| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 11, September 2020, pages 57-65
Pytochemical profile and antioxidant activity of chiltepin chili (Capsicum annuum var. glabriusculum), Sonora, Mexico
Alma A. Vazquez-Floresa, Oneydi Góngora-Péreza, Izamar Olivas-Orduñab, Óscar A. Muñoz-Bernala, Pedro Osuna-Avilaa, Joaquín Rodrigo-Garcíab, Laura A. de la Rosaa, Emilio Alvarez-Parillaa, *
aDepartamento de Ciencias Químico Biológicas, Universidad Autónoma de Ciudad Juárez, Anillo envolvente del PRONAF y Estocolmo s/n. Ciudad Juárez, Chihuahua 32310, Mexico
bDepartamento de Ciencias de la Salud, Universidad Autónoma de Ciudad Juárez, Anillo envolvente del PRONAF y Estocolmo s/n. Ciudad Juárez, Chihuahua 32310, Mexico
*Corresponding author: Emilio Alvarez-Parrilla, Departamento de Ciencias Químico Biológicas, Universidad Autónoma de Ciudad Juárez, Anillo envolvente del PRONAF y Estocolmo s/n. Ciudad Juárez, Chihuahua 32310, Mexico. Tel: +52 656 688 1800 Ext. 1562; E-mail: ealvarez@uacj.mx
DOI: 10.31665/JFB.2020.11237
Received: February 19, 2020
Revised received & accepted: March 31, 2020
| Abstract | ▴Top |
Quantification of chiltepin hot pepper (Capsicum annuum L. glabriusculum) phytochemicals provides a tool to evaluate the fruit quality and health impact. This study evaluates the phytochemical content and antioxidant activity of chiltepin from different locations of Sonora, Mexico, at two ripening stages (immature and mature). Seeds from Cumpas and Sahuaripa, were grown under greenhouse conditions and phenolic compounds, flavonoids, carotenoids, chlorophylls, and ascorbic acid were determined by spectrophotometric techniques. Capsaicinoids were determined by HPLC-DAD. The antioxidant activity was determined through DPPH and ABTS radical scavenging and by FRAP techniques. The origin of the seed influenced the antioxidant activity and phytochemical content. Samples from Cumpas, were superior in phytochemicals compared with Sahuaripa. Antioxidant activity and phytochemicals were higher in mature stage. Antioxidant activity correlates mainly with phenolic compounds and carotenoids. This study highlights that mature chiltepin pepper from Sonora could grow under controlled conditions develop bioactive compounds with antioxidant potential.
Keywords: Seed origin; Phytochemical compounds; Mature stage; Immature stage; Antioxidant activity
| 1. Introduction | ▴Top |
Pepper (Capsicum annum) is consider one of the most important vegetable in Mexico for its gastronomic-cultural relevance. Besides, is valued for its high nutritional content, been an important source of vitamins (C, B1 and B2), minerals, carotenoids and phenolic compounds (Vera-Guzman et al., 2018). The annual Mexican per capita consumption of pepper is 8 to 9 kg, due to their high sensory acceptability, together with its high nutritional content. The sensory characteristics of pepper such as size, pungency and taste are the main factors for the consumer choice and making chiltepin and jalapeño pepper among the preferred hot peppers in Mexico (Rodríguez-del Bosque, 2005).
Chiltepin pepper (Capsicum annum var. glabriusculum) is wild perennial bush plant that grows in dry and warm conditions, at heights below 1,300 meters below sea level, which presents a fruit in form of berries, with 3 to 6 millimeters in diameter (Araiza et al., 2011). At the ripening stage has a reddish color and high pungency (spicy taste). Chiltepin growths in the wild and is widely distributed in Mexico, being Sonora the main chiltepin producer. Has different names depend on the region, such as chiltepin, piquin pepper, mountain pepper among others (Hayano-Kanashiro et al., 2016). Until recently, chiltepin could not be tamed due its specific conditions of growth like moisture, soil and daylight that only can be found in their natural habitat. In the present, is under anthropogenic pressure, since the harvest of the fruit by farmers lacks of an appropriate technique that compromises the root, and consequently the plant dies during harvesting (Araiza et al., 2011). Furthermore, in the natural environment, for the germination of seeds, it is necessary that they are eaten and digested by birds, because the acidity of their stomach promotes its germination. Such digestive process by birds promotes both seed germination and plant propagation. For this reason, the cost of chiltepin in the market is high and studies are undertaken to achieve chiltepin production under greenhouse controlled conditions (Reyes-Acosta et al., 2019), in order to conserve the resource and reduce its commercial price (Araiza et al., 2011; Vera-Guzman et al., 2018).
