| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 11, September 2020, pages 75-83
Phenolic antioxidants of bael fruit herbal tea and effects on postprandial glycemia and plasma antioxidant status in healthy adults
Anoma Chandrasekaraa, *, Thavanthen Janukaa, Disna Kumaria, Adriano Costa de Camargob, Fereidoon Shahidic
aDepartment of Applied Nutrition, Wayamba University of Sri Lanka, Makandura, Gonawila, Sri Lanka, 60170
bLaboratory of Antioxidants, Nutrition and Food Technology Institute, University of Chile, Santiago, Chile
cDepartment of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada, A1B 3X9
*Corresponding author: Anoma Chandrasekara, Department of Applied Nutrition, Wayamba University of Sri Lanka, Makandura, Gonawila, Sri Lanka, 60170. Tel: +94 (031) 2298120; E-mail: anomac@wyb.ac.lk, anomapriyan@yahoo.ca
DOI: 10.31665/JFB.2020.11239
Received: August 11, 2020
Revised received & accepted: September 13, 2020
| Abstract | ▴Top |
Herbal teas are globally popular among health conscious consumers. In this study the phenolic content and potential antioxidant activities of bael fruit herbal tea prepared with dried immature bael fruit cuts (Aegle marmelos), traditionally used in the Asia, were determined. Phenolic compounds of the herbal extracts were identified using liquid chromatography-tandem mass spectrometry. The total phenolic content (TPC) and antioxidant activities of the tested herbal extract was determined. The amount of herbal material to be used in the tea preparation was established based on the sensory evaluation conducted with 50 untrained adult panelists. The single dose efficacy of the bael fruit herbal tea on postprandial glycemic response and plasma antioxidant capacity (PAC) of healthy adults were investigated. A randomized crossover study was carried out with 16 healthy adults who consumed 250 mL bael fruit tea with 50 g glucose challenge and the control (50 g glucose in 250 mL water) randomly within two visits. Blood samples were collected at the baseline and postprandial at 30, 45, 60, 90, and 120 min using microcapillary tubes. The plasma was analyzed for glucose concentration (PGC) and PAC. The TPC of bael fruit tea extract was 108.3 µmol gallic acid equivalents/g of extract. There was a reduction trend in mean PGC of those subjects who consumed bael fruit tea compared to the glucose added water (control) at each time point. Furthermore, the bael fruit tea significantly increased PAC at the end of 120 min post ingestion. Further research is warranted to examine the long-term efficacy of multiple dose ingestion of bael fruit herbal tea in the control and management of diseases associated with oxidative stress.
Keywords: Aglae marmelos; immature fruits; HPLC; TPC; single dose ingestion; Sri Lanka
| 1. Introduction | ▴Top |
Oxidative stress is a known underlying factor of metabolic syndrome and associated health conditions such as cardiovascular disease, diabetes, hypertension, as well as liver and kidney disorders (Iannitti and Palmieri, 2009). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) play a pivotal role in production of secondary complications of diabetes. Hyperglycemia leads to the generation of ROS which can damage cell membranes and form lipid peroxides which lead to atherosclerosis.
There is a renewed recent interest on certain plants as complementary alternative medicine to prevent and manage non-communicable disease (NCD) conditions associated with dietary and lifestyle behavior. Plant products are rich in bioactive compounds and are more active in natural formulations than those of isolated components due to the synergistic, additive or antagonistic actions among compounds (Caesar and Cech, 2019).
Herbal teas play an important role among commonly consumed beverages such as tea, coffee and cocoa in the food cultures of many countries (Chandrasekara and Shahidi, 2018). Sri Lankan herbal teas are conventionally used as an alternative to regular tea (Camellia sinensis) and are popular among health-conscious consumers. In recent years, herbal teas have received much attention as natural health promoting products (Li et al., 2019). The type of herbal teas commonly consumed in different countries may be affected by local habits and availability of the respective plants. Herbal teas contain a number of bioactive compounds belonging to the group of carotenoids, phenolic acids, flavonoids, coumarins, alkaloids, polyacetylenes, saponins and terpenoids, among others. These bioactive compounds are responsible for several health effects, such as antioxidant, antibacterial, antiviral, anti-inflammatory, antihyperglycemic, antihyperlipidemic, anticarcinogenic and antiaging activities (Akalin et al., 2019; Chandrasekara and Shahidi, 2018; Chandrasekara et al., 2018; Kamalakkannan and Prince,2005, 2003).
