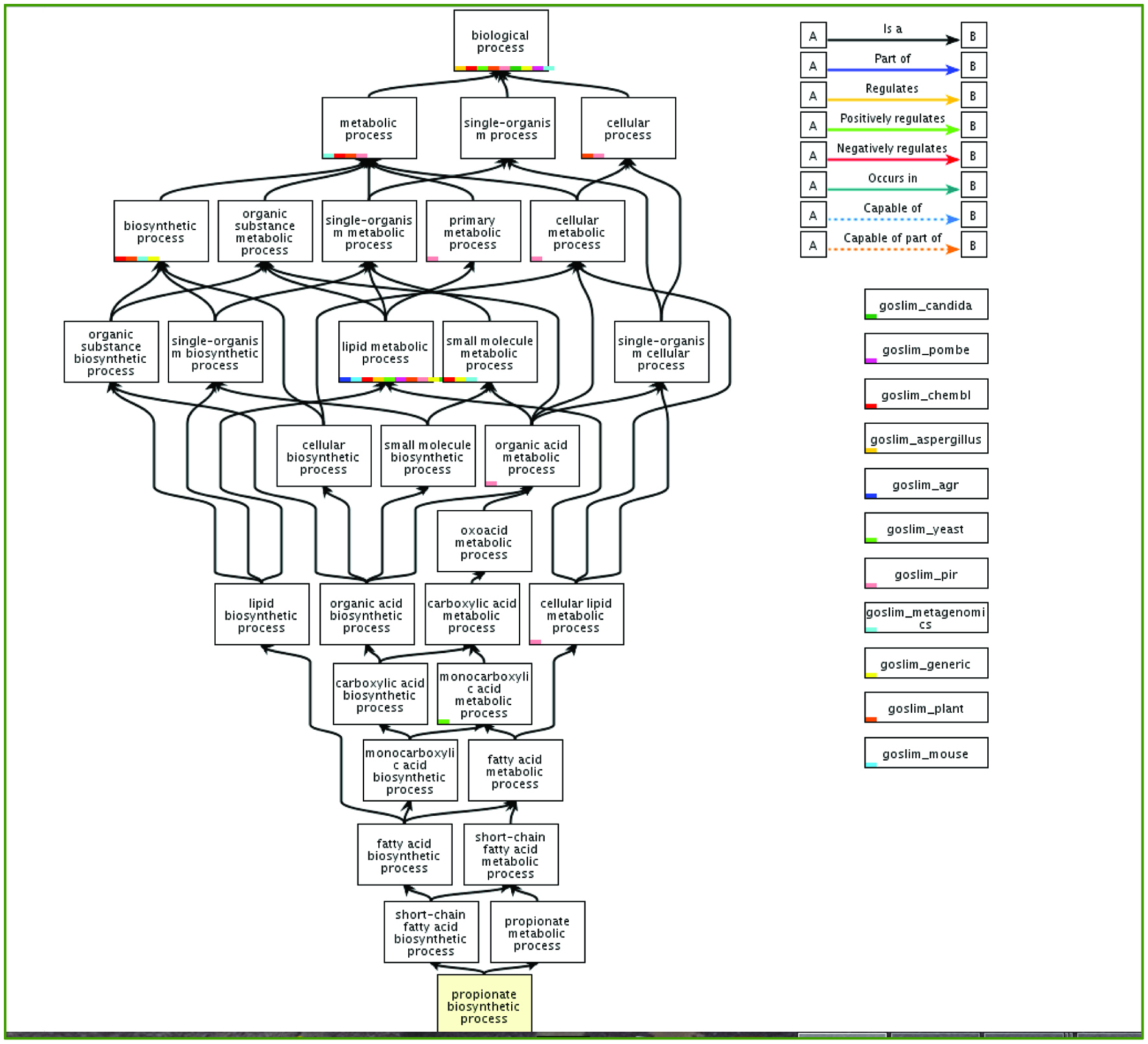
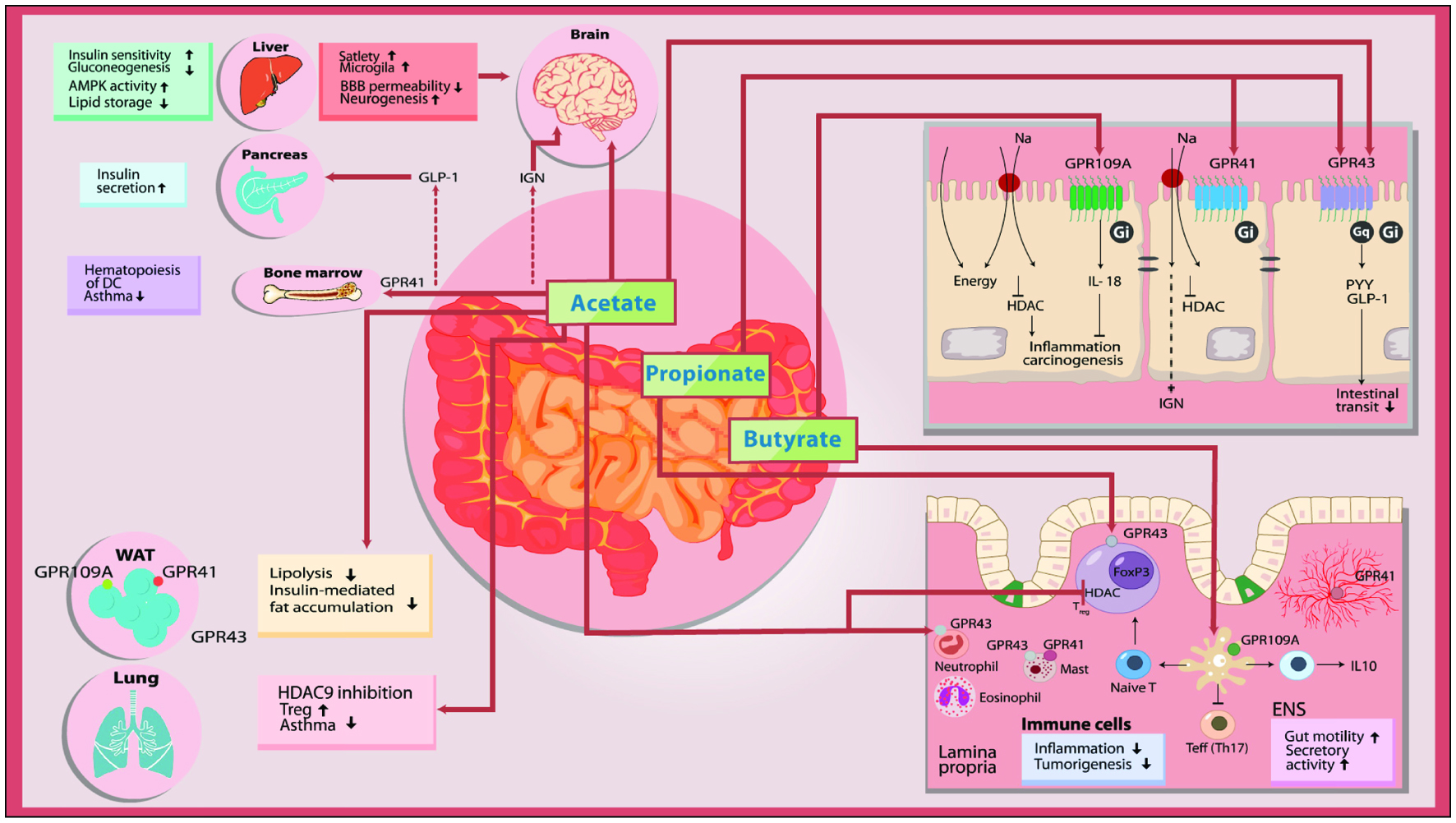
Healthy African Americans, 50–65 y (20)
Healthy South Africans, 50–65 y (20) | Own diet (2 w) followed by exchange to high-fiber, low-fat African-style (2 w)
Own diet (2 w) followed by high-fat, low-fiber Western-style (2 w) | African style diet: ↑ butyrate; reciprocal changes in colon cancer risk biomarkers | O’Keefe et al., 2015 |
| Healthy volunteers (23) | Cross-over: high red meat (HRM) diet vs. HRM plus butylated high-amylose maize starch (HAMSB) (4/4 w wash-out) | HRM+HAMSB diet:↑ excretion of SCFAs and microbiota composition changes | Le Leu et al., 2015 |
| Healthy active volunteers (51) | Parallel-groups: butylated high amylose maize starch (HAMSB) vs. low-AMS (28 d) | HAMSB diet:↑free, bound and total butyrate and propionate | West et al., 2013 |
| Healthy volunteers, 20–50 y (17) | Cross-over: whole-grain (Newgard et al.) vs. refined grain (2/5 w wash out) | WG diet: ↑acetate and butyrate | Ross et al., 2013 |
| Healthy volunteers, 18–85 y (63) | Cross-over: wheat bran extract (WBE) (3 or 10 g WBE) vs. placebo (0 g WBE; 3 w, 2 w wash-out) | Daily 10 g intake WBE:↑bifidobacteria;↑ fecal SCFAs and ↓ fecal pH | Francois et al., 2012 |
| Healthy volunteers, 18–24 y (60) | Parallel-groups: xylo-oligosaccharide (XOS) vs. inulin-XOS mixture (INU-XOS) vs. placebo (maltodextrin; 4 w) | XOS: ↑bifidobacteria and butyrate, and ↓acetate INU-XOS: ↑SCFAs, especially propionate, maintain acetate level | Lecerf et al., 2012 |