Sensorial characteristics of chiltepin depends on its phytochemical composition (Vera-Guzman et al., 2018). Phytochemicals are compounds with specific physiologic functions in vegetables. They include phenolic compounds, carotenoids, capsaicinoids and vitamins. Moreover, the phytochemical content in edible fruits its related to their health beneficial effects, due to their antioxidant and anti-inflammatory activities, since such compounds may prevent oxidative stress in cells (Rodrigo-García et al., 2011). It has been reported that phenolic compounds present in fruits may affect their color and taste (Sarafi et al., 2018). Carotenoids are responsible for the reddish color at ripening stage. Capsaicinoids only present within the Capsicum genus, are responsible for the pungent sensation that mammals experience when hot peppers are ingested (Hayano-Kanashiro et al., 2016). Phytochemical content among vegetables present high variability due to differences in ripeness, U.V. radiation exposure, water availability and genetical modification seed, harvest season (González-Ayala et al., 2012) and in consequence, the sensorial characteristics and the consumer preferences on certain types of peppers, specially chiltepin pepper, will vary depending mainly on harvesting location (Gao et al., 2011). Even though, the environment conditions affect considerably the phytochemical content, there is a lack of studies that evaluate the effect of chiltepin recollection region on its phytochemical content. In this context, the aim of this study was to evaluate the phytochemical content and antioxidant activity of chiltepin peppers from seeds from two regions of Sonora, Mexico, at two ripening states (immature and mature) grown under controlled greenhouse conditions.
| 2. Materials and methods | ▴Top |
2.1. Chemicals
Reagents and standards used for spectroscopy determinations as Folin-Ciocalteu reagent, sodium carbonate, sodium nitrate, aluminum chloride, sodium hydroxide, potassium chloride, sodium acetate, iron chloride hexahydrate, 2,4,6 tripyridyl-s-triazine (TPTZ), 2,2-diphenyl-1picryl-hydrazyl (DPPH), 2,2′-azino-bis-[3-ethyl-benzothiazoline]-6-sulfonic acid (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), sodium phosphate monobasic, sodium phosphate dibasic, sodium chloride, potassium persulfate, solvents as methanol, acetone and chloroform were purchased from Sigma-Aldrich (St. Louis, MO, USA). Only acetone, methanol, and acetonitrile used in chromatography were acquired from TEDIA® (Fairfield, OH, USA).
2.2. Samples
Chiltepin (Campsicum annuum var. Glabriusculum) from two regions: Sierra de Cumpas, Sonora (29°59′38″ N y 109°46′55″ West, 760 meters above the sea level) Sahuaripa, Sonora (29°03′00″ N y 109°14′00″ West, 440 meters above the sea level, from a backyard), were collected, identified and kept as reference samples in the greenhouse facilities. Seeds were cultivated under greenhouse-controlled conditions at the Universidad Autónoma de Ciudad Juárez experimental greenhouse (summer of 2015). Both seeds were germinated in plastic bags (3.7 L capacity) with substrate mix composed of 50% peat moss and 50% agricultural soil. Substrate and seeds were fertilized with Miracle-Growth® 24-8-16 (3.5% ammoniacal nitrogen and 20.5% urea, 8% P2O5, 16% soluble potassium (K2O), 0.02% boron, 0.07% CuSO4, 0.15% chelated iron, 0.05% chelated manganese, 0.0005% sodium molybdenum and 0.06% CuSO4, water soluble) three times at manufacturer recommended dose (3 g l−1). Seeds were germinated for 115 days previous harvesting green peppers (immature). Peppers were harvested in the production stage. A total of 90 chiltepin peppers from Cumpas (wild) seeds were harvested, 38 from immature stage (green) and 58 from the mature stage (red). Thirty-three chiltepin peppers were harvested from Sahuaripa seeds: 14 immatures and 19 mature.
2.3. Morphological characteristics of fruit from Capsicumm annuum L. glabriusculum
Morphological characteristics of peppers were analyzed in function of weight, diameter and length for each type and ripeness stage.
2.4. Sample treatment
After morphological characterization of peppers, samples were frozen at −80 °C for at least 24 h (Thermo Scientific®, EXF32086D, Wattham, MA, USA). Then, samples were freeze-dried for 48 h (LABCONCO®, Freezone 6, Kansas, MO, USA). Moisture content was determined by difference of fresh weight and after lyophilized weight to express results in grams per dried matter (DM). Dried samples were grinded in a commercial coffee mill, and homogenized in a sieve (mesh no. 40, 20 µm). Homogenized sample were vacuum stored at −80 °C until further analysis.
2.5. Total soluble phenolic extraction
One g of dried chiltepin peppers were homogenized with 80% methanol at a 1:10 (w/v) ratio for immature peppers and 1:25 (w/v) for mature peppers. Samples were sonicated for 30 min at room temperature and centrifugated for 10 min at 420 × g at 4 °C. Supernatant was removed and extraction process was repeated. Supernatants were mixed and methanol was removed by rotovaporation (BÜCHI®: R-3, New Castel, NJ, USA). Finally, the extract was frozen at −80 °C for 24 h and freeze-dried for 48 h. Solid extracts were vacuum stored at −80 °C until further analysis (Moreno-Escamilla et al., 2015).
2.6. Total soluble phenolic content
Total soluble phenolic compounds were determined by the Folin-Ciocalteu reagent method according to Moreno-Escamilla et al. (2015) with slightly modifications. Briefly, 250 µl of extract (1 mg ml−1 in methanol) were mixed with 1,000 µl of sodium carbonate (7.5%) and incubated for 3 min at room temperature. After this time, 1,250 µl of Folin-Ciocalteu reagent (10% in distilled water) were added. Mix was incubated for 15 min at 50 °C in the dark. Finally, chill in cold water for 10 min, and 300 µl of reaction were transferred to a microplate well and absorbance was read at 760 nm in a microplate spectrophotometer (BioRad® Xmark, Hercules, CA, USA) Gallic acid was used as standard and results were expressed as milligrams of gallic acid equivalents per gram of dried matter (mg GAE g−1 DM).
