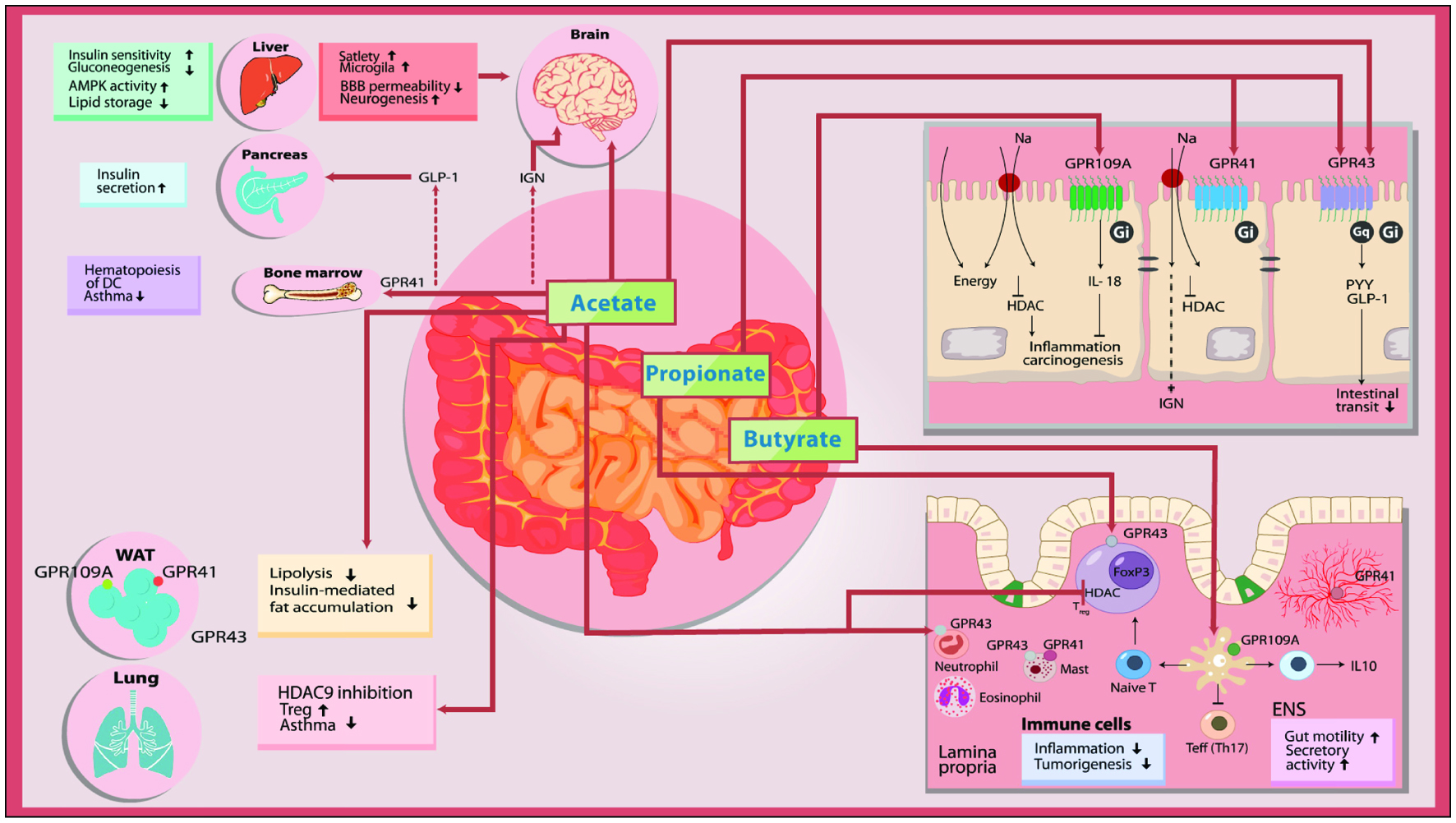 Click for large image | Figure 7. Schematic view of the mechanism of microbial SCFAs. |
After gastric bypass surgery, in addition to the increased incretins such as GLP-1, there was also large amounts of the gut hormone peptide YY3-36 and oxyntomodulin (OXM) that were secreted by intestinal endocrine L cells (Laferrere et al., 2011). These two hormones of course regulate food intake, but also modulate liver and adipose tissue metabolic pathways, which include BCAA metabolism (Laferrere et al., 2011). This rise in gut hormones was not seen in the closely controlled dietary intervention group in this study (Laferrere et al., 2011), suggesting that these are intrinsic properties and not highly diet dependent. In other words, the metabolic benefits particularly for BCAA rebalancing and T2D in the gastric bypass group were not observed in the dietary intervention group. On the other hand, since the dietary intervention was a generic dietary modification, it is possible that certain special diets and increased ingestion in the amounts of special substances from the diet can have similar beneficial effects of gastric bypass. The authors of the bypass surgery study acknowledged that the metabolic pathways and mechanisms involving gut hormones having a major influence on the staggering changes observed after gastric bypass surgery should be thoroughly investigated (Laferrere et al., 2011). Meanwhile, perhaps a particular specific substance ingested in high amounts, can also play a similar central role in metabolism especially via actions on the microbiome, BCAA breakdown, and insulin sensitivity. This can then not only be a novel approach in treating T2D, but could also result in similar affects to that of gastric bypass surgery such as increased satiety (Laferrere et al., 2011) hence decreasing the need for this procedure. The high amount of a specific substance in diet can potentially have similar effects to gastric bypass surgery, in particular the rebalancing of BCAAs (Laferrere et al., 2011). These similar effects can be as a result of increasing gut hormones which can affect food intake via actions on satiety centers, food absorption by acting on intestinal lumen, and metabolism by acting on organs of metabolism via systemic circulation.