Ulcerative colitis (UC) remission patients (19)
Healthy volunteers (10) | Cross-over: Australian diet vs. plus wheat bran-associated fiber and high amylose-associated resistant starch (8 w) | Intervention diet: did not correct the low gut fermentation in patients with UC | James et al., 2015 |
Irritable bowel syndrome
with constipation woman, 20–69 y (32) | Parallel-groups: Milk acidified product vs. Fermented Milk product (FMP) (4 w) | FMP:↑potential butyrate producers, and ↑Total SCFAs in vitro↑butyrate | Salazar et al., 2015 |
IBS patients (27)
Healthy volunteers (6) | Cross-over: Australian diet vs. low FODMAP (Fermentable Oligo-, Di-, Mono-saccharides And Polyols) diet (21/21 d wash-out) | Australian diet:↑ relative abundance ClostridiumclusterXIVa (butyrate-producer)
Low FODMAP diet:↓total bacterial abundance | Halmos et al., 2015 |
| Cow’s milk protein allergy infants (16) | Cross-over:hydrolysed whey protein formula (eHF) without lactose vs. eHF containing 3.8% lactose (2 m) | Addition of lactose: ↑SCFAs; ↑LAB and bifidobacteria; ↓Bacteroides/clostridia | Di Cagno et al., 2011 |
| Obese women 18–65 y (30) | Parallel-groups: ITF vs. placebo (maltodextrin) (3m) | ITF:↓ total SCFAs, acetate and propionate; ↑bifidobacteria | Salazar et al., 2015 |