2.7. Total soluble flavonoid content
Total soluble flavonoids were measured by the aluminum complexation method (Alvarez-Parrilla et al., 2011). In brief, 31 µl of sample (1 mg ml−1) was poured in a microplate well and mixed with 125 µl of distilled water and 9.3 µl of sodium nitrite (5%) and incubated for 3 min at room temperature. 9.3 µl of aluminum chloride (10%) was added and reaction was incubated 3 min at room temperature. Finally, 125 µl of sodium hydroxide (0.5 M) was added and incubated for 30 min at room temperature in the dark. Absorbance was determined at 510 nm in a microplate spectrophotometer. Catechin was used as standard and results were expressed as milligrams of catechin equivalents per gram of dried samples (mg CE g−1 DM).
2.8. Carotenoid extraction and quantification
Two hundred and fifty milligrams of samples were mixed with 10 mL of acetone and sonicated for 20 min at room temperature in the dark. The extract was centrifugated at 420 × g for 10 min at 4 °C. Supernatant was removed and extraction process was repeated. Both supernatants were mixed and diluted to 50 mL volumetric flask with acetone. Extract was diluted 1:10 (v/v) with acetone, and absorbance was determined at 454 nm. β-carotene was used as standard and results were expressed as milligrams of β-carotene equivalents per gram of dried matter (mg βCE g−1 DM) (Moreno-Escamilla et al., 2015).
2.9. Capsaicinoids extraction and quantification
Capsaicinoids were extracted and quantified according to Moreno-Escamilla et al. (2015). One hundred milligrams of sample were mixed with 1 ml of methanol (100%). Mixture was sonicated for 30 min at room temperature in the dark. Then, mix was centrifugated at 420 × g for 7 min at room temperature. Supernatant was removed and extraction process was repeated. Both supernatants were mixed and stored at −20 °C until further analysis (no more than 24 h). Identification and quantification of capsaicinoids was performed by high performance liquid chromatography (HPLC, PerkinElmer® series 200 with diode array), equipped with a SupercosilLC-18 collum (5 μm particle size, 250 × 4.60 mm). Capsaicinoids extract (2 ml) were passed through nylon filter (45 µm) and poured in a vial. Ten µL of each sample (1 mg ml−1) was injected into the HPLC system and eluted using an isocratic mobile phase (50:50 v/v, acetonitrile 100% and formic acid 1% in water), at a 1 ml min−1 flow rate. Capsaicinoids were monitored at 280 nm wavelength. Identification of compounds in samples was determined using retention times (Rt) of commercial standards (capsaicin (Cap) and dihydrocapsaicin (DHC), and results expressed as micrograms of capsaicin or dihydrocapsaicin equivalents per gram of dried matter (µg CapE or DCE g−1 DM).
2.10. Ascorbic acid extraction and quantification
Ascorbic acid (vitamin C) content in chiltepin pepper samples was determined according to Alvarez-Parrilla et al. (2011). Two hundred mg of each dried sample was mixed with 5 ml of metaphosphoric acid (5%) and sonicated for 20 min at room temperature in the dark. Samples were centrifugated at 1,300 × g for 10 min at 4 °C, and supernatant was collected. Ascorbic acid was quantified by mixing 300 µl of supernatant with 200 µl of trichloroacetic acid (6.65%) and 75 µl of dinitrophenylhydrazine (DNPH) (2 g of DNPH, 230 of thiourea and, 270 mg cuprum sulphate pentahydrate in 100 ml of sulfuric acid 0.5 M). Reaction was incubated for 3 h at 37 °C. After this incubation period, 500 µl of sulfuric acid (65%) was added, and absorbance was measured at 520 nm in a microplate spectrophotometer. Ascorbic acid was used as standard, and results were expressed as milligrams of ascorbic acid per gram of dried matter (mg AA g−1 DM).
2.11. Chlorophylls extraction and quantification
Chlorophylls were extracted by mixing 100 mg of dried chiltepin peppers with 10 mL of chloroform-methanol (2:1, v/v) solution and stirred for 3 min. Mixture was filtered, and the solid phase was extracted once again. Supernatant were mixed in a 25 ml volumetric flask and filled with the chloroform-methanol solution and stored at −80 °C. Chlorophyll was quantify in a microplate by measuring the absorbance at 663 and 645 nm. Chlorophyll content was calculated using Equation 1 and, results were expressed as milligrams of total chlorophylls per 100 g of dried matter (mg TC g−1 DM), as result of the sum of chlorophyll a and b present in samples (Sumanta et al., 2014).
2.12. Antioxidant activity of Capsicumm annuum L. glabriusculum
Antioxidant activity of chiltepin peppers was determined by three techniques: radical scavenging 2,2-diphenyl-1pycrilhydrazil (DPPH) and 2,2′-azino-bis (3 ethylbenzotiazolin-6 sulfonate) (ABTS) and by ferric reduction antioxidant power (FRAP). In all cases, phenolic extracts were used (1 mg ml−1 in methanol).