To reiterate the notion that insulin resistance is associated with intrinsic microbiome genetic properties independent of diet, studies have shown that replacement of fecal microbiota from lean to obese subjects improved insulin sensitivity (Vrieze et al., 2012). Specifically, modulation of the gut microbiota composition leads to increased butyrate production and result in suppression of body weight gain and insulin resistance in high fat fed and obese mice and these effects were shown to depend on FFAR2 expression (Kimura et al., 2011). Additionally, gut microbiota “transplantation” independent of diet, variations in plasma levels of BCAAs and aromatic amino acids all have been correlated with insulin resistance and T2D (Neis et al., 2015).
Other studies have shown that dietary changes can indeed modulate the composition of the gut microbiota, and its metabolic products (Musso et al., 2010). Dietary fibers through SCFAs can also exert the corrective metabolic actions via their anti-inflammatory effects particularly in the gut (Puddu et al., 2014). This anti-inflammatory effects of SCFAs is evidenced via induction of anti-inflammatory cytokines (Puddu et al., 2014). Even the enterocyte receptors that the SCFAs project their metabolic and anti-inflammatory influences have specifically been identified which turn out to be G protein coupled receptors (Puddu et al., 2014). The receptors are FFAR2 and FFAR3, and upon stimulation relay information via 2nd messenger cascades to downregulate CXC chemokine receptor 2 (CXCR2) expression on enterocytes, a receptor that attracts white blood cell migration (Maa et al., 2010). Further studies on these receptors showed that diets rich in propionate via stimulation of the same FFAR3 receptor can lead to decreased food intake (Puddu et al., 2014). Interestingly this was supported by (Musso et al., 2010)) via their demonstration and derivation of the following equation: F + 10l – 120 = 0, in which L represents the level of propionate and F represents Food intake. In addition, these receptors also play a minor role in propionate-dependent induction of glucose-dependent insulinotropic peptide. In a different study and as another example, the SCFAs propionate and butyrate exert stimulatory effects on anorexigenic gut hormones (Musso et al., 2010). Once again this was demonstrated by (Musso et al., 2010) with the derivation of yet another equation: A + 10L − 110 = 0, in which A refers to anorexigenic gut hormones in human and L refers to between level of propionate.