Aegle marmelos, known as Bengal quince, Indian quince, holy fruit, golden apple in English, and bael in Hindi and bilva in Sanskrit, belongs to the family Rutaceae. Bael is a tropical plant and is native to the Southeast Asian region in India, Sri Lanka, Pakistan, Bangladesh, Mianmar, and Thailand. Although the ripen fruit is most popular, different parts of bael tree, namely flowers and buds, unripe fruit, seeds, leaves, bark, branches, and roots are used for the treatment of a number of ailments in traditional Ayurvedic medical system (Dhankhar et al., 2011). The bark as well as fruit are used as ethnomedicine for dysentery and various intestinal complaints. Several bioactivities, such as antiulcer, antimalarial, anti-inflammatory, radioprotective, analgesic, antibacterial, antiviral, anti-dyslipidemic, anticancer and antidiabetic activities of parts of bael tree have been reported (Dhankhar et al., 2011; Kamalakkannan and Prince, 2005; Rajadurai and Prince, 2005; Arul et al. 2005; Jagetia et al. 2004; 2005; Dhuley, 2004; Badam et al. 2002; Misra et al. 1991). Coumarins, alkaloids, tannins and other phenolic compounds, and carotenoids, are reported in different parts of bael tree (Baliga et al. 2011; Dhankhar et al. 2011; Maity et al. 2009). In a previous study, we reported the presence of several phenolic acids, namely vanillic, p-coumaric, chlorogenic, caffeic, and gentisic acids along with some flavonoids, mainly catechin, and quercetin in the extracts of dried bael flowers and buds (Chandrasekara et al. 2018).
Bael fruit is a source of vitamin C, vitamin A, thiamin, niacin and riboflavin as well as calcium and phosphorus. The ripe fruit is slightly sweet and is characteristically aromatic. The pulp of fully ripen Thai bael fruit contain both soluble, and insoluble dietary fiber at 11.2, and 8.6 g/100 g dry weight (dw), respectively (Suvimol and Pranee, 2008). Fruits of bael have been reported to contain several bioactive compounds such as marmelosin, marmelide, luvangetin, aurapten, psoralen, aegelin, scoparone, scopoletin, tannic acid, xanthotoxol and β-sitosterol and tannin (Maity et al., 2009; Kamalakkannan and Prince, 2005). Immature bael fruit cuts are dried and used to prepare tea by open boiling or as tea bags after making a powder in Sri Lanka.
A number of therapeutic effects of mature bael fruit are reported (Baliga et al., 2011; Dhankhar et al., 2011; Krushna et al., 2012). However, studies on beneficial health effects improved plasma antioxidant activities and hypoglycemic response of dried immature bael fruit tea upon ingestion have not been reported. The present study aimed to determine the phenolic content and antioxidant activities of teas prepared with dried immature bael fruits. Furthermore, the potential short-term efficacy of bael fruit herbal tea on postprandial glycemic response and plasma antioxidant capacity of healthy adults were evaluated.
| 2. Materials and methods | ▴Top |
2.1. Herbal samples
Dried cuts of immature bael fruits (Aegle marmelos) were purchased from a local market in North Western Province in Sri Lanka. All samples were cleaned to remove debris and dust and dried under sunlight before use.
2.2. Chemicals
Sodium carbonate and ferric chloride were procured from Thomas Baker (Chemicals) Ltd. (Mumbai, India). Folin-Ciocalteu’s reagent and sodium nitrite were purchased from Research Lab Fine Chem Industries (Mumbai, India). The compounds 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate)(ABTS), trolox, ferric chloride, ferrous chloride, 2,2-azobis (2-methylpropionamidine) dihydrochloride (AAPH), potassium ferricyanide, sodium chloride, gallic acid, catechin, ascorbic acid and methanol, were purchased from Sigma-Aldrich, (St Louis, MO, USA). The compound 3-(2-pyridyl)-5, 6-diphenyl-1,2,4-triazine-4,4-disulfonic acid sodium salt (Ferrozine) was bought from SERVA Electrophoresis GmbH (Heildberg, Germany). Aluminum chloride, and dibasic potassium phosphate were purchased from Techno Pharm Chem (Delhi, India). Sodium hydroxide (NaOH), and monobasic potassium phosphate, were bought from Loba Chem Pvt Ltd (Mumbai, India). Ethylenediaminetetraacetic acid trisodium salt (Na3EDTA) was purchased from Needham Market (Suffolk, UK). Ellagic acid, protocatechuic acid (+)-catechin, (−)-epicatechin were purchased from Sigma Aldrich Canada Ltd (Oakville, ON, Canada). Acetonitrile, and formic acid, were procured from Fisher Scientific Ltd (Ottawa, ON, Canada). Glucose GOD-PAP reagent was purchased from BIOLABO (Maizy, France).
2.3. Herbal aqueous extracts preparation
The hot water extracts were prepared by boiling dried immature fruit cuts of bael in water (1:15; w/v) at 100 °C for 20 min. The residues were filtered through medium porosity filter papers and extracts were freeze dried at −55 °C, and 0.012 mbar (Alpha 1-4 LD plus CHRIST, Osterode am Harz, Germany). Lyophilized extracts were stored at −80 °C until used for further analysis.
2.4. Identification and quantification of phenolic compounds—HPLC-DAD-ESI-MSn analysis
Phenolic composition of herbal tea aqueous extracts was determined by RP-HPLC analysis using an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with a G1311A quaternary pump, a G1379A degasser and a G1329A ALS automatic sampler, a G1330B ALS Therm, a G1316A COLCOM column compartment, a G1315B diode array detector (DAD) and a system controller linked to Chem Station Data handling system (Agilent Technologies) as explained by de Camargo et al. (2015). Separations were conducted with a SUPELCOSILTM LC-18 column (4.6 × 250 mm, 5 μm; Merck, Darmstad, Germany). The binary mobile phase consisted of 1% formic acid (eluent A) and 1% formic acid in acetonitrile (eluent B). Gradient elution was used as follows: 0 min, 100% A; 5 min, 90% A; 35 min, 85% A; 45 min, 60% A; 45 to 50 min 60% A was maintained from 50 to 55 min eluent A was increased and 100% A at 55 min. The flow rate was adjusted to 0.5 mL/min and the detection of compounds was achieved at 280 nm. Samples were filtered through a 0.45 µm PTFE membrane syringe filter (Thermo Scientific, Rockwood, TN, USA) before injection.