DPPH assay. Twenty-five 25 µl of sample (or Trolox standard) were mixed with 200 µl of DPPH radical (190 µM in methanol) in a microplate well. Absorbance was measured every 20 s for 10 min at 517 nm. Inhibition percent was calculated using Equation 2:
ABTS: ABTS radical was prepared at 7 mM in phosphate buffer solution 0.1 M (PBS, pH 7.4, 0.15 M potassium chloride) then potassium persulfate was added 2.45 mM (final concentration). Radical was incubated for 12 to 16 h at room temperature in the dark. After this period, radical absorbance was adjusted to 0.7 ± 0.1 at 734 nm with PBS. Antioxidant activity was performed in a 96 well-microplate. Twelve µl of sample were mixed with 285 µl of adjusted ABTS radical, and the absorbance was measured every 30 s for 6 min at 734 nm. Inhibition percent was determined using Equation 2. Results were expressed as micromoles of Trolox equivalents per gram of dried matter (µmol TE g−1 DM) (Moreno-Escamilla et al., 2015).
FRAP: FRAP reagent (2,4,6-trypyridil-s-triazine, TPTZ 0.3 M) was prepared in a acetate buffer solution (pH 3.6), hydrochloric acid (40 mM) and ferric chloride (20 mM) in a 10:1:1 ratio and heated at 37 °C for 30 min. twenty-five µl of sample mixed in a microplate well with 180 µl of FRAP reagent. Absorption was measured at 595 nm every 60 s for 30 min. Trolox was used as standard and results were expressed as micromoles of Trolox equivalents per gram of dried matter (µmol TE g−1 DM) (Moreno-Escamilla et al., 2015).
2.13. Statistical analysis
All analyses were carried out by triplicate. Results are expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) and Tukey analyses were performed in order to determine statistical differences (p < 0.05) between seeds origin and ripening stage. All data were analyzed using SPSS 21 statistical software (SPSS Inc. Headquarters, Chicago, IL, USA). Pearson correlation was performed using Prisma software at p < 0.05.
| 3. Results | ▴Top |
In order to evaluate the effect of seeds origin on the phytochemical characteristics of chiltepin hot pepper (Campsicum annuum var. Glabriusculum), seeds collected in two regions of Sonora were cultivated under controlled greenhouse conditions at the Universidad Autónoma de Ciudad Juárez greenhouse, and harvested at two commercial ripening stages: immarure (green) and mature (red), and their morphological and phytochemical characteristics evaluated.
Table 1 summarizes the morphological differences found in chiltepin peppers from Cumpas (wild, 29°59′38″ N y 109°46′55″ West, 760 meters above the sea level) and Sahuaripa (29°03′00″ N y 109°14′00″ West, 440 meters above the sea level, from a backyard) Sonora at both immature (green) and mature (red) stages. These results show that both seeds origin and ripening stage affect the morphology of chiltepin peppers. Sahuaripa samples showed larger dimensions at both ripening stages. Interesting mature (red) peppers showed smaller sizes compared to immature peppers. No statistical differences were observed for the length at the mature stage. Similar results have been reported by Kissinger et al. (2005). Both mature peppers showed similar morphological characteristics, compared to other chiltepin peppers harvested in the northern states of Mexico (Hayano-Kanashiro et al., 2016).
 Click to view | Table 1. Morphology of wild chiltepin pepper (Campsicum annuum var. glabriusculum) from Cumpas (wild) and Sahuaripa (backyard), at two ripening stages: immature (green) and mature (red) |
Total phytochemical content of chiltepin pepper samples, determined by spectrophotometric techniques, are summarized in Table 2. From the analysis of this table, it is possible to observed that phytochemical content is affected by the seed origin, as well as the chiltepin ripening stage. Chiltepin pepper grown from seeds collected in Cumpas showed a higher content of total phenols, total flavonoids, carotenoids, total chlorophylls and ascorbic acid, at both ripening stages. For both cultivars, higher phytochemical content was observed for mature stage, except for total chlorophylls, which were not identified in this stage.
 Click to view | Table 2. Phytochemicals quantified in chiltepin pepper (Campsicum annuum var. glabriusculum) from Cumpas (wild) and Sahuaripa (backyard), at two ripening stage: immature (green) and mature (red). |
Among all phytochemicals, phenolic compounds have probably been the most studied, due to large experimental evidence that link these compounds with different biological activities (Bhat and Rajanna, 2017). Total phenolic content in chiltepin samples from Sahuaripa, Sonora was in the range of 14 to 26 mgGAE g−1 DM for immature and mature stage respectively, while it ranged from 24 to 42 mgGAE g−1 DM for the samples from Cumpas, Sonora. These results may indicate that seeds harvested from wild chiltepin plants showed higher total phenolic content compared to those harvested in semi-domesticated plants (backyard). On the other hand, for both samples, their phenolic content increased up to 50% when they reached fruit maturity (red). In the case of flavonoids, a similar trend as those for total phenolic compounds was observed, even though no significant difference was observed at the mature stage. Considering that homogeneous greenhouse growing conditions were applied to both samples, differences can be attributed to environmental conditions at which seeds were exposed.
Total carotenoids content (reported as β-carotene) showed significant differences both between seeds location and ripening stage (Table 2). As in the case of phenolic compounds, Cumpas chiltepin peppers showed higher carotenoids content (1.6–6.06 mg βCE g−1 DM), and their values increased in mature samples. Total chlorophylls content was only detected in immature samples, with values of 5.0 and 2.5 mg TC g−1 DM, for chiltepin pepper from Cumpas and Sahuaripa, respectively. As expected, no chlorophyll was detected in mature samples. It was observed that the chlorophyll contents were significantly more abundant in the green chiltepin peppers of Cumpas than in those of Sahuaripa. No significant differences in ascorbic acid content among seeds from Cumpas and Sahuaripa (Table 2). However, mature samples showed approximately 7 times more ascorbic acid content than immature peppers for both samples.