Specifically, dietary fiber intake is related to increased SCFAs profiles while a high-fat diet in contrast has been associated with reduced SCFAs levels and even an increase in gut lipopolysaccharide (LPS) levels (Amar et al., 2008) which is found on the outer membrane of Gram-negative bacteria. LPS can influence intestinal permeability and this is evidenced by metabolic endotoxemia a condition when endotoxins can enter the circulation because of LPS induction on enterocytes leading to release of pro-inflammatory cytokines (Mariadason et al., 1997; Peng et al., 2009) and subsequent increased permeability. In contrast, incubation of a human colonic epithelial cell line with butyrate increased transepithelial resistance by promoting the assembly of tight junctions (Mariadason et al., 1997; Peng et al., 2009) and thus decreased intestinal permeability and absorption. It is worth highlighting that LPS has also mediate critical activities underlying insulin resistance (Cucak et al., 2014; Garay-Malpartida et al., 2011) and of course, whether this is through their direct or indirect actions on BCAAs remains to be clarified.
| 5. Conclusions | ▴Top |
The type of food can stimulate different gut hormones or microbiota’s secretion of products, which then can influence metabolism. Thus, it may not necessarily be the diet per se, but the diet’s effect on the metabolic characteristics of the individual, which regardless of the diet, has unique characteristics and can respond differently to different diets. Still this will not stop further research in finding at least some universal common denominators such as SCFAs that can influence the gut, gut hormones, and microbiome uniformly that in this specific case for example, allow for less BCAA or increased metabolism. The identification of the microbiome content of a diabetic individual using PCR could have been a major piece of the puzzle that was overlooked and perhaps the major role player in the intrinsic metabolism of any individual. Perhaps again, a common denominator such as SCFAs can have general positive metabolic effects regardless of the vast genetic variability of a person’s microbiome or have similar effects in persons with similar disorders such as diabetes. As discussed in the report some studies compared the genetic make-up of the subject’s microbiota to delineate microbiota acts dependent or independent of specific diets such as SCFAs in metabolic disorders. If future studies were to be conducted that compared two controlled groups, perhaps one of the controlled variables should be the genetic make-up of the microbiota. Then a further step can be made to compare those with a “good” microbiome genetic make-up and the type of species they carry in their gut, with those who have a “less good” make-up. Similar to gut hormones, products secreted by the microbiota, can be the deal breaker in how much they directly act upon the BCAAs in the lumen before absorption, how much of the BCAAs get absorbed, and later how much the BCAAs will be acted upon indirectly through gut hormone or microbiota product actions on enterocytes, hepatocytes, adipocytes, myocytes, pancreatic B-cells, or even on the central command center: the hypothalamus (fat soluble products can be examined with potential to cross blood brain barrier). Further studies can settle some of the dust by having groups with similar BCAA blood compositions and numerous other metabolic variables, and ingestion of the same diet, examined after the experiment for glycemic, metabolic, and insulin type variables, but also for their gut microbiota and gut hormones. If one group was to have better glycemic controls, it may very well originate from differences in the gut behavior and composition and be the focal point overshadowing the responsibility of metabolic imbalances. Then upon identification of commonalities in gut traits, one can target specific variables using specific diets such as SCFAs. This can lead to novel approaches with the focus on BCAA re-balancing in the treatment of diabetes and other metabolic syndromes. Simultaneously, if BCAAs are major role players, they can also be used for newer and more sensitive preventative measurements for future risk assessment of diabetes as well as vascular or inflammatory stress-induced pathologies.
This article is dedicated in the memory of Dr. Vahid Hosseinian, a pediatrician who helped many sick children.
| References | ▴Top |