Phenolic compounds were identified by comparing their relative retention times (RT), and UV spectra and ESI-MS spectra with authentic compounds. HPLC-MS analysis was performed under the HPLC analytical conditions explained above using an Agilent 1100 series capillary liquid chromatography/mass selective detector (LC/MSD) ion trap system in electrospray ionization (ESI) negative ion mode. Complete system control and data evaluation were achieved with Agilent LC/MSD Trap software (Agilent Technologies). The mass spectrometer was operated in scan range of m/z 50–2,000; smart parameter setting using a drying gas (N2) temperature of 350 °C, drying gas flow 12 L/min, and nebulizer gas (N2) pressure of 70 psi. A number of compounds, present in the extracts were tentatively identified using ESI-MSn and UV spectral data and literature and quantified as equivalents of the closely related available standards. The external standard method in which reference compounds were chromatographed under similar chromatographic conditions separately from samples was used for quantification purposes.
2.5. Chemical analysis of test herbal extract
2.5.1. Determination of total phenolic content (TPC)
The total phenolic content of herbal extracts was determined according to the method of Singleton and Rossi (1965) with slight modifications (Chandrasekara et al, 2018). Briefly, the lyophilized crude extract of herbal tea was dissolved in methanol to obtain a concentration of 1 mg/mL. Folin Ciocalteu reagent (0.25 mL) was added to 50 mL centrifuge tubes containing 0.25 mL of extract and the contents were mixed thoroughly by vortexing. To the reaction mixture 0.5 mL of saturated sodium carbonate solution was added followed by the addition of distilled water (4 mL) with thorough mixing. Tubes were allowed to stand at room temperature in the dark for 35 min followed by centrifugation at 4,000 × g for 10 min (Refrigerated centrifuge 3-18R TOMOS Life Science Group, Belmont, MA, USA). The absorbance of the resulting blue color supernatant was read at 725 nm (UV-VIS Spectrophotometer, Labomed Inc, Culver City, CA, USA) using appropriate blanks for background subtraction. The content of total phenolics in the extract was determined using a standard curve prepared for gallic acid and expressed as µmol gallic acid equivalents (GAE) per gram of crude extract.
2.5.2. Determination of total flavonoid content (TFC)
Total flavonoid content was determined using a colorimetric method as previously described (Kim et al, 2003). In brief, a known volume (1 mL) of aliquot of the extract, dissolved in methanol (1 mg/mL), was mixed with 4 mL of distilled water in a 50 mL centrifuge tube and 0.3 mL of 5% NaNO2 was then added to the tube and allowed to react for 5 min. Subsequently, 0.3 mL of 10% AlCl3 was added to the reaction mixture and allowed to stand for 1 min. Finally, 2 mL of 1M NaOH and 2.4 mL of distilled water were added and mixed immediately. Centrifuge tubes were kept in the dark at room temperature for 15 min followed by centrifugation at 4,000 × g for 5 min. The absorbance was read at 510 nm against a blank prepared in a similar manner by replacing the extract with distilled water. A standard curve prepared with catechin was used to calculate total flavonoid content which was expressed as µmol catechin equivalents (CE) per gram of crude extract.
2.5.3. DPPH radical scavenging activity (DRSA)
The DPPH radical scavenging ability was determined by the method of Lee et al. (2007). The sample of 0.04 mL of extract (1 mg/mL in methanol) was added to the 1.96 mL of methanolic DPPH (60 µM) solution. The mixture was vortexed and allowed to stand at room temperature in the dark for 20 min. The absorbance of the solutions was read at 517 nm with appropriate blank. The DPPH radical scavenging activity was expressed as µmol trolox equivalents (TE) per gram of crude extract.
2.5.4. Trolox equivalent antioxidant capacity (TEAC)
The TEAC of the extract was determined as previously explained (Chandrasekara and Shahidi, 2010). AAPH (2.5 mM) was mixed with ABTS (100 mM) in saline phosphate buffer (PBS) (pH 7.4, 0.15 M NaCl) to prepare the ABTS•+ solution. The solution was kept in a water bath at 60 °C for 16 min and the flask was covered by aluminum foil to protect it from light. Medium porosity filter papers were used to filter the prepared ABTS•+ solution before mixing with the extract. A separate blank was used to compensate for the diminished absorbance of radical solution. PBS solution was used to prepare herbal extract (1 mg/mL) and further diluted to fit within the range of 6.25–50 μM of the standard curve prepared using trolox. To measure the TEAC, 40 μL of the extract were mixed with 1,960 μL of the ABTS•+ solution. The absorbance of the reaction mixture was read at 734 nm immediately at the point of mixing (t0) and after 6 min (t6). The decrease in absorbance at 734 nm after 6 min of addition of trolox and extract was calculated using the equation: ΔA trolox = (At0, trolox − At6, trolox) − (At0, blank − At6, blank), where ΔA is the reduction of absorbance and A is the absorbance at a given time. TEAC values were expressed as µmol trolox equivalents (TE)/g of crude extract.