Capsaicin and dihydrocapsaicin were identified and quantified in Cumpas and Sahuaripa chiltepin peppers by HPLC-DAD. Figure 1 shows the chromatograms of chiltepin pepper extracts from the mature stage from Cumpas (1a) and Sahuaripa (1b). capsaicin and dihydrocapsaicin were identified in the samples by comparison of their retention times (13.6 and 19.5 min, respectively) and absorption spectra with those of commercial standards. Capsaicin and dihydrocapsaicin and total capsaicinoids (sum of both capsaicinoids) are reported in Table 2. All samples showed higher capsaicin content than dihydrocapsaicin. Also, both capsaicin and dihydrocapsaicin values were higher in the mature stage. No statistical differences were observed between samples from Cumpas and Sahuaripa. The reported capsaicinoid content for chiltepin peppers of Cumpas and Sahuaripa position them among the most pungent pepper commercially available in northern Mexico (4.17 to 8.2 mg g−1 DM) (Hayano-Kanashiro et al., 2016).
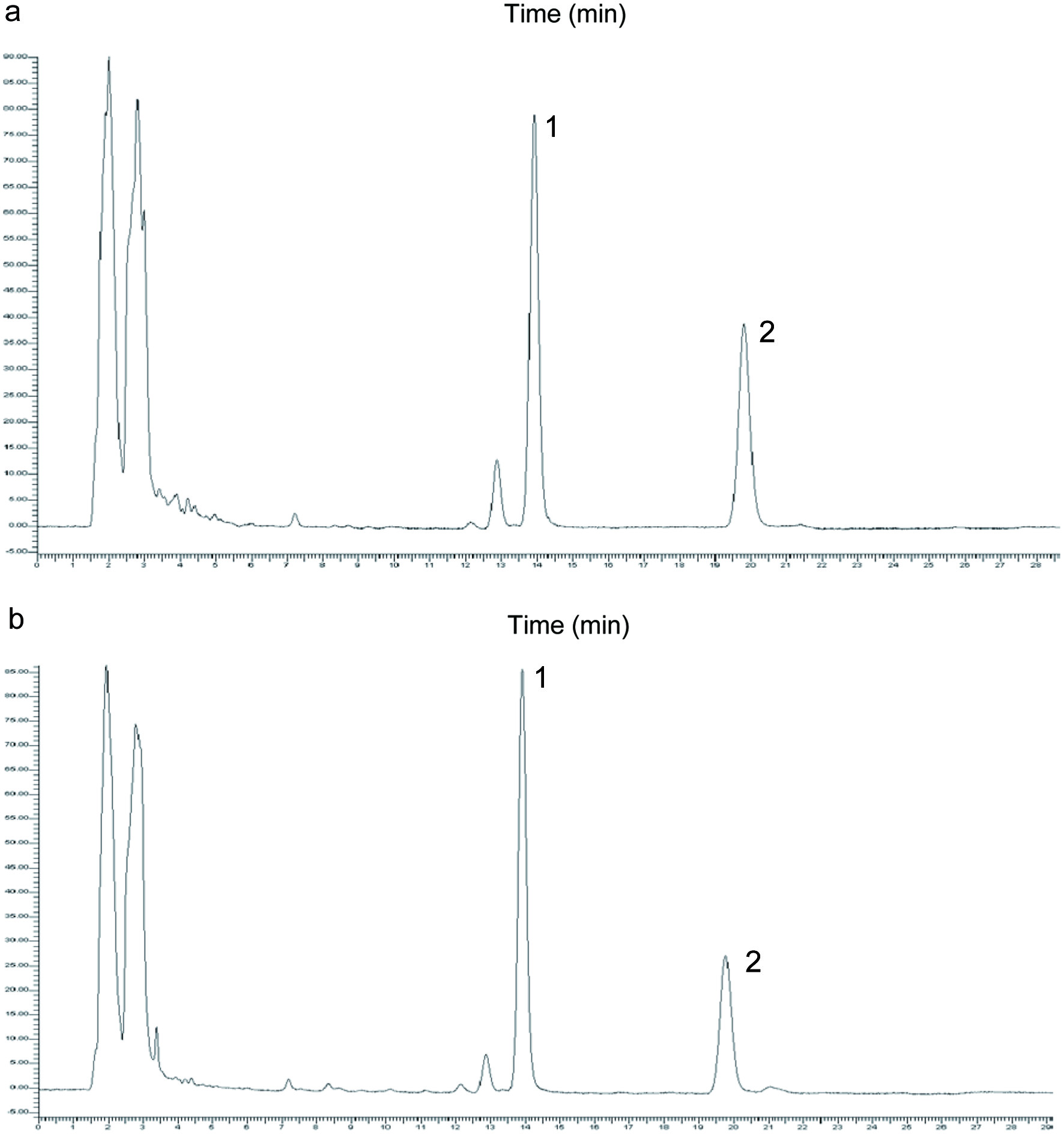 Click for large image | Figure 1. Chromatogram of chiltepin pepper (Campsicum annuum var. glabriusculum) from (a) Cumpas (wild) and (b) Sahuaripa (backyard) in mature stage. 1 Capsaicin, 2 Dihydrocapsaicin. |
3.1. Antioxidant activity
Antioxidant activity of chiltepin pepper from Cumpas and Sahuaripa, Sonora at two ripening stages (immature and mature) was evaluated by three spectrophotometric techniques: DPPH and ABTS radical scavenging and FRAP, based on iron reduction, and results are presented in Table 3. Antioxidant activity showed different results depending on the method used. DPPH showed de lowest values in all samples. DPPH values were higher for mature samples compared to immature and for both ripening stages, Cumpas samples were higher than those from Sahuaripa. Antioxidant activity measured by FRAP showed that there were only significant differences (p < 0.05) due to sample ripening stage, being higher for mature samples (∼200 μmol TE g−1 DM), compared to immature stage (∼100 μmol TE g−1 DM) for both seeds. A completely different effect was observed by ABTS, where the immature stages presented the highest antioxidant activity, likewise, the fruits from Sahuaripa seeds were above those of Cumpas with 209 and 135 μmol TE g−1 DM respectively, while for mature stage the place of origin did not affect the antioxidant activity (∼70 to 80 μmol TE g−1 DM).
 Click to view | Table 3. Antioxidant activity of chiltepin pepper (Campsicum annuum var. glabriusculum) from Cumpas (wild) and Sahuaripa (backyard) in two ripeness stages: immature and mature |
A large variety of bioactive compounds in chiltepin pepper (C. anumm var. glabriusculum) such as flavonoids, carotenoids, capsaicinoids, ascorbic acid and chlorophylls may be responsible for their antioxidant activity (Hayano-Kanashiro et al. 2016; Vera-Guzman et al. 2018). To determine which phytochemical present in chiltepin pepper have a greater impact on antioxidant activity, a correlation analysis was performed between each of the six phytochemicals determined in this study and the three antioxidant activity methods (DPPH, ABTS and FRAP), and results are shown in Table 4. Results describe that there is a significant correlation coefficient between antioxidant activity by DPPH and caroteonid content, (r = 0.99), followed by capsaicinoids (r = 0.98), and flavonoids (r = 0.98). While for ABTS method, flavonoids (r = 0.97) seems to be the only phytochemical that correlates with this antioxidant activity method. Finally, FRAP showed correlation with carotenoids (r = 0.98), ascorbic acid (r = 0.995) and capsaicinoids (r = 0.997). These results allow to infer that flavonoids and capsaicinoids are the main phytochemical compounds present in chiltepin responsible for its antioxidant activity determined by DPPH and ABTS, while capsaicinoids affect the activity of DPPH and FRAP.
 Click to view | Table 4. Correlation coefficients (r) between phytochemicals and antioxidant activity in chiltepin pepper (C. anuumm var. glabriusculum) |
| 4. Discussion | ▴Top |
Considering the growing demand of chiltepin peppers (Capsicum annuum var. glabrisculum) among consumers, and that this hot pepper is mainly exploited as a wild crop by cutting all the plant before collecting the fruits, chiltepin peppers are overexploited and at risk of becoming endangered. For this reason, several studies have been undertaken to produce this pepper under controlled conditions both under normal field and greenhouse conditions, without losing sensory and quality properties (Rodríguez-del Bosque, 2005).
An important agronomical and economical factor for chiltepin peppers is their morphological changes during ripening (Gao et al., 2011). Interesting, a decrease in dimensions was observed from immature to mature stage in peppers from seeds from both Cumpas and Sahuaripa grown under greenhouse conditions. This phenomenon is explained by some authors due to the ripening process itself, where the fruit ceases its growth and stops the accumulation of reserves. For example, water is incorporated into metabolic processes that lead to maturity (Kissinger et al., 2005). It is interesting to state that the dimensions of mature chiltepin peppers cultivated under greenhouse conditions were in the range reported for commercial chiltepin peppers (6 and 8 mm in diameter) (Hayano-Kanashiro et al., 2016). In this way, both states have chiltepin seeds variety that can grow within normal size range.
The increase in phenolic compounds during ripening from immature to mature fruit for Cumpas and Sahuaripa was to be expected since the production of phenolic compounds as secondary metabolism, responds to the abiotic stress to which the fruit growth is subjected (Navarro et al., 2006). Although both seeds belong to the same species, they belong to different geographical areas that can modified the phenolic content in each seed, even growing under controlled greenhouse conditions. Likewise, backyard samples can also be considered wild, because they are not commercially cultivated, since they are in a community, they have greater access to water and irrigation, which is subject to less water stress. Similar results have been observed for jalapeño peppers, where the stage of maximum maturation (red) presented higher phenolic compounds content (Moreno-Escamilla et al., 2015). The reported phenolic content for both seeds location and ripening stage are between the range of those reported for chiltepin peppers collected in different Mexican states. Total phenolic content of 250–500 mg GAE g−1 DM were reported for samples from Tamaulipas (Moreno-Ramírez et al., 2018), 34–54 mg GAE g−1 DM for samples from Coahuila (Reyes-Acosta et al., 2019) and 4.85 mg GAE g−1 DM for samples from Baja California (Rodríguez-Maturino et al., 2012). This wide difference in total phenolic content may be due not only to the conditions of handling the samples during their collection and commercialization, but also the influence of the geographical area in which the fruits grow.