2.5.5. Reducing power (RP)
The RP of the sample was determined using a spectrophotometric method as explained by Kumari et al. (2017). Briefly, the extract (0.5 mL) was mixed with 1.25 mL of phosphate buffer solution (0.2 M, pH 6.6) and 1.25 mL of potassium ferricyanide in a centrifuge tube. The mixture was incubated for 20 min at 50 °C and 1.25 mL of 10% TCA were added followed by centrifugation at 1,750 × g for 10 min. The supernatant (1 mL) was transferred into a tube containing 1.25 mL of deionized water and 0.25 mL of 0.1% (w/v) FeCl3, and the absorbance was read using a spectrophotometer at 700 nm. The results were expressed as µmol ascorbic acid equivalents (AAE) per gram of crude extract using a standard curve prepared using ascorbic acid.
2.5.6. Ferrous ion chelating activity (FICA)
The ability of herbal tea extract to chelate ferrous ions was measured according to the method described by Kumari et al. (2017). The crude extract in distilled water (1 mg/mL) was used to measure chelating activity of ferrous ions. Briefly, 0.4 mL of extracts was added to a solution of 2 mM FeCl2 (0.05 mL) followed by addition of 5 mM ferrozine (0.2 mL). The total volume was adjusted to 4 mL with distilled water followed by vigorous vortexing. After standing for 10 min at room temperature, the absorbance of the reaction mixture was read at 562 nm. Distilled water was used in place of the extract as the control. Appropriate blanks were prepared with 0.4 mL of the sample and 3.6 mL of distilled water for background subtraction. The inhibition percentage of ferrozine-ferrous ion complex formation was calculated using the following equation. Metal chelating effect (%) = {1 − (Absorbance of the sample/Absorbance of the control)} × 100. The percent inhibition of ferrozine-ferrous ion complex formation was calculated by the following equation: metal chelating effect (%) = [1 − (absorbance of the sample − absorbance of the control)] 100. The results were expressed as µmol EDTA equivalents/per gram of crude extract
2.6. Herbal tea preparation
Three formulations of bael fruit tea were prepared with dried immature fruit cuts of bael in water (1:40, 1:20, and 1:10; w/v). The quantities were based on the customary use of ingredients in Sri Lankan households. In preparation of the tea, ingredients were added to water and then allowed to boil for 20 min in medium heat followed by 20 min standing at room temperature. Residues were discarded by straining and the warm tea was used.
2.7. Sensory evaluation of herbal teas
Sensory properties were evaluated to determine the optimum level of herbal ingredient in the tea preparation. Fifty untrained panelists (25 males and 25 females in the age range of 24–40 years) were recruited for the sensory evaluation. The panelists were mainly students (80%) and university staff (20%) of the Makandura premises of Wayamba University of Sri Lanka. Three herbal teas, 50 mL each and coded with three digits, were served randomly to each panelist. Teas were served at the temperature range of 40–50 °C in transparent glass cups. The panelists rinsed their mouths with warm water before the commencement of tasting and in between each tasting of herbal teas. The panelists were instructed to wait 90 seconds before tasting the next sample and cracker biscuit was provided between tastings to eliminate any carry over effect. The panelists were not required to drink 50 mL of each bale fruit tea. They were asked to keep one sip of tea for 5 seconds in the mouth and swallow in small quantities. The panelists repeated tasting of the same tea whenever necessary.
The panelists scored in a 7 point hedonic scale for five attributes of the herbal tea. These included color, taste preference, after taste, overall acceptance, and willingness to drink regularly. In the hedonic scale 1 meant ‘dislike very much’ and 7 meant ‘like very much’. The herbal tea performed with high score with majority of attributes favorably was selected for further experimentation based on the results of Frideman ranking test.
2.8. Determination of phenolic content of the selected herbal tea
The total phenolic content of the selected herbal tea based on sensory evaluation was determined as explained by Chandrasekara et al. (2018) using 0.25 mL of herbal tea. The TPC in the herbal tea was expressed as µmol GAE per 250 mL serving.
2.9. Determination of antioxidant activity of the selected herbal teas
Antioxidant activity of the selected herbal tea was determined by TEAC as explained by Chandrasekara and Shahidi (2010). The TEAC was expressed as µmol TE per 250 mL serving.
2.10. Determination of glycemic response and plasma antioxidant capacity
2.10.1. Subjects
Volunteers for the study were recruited through an opened advertisement from Makandura premises of Wayamba University of Sri Lanka. Written consent was obtained from each subject after explaining the study protocol before recruiting to the study. Ethical approval was obtained from the Ethics Review Committee of Faculty of Livestock, Fisheries and Nutrition, Wayamba University of Sri Lanka (201810HI06). Sixteen healthy individuals with fasting blood glucose between 70–110 mg/dL were recruited to the study after screening. Exclusion criteria included presence of any microvascular complications, alcohol and cigarette consumption, known allergies to herbal teas, and continuous use of any kind of medications.