The differences in the content and composition of flavonoids define the sensory characteristics of each type of pepper and, therefore, is related to consumer use and preferences (Vera-Guzman et al., 2018). In agreement with results for Cumpas and Sahuaripa chiltepin peppers, it has been reported that the amount of flavonoids tends to increase with fruit ripening (transition from green to red) (Adedayo et al., 2010). This may be because the metabolism of flavonoids in chiltepin pepper depends on the geographical area of growth (variables studied in this research), agroecological characteristics and post-harvest management are also important. Even so, published studies indicate that high variability in flavonoid content in some peppers is due to growing conditions, soil, climate and maturity level (Alvarez-Parrilla et al., 2011).
The capsaicinoid content in chiltepin pepper is one of the essential quality parameters, because it is associated with the appreciated pungent sensation of hot peppers. From the analysis of the data reported in Table 2, it can be deduced that the ripening stage highly influenced capsaicinoids content in chiltepin peppers, since, independently of the seeds region, capsaicinoid content increased 5–6 times during maturation from an immature (green) to mature (red) fruit. Similar results have been reported for samples of green and red wild chiltepin pepper from Sonora (Montoya-Ballesteros et al., 2009), Coahuila (Reyes-Acosta et al., 2019) and Tamaulipas (Moreno-Ramírez et al., 2018). Little differences may be a product of the response of varieties of the same species that have grown under different abiotic conditions and that express differences in the phytochemical content, including capsaicinoid compounds. The change of capsaicinoids from the green to the red stage justifies the high pungency of the red chiltepin pepper from wild areas such as the Sonoran desert region, where its consumption is mainly as a condiment on some typical foods such as fruits or corn, but not as the main ingredient of dishes such as mole, sauces and chilaquiles (Vera-Guzman et al., 2018). The high amount of capsaicinoids in the mature stages of both samples tested, propose an advantage over other types of peppers, since they can provide a very spicy seasoning with small amounts as an ingredient and without losing the original flavor of the dishes (Hayano-Kanashiro et al., 2016), unlike other peppers as jalapeño red and serrano with lower capsaicinoid content (Alvarez-Parrilla et al., 2011; Moreno-Escamilla et al., 2015). When total capsaicinoids content were compared with other studies carried out with wild chiltepin samples, it is possible to observe that the reported values were below those previously reported for both green and red peppers (16–31.84 mg Caps g−1 DM) (González-Zamora et al., 2013; Moreno-Ramírez et al., 2018). These differences may be because in the present study greenhouse controlled growing conditions were used, and consequently less heat and hydric stress was applied. It has been reported that the accumulation of capsaicinoids in peppers increases when the environment temperature rises, especially in desertic conditions, altering the genotype of the seed (Moreno-Ramírez et al., 2018).
Carotenoid, chlorophyll and ascorbic acid content was higher in mature stage of chiltepin pepper. The increase of carotenoids, up to 6 times, is consistent with previous studies (Rochín-Wong et al., 2013). Carotenoid content in chiltepin from Cumpas (1.6–6.03 mg βCE g−1 DM for immature and mature peppers, respectively) and Sahuaripa (0.7–5.7 βCE g−1 DM) was higher than that of red jalapeno (2.04 mg βCE g−1 DM), harvested in Chihuahua (Moreno-Escamilla et al., 2015). It has been reported that the color of the peppers is attributed to a wide variety of pigments, that include flavonoids, chlorophylls and carotenoids, and that their content varies as a function of the ripening stage of the fruit, and geographical and agronomical conditions (Hayano-Kanashiro et al., 2016). The absence of chlorophyll in the mature stage is in agreement with the literature, which states that pigments such as chlorophylls decrease with fruit ripening, rising in fruit formation (immature), and disappearing at commercial maturity (post-harvest) (Gallardo-Guerrero et al., 2002). The different concentration between chlorophyll content in immature samples from Cumpas (higher content) and Sahuaripa can be attributed to different environmental conditions between Cumpas (760 masl) and Sahuaripa (440 masl), which triggers different phytochemical synthesis (Hayano-Kanashiro et al., 2016). In agreement with previous studies, ascorbic acid increased 6–7 times in the mature stage (Rodríguez-Maturino et al., 2012). These results confirm that hot peppers are one of the highest sources of ascorbic acid (Alvarez-Parrilla et al., 2011).
The variability observed in the phytochemical content of fruits from different geographical areas has already been observed for Capsicum annuum fruits (Vera-Guzman et al., 2018). Therefore, the difference in phytochemical content between Cumpas and Sahuaripa samples coincides with those described in the scientific literature. In this way, not only the shape and size of the fruits are modified, but also the content of molecules responsible for color and taste are modified (Vera-Guzman et al., 2018). Considering that both seeds (Cumpas and Sahuaripa) were grown under controlled conditions (greenhouse), the variability observed in the phytochemical content in chiltepin pepper can be attributed not only to the ripening stage, but to genetic differences between the seeds collected from each location. Among differences in environmental conditions between seeds from Cumpas and Sahuaripa may be the water stress that would be greater in Cumpas than in Sahuaripa.