2.10.2. Protocol
Two days prior to the test, participants were asked to restrict their intake of tea, including any herbal teas. Furthermore, they were restricted in intensive physical activities such as exercise and running. Each person made three visits and during the first visit anthropometric measurements (weight and height) and 24 hour dietary recall were taken from each participant. On each study visit, after 10–12 hours of fasting a blood sample was collected immediately before administering the herbal tea or control. The bael fruit tea (250 mL of serving with 50 g glucose) and control (250 mL water with 50 g of glucose) were administered to participants randomly. The herbal tea and control were served in amber glass tumblers at same temperature level and panelists were advised to drink within 10 min.
Finger prick blood samples were obtained at 30, 45, 60, 90 and 120 min after completing the drink using glass micro-hematocrit capillary tubes (75 µL, sodium heparinized) for the determination of postprandial plasma glucose concentration (PGC). Only fasting and end point blood samples were taken for the determination of plasma antioxidant capacity (PAC) by finger pricking. The plasma was separated using micro-hematocrit centrifuge (Model HC-12A, Zenith Lab Inc, Brea, CA, USA) at 15,300 ×g for 3 min and was stored in Eppendorf tubes at −80 oC for subsequent analysis of PGC and PAC.
2.10.3. Determination of plasma glucose concentration
Plasma glucose concentrations were determined by spectrophotometric method using a commercial kit (Glucose GOD-PAP liquid ready for use from BIOLABO (Maizy, France). Briefly, 10 µL of plasma were mixed with 1 mL of reagent and was left for 20 min at room temperature (30 °C). The absorbance of the mixture was measured at 500 nm against the reagent blank. Plasma glucose values were calculated using the given standard concentration and expressed as mg/dL.
2.10.4. Determination of plasma antioxidant capacity
The TEAC assay described by Re et al. (1999) was used with minor modifications. The ABTS•+ solution was prepared as explained by Chandrasekara and Shahidi (2010). The TEAC was measured by mixing 20 µL of the plasma sample with 980 µL of the ABTS• solution. The absorbance value of the reaction mixture was read at 734 nm immediately at the point of mixing (t0) and after 6 min (t6). The decrease in absorbance at 734 nm after 6 min of addition of trolox and plasma was calculated using the following equation: ΔA trolox = (At0, trolox − At6, trolox) − (At0, blank − At6, blank), where ΔA is the reduction of absorbance and A is the absorbance at a given time. TEAC values were expressed as µmol TE per L.
2.11. Statistical analysis
All experiments of chemical analysis of the herbal extract were carried out in triplicates and data were reported as mean ± standard deviation. The mean ranking of sensory scores was determined using Friedman test (Milton, 1937). In the glycemic response efficacy study data were reported as mean ± standard error of mean. The glycemic responses of the control and the herbal tea was compared using Bonferroni multiple comparison with 95% confidence interval (CI). The differences of group means at each time point were analyzed by paired t-tests with 95% CI. Plasma antioxidant capacities at the baseline and end of the group were compared using paired t-tests. All statistical analysis was performed by using SPSS version 23 (IBM Analytics, USA).
| 3. Results and discussion | ▴Top |
In this study, phenolic content and antioxidant activities of immature dried bael fruit extracts were determined using different in vitro methods along with identification of compounds by HPLC-DAD-ESI-MSn analysis. Subsequently, a bael herbal tea preparation, based on sensory characteristics, was selected and tested in a randomized single blind cross over design to determine the single dose efficacy on postprandial glycemic response and plasma antioxidant capacity of healthy adults.
3.1. Identification of phenolic compounds
Table 1 summarizes individual phenolic compounds and their contents from aqueous extracts of dried immature bael fruit cuts. Three compounds belonging to phenolic acids, and stilbenes were identified. Phenolic acids identified were ellagic acid and a derivative of protocatechuic acid. Ellagic acid was identified using an authentic standard and also due to the presence of a deprotonated ion at m/z 301 and MS2 at 283 (de Camargo et al., 2015). The second phenolic acid showed a deprotonated ion at m/z 337 and gave product ions at m/z 153, 119 (protocatechuic acid) in MS2, which was confirmed using an authentic standard and literature data (de Camargo et al., 2015), thus tentatively identified as a protocatechuic acid derivative. Furthermore, a resveratrol derivative belonging to the group of stilbenoids was tentatively identified in the aqueous extract of immature bael fruits due to its m/z of 433 in MS and m/z of 227 in MS2, the latter being typical of resveratrol (Urpí-Sardà et al., 2005). In contrast to protocatechuic acid derivative, which gave product ions that allowed a better identification in MS2, the third compound (deprotonated ion at m/z 301) only showed one product ion (m/z at 227) in MS2. Therefore, to provide a putative information the compound was only tentatively identified as a resveratrol derivative and its further isolation and characterization by nuclear magnetic resonance (NMR) is deemed necessary. However, this is beyond the mandate of the present study and may be addressed in the future.
 Click to view | Table 1. Phenolic compounds identified in bael fruit extracts |
The tentatively identified derivative of resveratrol made the highest contribution to the phenolic profile, showing a content of 497.8 µg/100 g. Resveratrol is a natural phytoalexin reported in a number of plant species and is mainly found in the skins and seeds of grapes as well as peanut skin (Salehi et al., 2018). Compounds identified in the matured bael fruit pulp in previous studies included monoterpenes, sesquiterpenes, coumarines, alkaloids, saponins, lignins, flavonoids and tannins, among others (Charoensiddhi and Anprung, 2008; Rajan et al., 2011). This is the first report on the presence of resveratrol or its derivatives in the extracts of bael fruit. It has been reported that resveratrol affects the nuclear factor kappa B (NF-κB) signaling pathway which is responsible for the regulation of inflammation and immune responses (Kundu et al. 2006). Furthermore, beneficial therapeutic efficacy of resveratrol in a number of diseases such as cancers, obesity, neurological disorders, type 2 diabetes, cardiovascular diseases and non-alcoholic fatty liver diseases has been in focus though the bioavailability is low (Berman et al., 2017). In the present work extracts of immature bale fruits were used whereas previous studies had used pulp which is generally obtained from mature fruits. Furthermore, a recent study demonstrated that composition of active compounds vary with the maturity stage of the bael fruit and was revealed that the level of phenolic compounds increased with the maturity of the fruit (Gurjar et al., 2019). Immature bale fruits contained higher levels of potassium, iron, marmelosin, psoralen and tannic acid compared with their mature counterparts (Gurjar et al., 2019).
3.2. Total phenolic contents (TPC) of bael fruit extract
Table 2 presents phenolic contents of the aqueous extract of immature bael fruit cuts. The TPC of bael fruit aqueous extract was 108.3 µmol GAE/g of extract. Flavonoids are known to possess antioxidant, anticancer, antiallergic, anti-inflammatory, and anti-neuro-inflammatory properties, among others (Shahidi et al., 2019; de Camargo et al., 2019; Zhang and Tsao, 2016). The total flavonoid content (TFC) of the bael fruit extract was 73.0 µmol CE/g extract (Table 2). According to Suvimol and Pranee (2008), TPC and TFC of 95% ethanolic extract of mature bael fruit pulp were 87.8 mg GAE/g (dw) and 15.2 mg CE/g (dw), respectively. Thus, the results in this study demonstrate that aqueous extracts of dried bael immature fruits were rich sources of phenolic compounds.
 Click to view | Table 2. Phenolic contents and antioxidant activitieof aqueous bael fruit extract |
3.3. Antioxidant activities of bael fruit extracts
Antioxidant activities of aqueous extracts of immature bael fruit cuts are presented in Table 2. TEAC of the bael fruit extract was 429.8 µmol TE/g of extract. This assay is commonly used to assess antioxidant capacity of food and other biological matrices that reduce the ABTS radical cation to its non-radical form. The results are compared with that of trolox, the water soluble analogue of vitamin E, thus only water-soluble compounds in the extracts are measured.
DPPH, a synthetic free radical, is widely used to evaluate radical scavenging properties of antioxidant compounds. In DRSA assay antioxidant compounds donate a hydrogen atom or an electron to the stable DPPH radical to convert it to the non-radical form (Yeo and Shahidi, 2019). DRSA of bael fruit extract was 27.4 µmol TE/g of extract. Krushna et al. (2012) previously showed that bael fruit extract scavenged DPPH and ABTS+ radical dose-dependently, in agreement with the findings of this study.
Activation of NF-κB is mediated by oxidative stress. A recent study showed that samples exhibiting scavenging activity of ABTS+ and DPPH radicals were effective in inhibiting the activation of NF-κB in RAW 264.7 macrophages (Falcão et al., 2019). Thus, the present study showed that aqueous extracts of dried immature bael fruit cuts containing resveratrol possessed the capacity to inhibit both ABTS+and DPPH radicals and could serve as a potential candidate for inhibiting the activation of NF-κB in biological systems.
Compounds with reducing ability are capable of donating electrons and reduce the oxidized intermediates of peroxidation, hence acting as antioxidants. Reductants in herbal extracts reduce ferric/ferricyanide complex to the ferrous form. RP of the tested extract of bael fruit herbal was 122.1 µmol ascorbic acid equivalents/g extract. The immature bael fruit extract tested in the present work exhibited a considerable RP thereby acting as effective reducing agents.
FICA of of bael fruit extracts showed values of 0.09 µmol EDTA equivalents/g extract in this study. By the action of ferrous ions in the body hydroxyl radicals are generated via Fenton’s reaction and cause destruction of biomolecules leading to degenerative disease conditions and aging. Compounds that can act as chelating agents, reduce the concentration of metal ions available for catalyzing peroxidation thus serve as effective secondary antioxidants. Presence of chelating agents decreases the intensity of the purple color of the complex of ferrous ions with Ferrozine in the assay.
3.4. Sensory evaluation
Table 3 presents the results of sensory evaluation conducted for the three bael fruit tea preparations. The product 780 with 1:20 ratio of ingredient to water (w/v) received significantly (p < 0.05) higher mean scores for taste preference, overall acceptability and willingness to drink regularly than others. The mean scores of untrained panelists for all attributes tested ranged between like slightly to like moderately for product 780. The mean scores of color and after taste were not significantly different between the product 780 and product 892 with 1:40 (w/v) ingredient to water ratio. The product 531 with 1:10 (w/v) ingredient to water ratio received the least (p < 0.05) mean scores for all attributes tested. According to the Friedman test rank the product 780 obtained the highest mean rank of 2.41 (p < 0.05) and was selected for further studies as the most preferred bael tea.