The phytochemical profile (amount of phenolic compounds, flavonoids, capasaicinoids, chlorophylls, carotenoids and ascorbic acid) of chiltepin peppers is essential to assess nutritional and sensory quality (color, flavor, aroma and texture) of the fruit and consequently may also affect consumers preference (Moreno-Ramírez et al., 2018; Vera-Guzman et al., 2018). Additionally, these phytochemicals are considered bioactive compounds that, included in the diet in sufficient quantities may show health benefits, promoting alternative strategies for prevention, management and treatment of chronic diseases (Baiano and Del Nobile, 2016). Normally these diseases are a result of an imbalance on the oxidative status in cells. One of the main strategies to reduce these oxidative damages is through the consumption of exogenous antioxidant compounds with the diet. It has been reported that phytochemicals present in chiltepin pepper have antioxidant potential, inhibiting or stabilizing free radicals (DPPH and ABTS) (Di Sotto et al., 2018; Hayano-Kanashiro et al., 2016; Moreno-Ramírez et al., 2018).
Antioxidant activity of Cumpas and Sahuaripa chiltepin peppers are reported in Table 3. DPPH values for Cumpas and Sahuaripa were above from those previously reported for chiltepin samples from Tamaulipas (70 μmol TE g−1 DM) (Moreno-Ramírez et al., 2018) and Coahuila (29 μmol TE g−1 DM) (Reyes-Acosta et al., 2019), but in the range of those reported in another study for Tamaulipas (57.3 mM TE g−1 DM) (Moreno-Ramírez et al., 2018). The antioxidant activity by the FRAP method has been reported for other varieties of C. annuum (fresh red jalapeño and smoked chipotle) with values below those obtained in this study (51 μmol TE g−1 DM) (Moreno-Escamilla et al., 2015). It has been reported in the literature that variations in antioxidant activity in C. annuum var. glabriusculum are dependent on the geographical origin of the fruit, where the agroecological characteristics modify the expression of the secondary metabolism to adapt to the abiotic conditions of the geographical region to which they are subjected, thus the synthesis of these phytochemicals that regulates antioxidant activity varies among regions (Moreno-Escamilla et al., 2015; Moreno-Ramírez et al., 2018; Vera-Guzman et al., 2018).
In this study, chiltepin pepper showed different antioxidant activity behavior by each of the 3 methods, which can be associated to the different mechanism involved in each method. DPPH is an antioxidant capacity method that measures mainly lipophilic compounds, while the ABTS is associated with the antioxidant activity of hydrophilic compounds in alcoholic extracts (Pérez-Nájera et al., 2013). In addition, both methods base their results on the neutralization of a free radical, which is more representative of the behavior of oxidative stress at biological level. The FRAP test is slightly different from the previous, since it measures antioxidant activity based on the ability that phytochemicals have to act in reducing the Fe3+ ion to Fe2+ in acidic conditions (Sarafi et al., 2018). Although, this mechanism does not neutralize an unstable radical, it does provide information about the importance of phytochemicals in the antioxidant activity of biological reactions that generate unstable molecules due to the presence of metals, complexing them and at the same time delaying production of free radicals (Fenton reaction) (Mardani-nejad et al., 2015). In previous studies, phenolic compounds have been correlated with antioxidant activity through the DPPH and neutralization of hydroxyl radical methods, with correlation coefficients of 0.84 and 0.75, for serrano and jalapeño peppers, respectively (Alvarez-Parrilla et al., 2011; Moreno-Escamilla et al., 2015). In another study, capsaicinoids have been correlated with antioxidant activity by DPPH and ABTS•+ methods with correlation coefficients of 0.9 and 1.0 respectively for chiltepin pepper (Rochín-Wong et al., 2013). Table 4 shows the correlation coefficients of phytochemicals with antioxidant activity values. Interesting, even though phenolic compounds correlated with DPPH and FRAP, they were not significantly, suggesting that other phytochemicals may be related to the antioxidant activity. Results shows that lipophilic compounds such as carotenoids and capsaicinoids exhibit a good relationship with the antioxidant activity of DPPH, while flavonoids that are more hydrophilic showed high correlation with ABTS (Ovando-Martínez et al., 2018). FRAP correlated with both hydrophilic and lipophilic compounds, suggesting that both compounds can reduce metals in vitro. Finally, some studies suggest the analysis of antioxidant activity by combining various methods to distinguish the dominant antioxidant mechanism (Shahidi and Zhong, 2015). The only phytochemicals present in chiltepin pepper that appear to have no antioxidant activity by any of the methods used, are chlorophylls. Although, it has been suggested that these compounds have antioxidant activity, they appear to exert it only in limited light conditions (Alvarez-Parrilla et al., 2011).
| 5. Conclusions | ▴Top |
The chiltepin pepper grown with seeds from Cumpas, Sonora, under controlled greenhouse conditions at both ripening stages (immature and mature) showed higher phytochemical content values, compared to peppers grown using seeds from Sahuaripa, Sonora. These greenhouse-controlled conditions showed that both ripening and seed origin influenced phytochemical content. Results suggest that greenhouse conditions may be a good alternative for the commercial production of chiltepin peppers.
High correlations were observed between DPPH and flaonoids, carotenoids and capsaicinoids, and between ABTS with flavonoids, while FRAP values were correlated with carotenoids, ascorbic acid and capsaicinoids.
Acknowledgments
Financing support by CONACYT (CB 2015 256009) is acknowledge. Authors wish to aknowledge Oneydi Góngora-Pérez and Izamar Olivas-Orduña for their financial support through a Mexican Academic of Sciences and Programa DELFIN Scientific Summer grant during 2015.
| References | ▴Top |