 Click to view | Table 3. Mean scores for sensory attributes and Friedman test ranks for different preparations of bael fruit tea |
3.5. Phenolic content and antioxidant activities of selected bale fruit tea
The TPC and TEAC of the selected bale fruit tea based on sensory evaluation were 1,576.0 ± 32.5 and 336.5 ± 26.5 µmol TE per 250 mL, respectively. The serving size used for the single dose ingestion (250 mL) for the determination of postprandial glycemic response and plasma antioxidant activity. The TPC and TEAC per g of raw ingredient (dried immature bael fruit cuts) were 16.1 mg GAE and 20.2 µmol TE, respectively. In a recent study Zhao et al. (2019) showed that TPC of 30 tea (Camellia sinensis) aqueous infusions ranged from 24.8 to 252.6 mg GAE per g of dry weight (g DW) of tea. The TEAC values of tea infusions varied from 166.3 to 2,532 µmol TE/g DW (Zhao et al., 2019). It should be noted that bael fruit tea used in the present work contained a lesser TPC and TEAC compared to tea infusions of green, black, oolong, white, yellow and dark teas (Zhao et al., 2019).
3.6. Effect of herbal teas on glycemic response
From the sixteen healthy subjects recruited in the study one withdrew after completing two visits due to change of personal work arrangement. In the final analysis 15 subjects (11 males and 4 females) were included. The mean age of subjects was 24 ± 2 y and the mean body mass index was 21.7 ± 3.4 kg/m2. Daily energy, carbohydrate, protein and fat intake of subjects were 1,322 ± 257 kcal, 216 ± 49 g, 39 ± 8, and 36 ± 9 g, respectively. No adverse effects due to the ingestion of herbal teas were reported during the study period.
Figure 1 and Table 4 present the change of mean PGC of healthy adults over the time from baseline (0 min) to 120 min. Bael fruit tea showed a lower PGC than the control at each postprandial time point except at the baseline (Figure 1). Several previous studies have shown that repeated administration of aqueous extracts of bael leaves, bark, seeds, fruits was effective in treating hyperglycemic conditions in rats (Kamalakkannan and Prince, 2003; 2005; Maity et al., 2009). According to Kamalakkannan and Prince (2003) oral administration of water extract of bael fruit two times per day for 4 weeks in streptozotocin induced diabeteic Wistar rats significantly reduced blood glucose, plasma TBARS, and hydroperoxides.
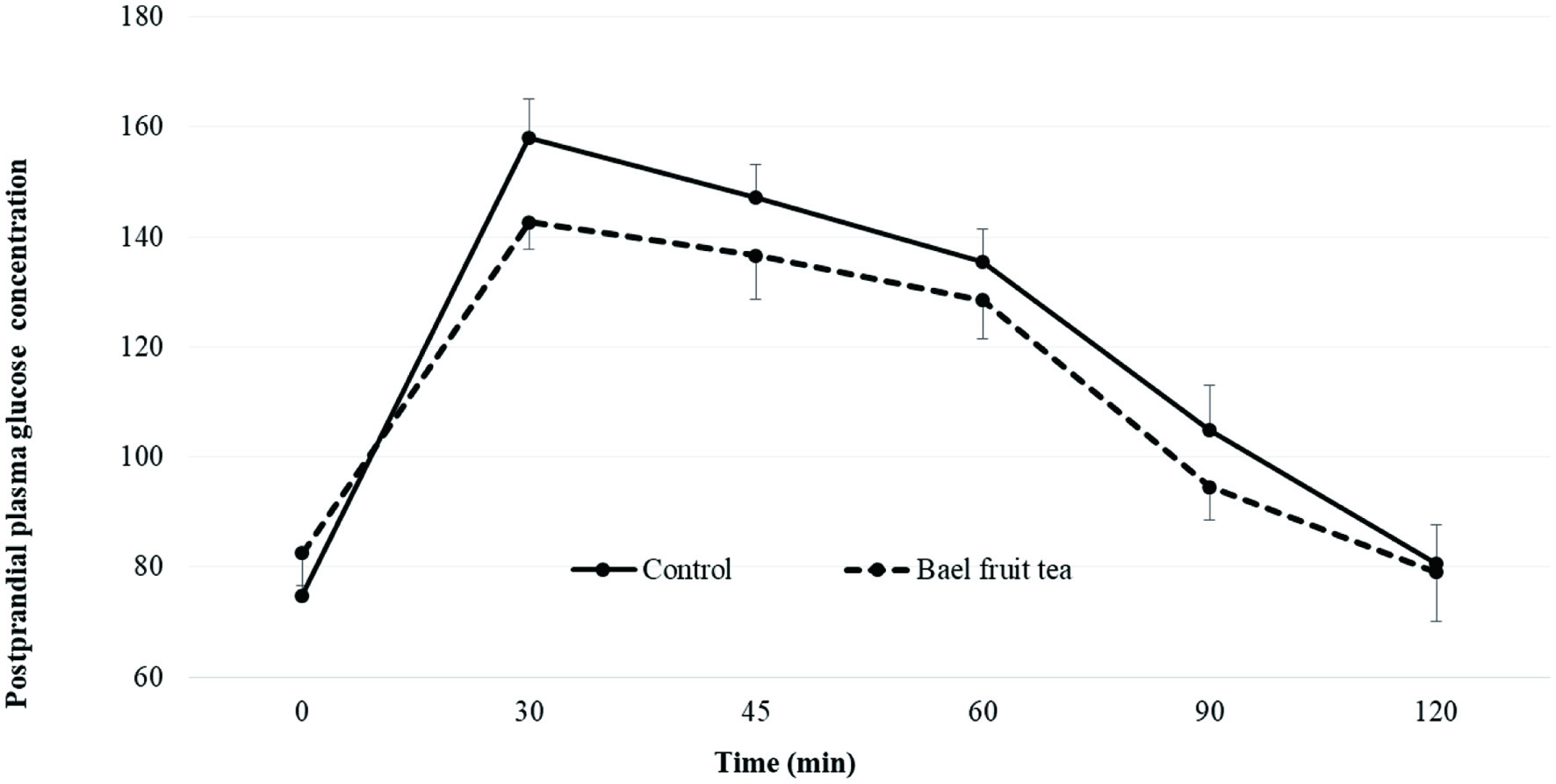 Click for large image | Figure 1. Mean plasma glucose concentration of healthy adults after consuming bael fruit herbal tea and the control. |
 Click to view | Table 4. Difference of postprandial plasma glucose response of healthy adults for bael fruit tea compared to the control |
To the best of our knowledge there are no reports of any human studies on how immature bael fruit tea may improve the postprandial glycemic control. In the present study, the efficacy of single dose administration of bael fruit tea was investigated in healthy humans. The results demonstrated that ingestion of a single dose of 250 mL of bael fruit tea with 50 g of glucose challenge had no significant (p < 0.05) effect on postprandial hypoglycemic response in healthy adults when compared with the corresponding values at each time point compared to the control.
3.7. Effect of herbal teas on plasma antioxidant capacity (PAC)
Plasma antioxidant capacity of healthy subjects was measured at the baseline and 120 min after ingestion of herbal teas. The test bael fruit herbal tea showed a significant (p < 0.05) increment of PAC compared to the baseline and the change was 74.6% (Table 5). The increment of PAC for bael fruit tea compared to the corresponding baseline was 168.1 TE/L. Villano et al. (2010) demonstrated an increment of PAC in healthy adults after a single dose load of 500 mL of fermented or unfermented rooibos tea. Furthermore, the increment of PAC was reported for green tea beverage and green tea extract in adults with metabolic syndrome (Basu et al., 2013).
 Click to view | Table 5. Plasma antioxidant capacity (µmol trolox equiv/L) of healthy adults at baseline and 120 min after ingestion of herbal teas |
The bael fruit tea showed a significantly higher PAC of 397.2 TE/L compared to the control at the end of 120 min post ingestion (Table 5). This was almost 75% increment of plasma antioxidant capacity compared to the baseline. In the present work the compositional analysis showed that bael fruit extract contained ellagic acid, protocatechuic acid derivative and resveratrol derivatives (Table 1). All these phenolic compounds are potent antioxidants and exert beneficial effects that prevent and manage non-communicable disease conditions in which oxidative stress plays a major role (Krushna et al., 2012; Muthukumaran et al., 2016; Salehi et al., 2018; Chandrasekara, 2019). Furthermore, the presence of resveratrol and its metabolites in human LDL-cholesterol after moderate intake of red wine has already been reported (Urpí-Sardà et al, 2005). Previously, Krushna et al. (2012) showed that bael fruit extract scavenged superoxide and hydroxyl radicals and nitric oxide in a dose dependent manner in vitro. In the same study, isoproterenol (ISO) induced oxidative stress of rats was ameliorated by repeated use of bael fruit extract for 45 d by reducing thiobarbituric acid reactive substances (TBARS) and tissue damaged marker enzymes, namely aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alanine transaminase (ALT) and creatinine kinase MB isoenzyme (CK-MB) in the serum. In addition, Krushna et al. (2012) further showed that bael fruit extract elevated antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase and reduced glutathione levels in serum that attenuated oxidative stress induced by ISO in rats.
The foregoing results suggest that bael fruit tea could serve as a potential source of antioxidant compounds and is effective in attenuating oxidative stress that leads to a plethora of non-communicable diseases, including type 2 diabetes.
| 4. Conclusion | ▴Top |
Herbal tea prepared with immature dried bael fruits is a rich source of water-soluble phenolic compounds with demonstrated potent antioxidant activities. Bael fruit tea tested in this work was found effective in increasing plasma antioxidant activity with the potential of reducing oxidative stress and help controlling secondary complications of type 2 diabetes and maintaining health and wellness.
Acknowledgments
This research was supported by the Wayamba University of Sri Lanka through a University research grant (SRHDC/RP/04/18-03) to AC. Dr Gamika Prathapasinghe, Department of Livestock and Avian Sciences, Wayamba University of Sri Lanka was acknowledged for generous support extended by providing the facility of micro centrifuge.
Conflict of interest
The authors state that there is no conflict of interest to declare.
| References | ▴Top |