| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 10, June 2020, pages 77-85
The chemical composition, antioxidant activity, and antiproliferative activity of selected seed flours
Zhangyi Songa, †, Yanfang Lib, †, Boyan Gaob, Jihye Leea, Yanbei Wuc, Jianghao Sund, Monica Whenta, *, Pei Chend, Seong-Ho Leea, Liangli Yua
aDepartment of Nutrition and Food Science, University of Maryland, College Park, MD 20742, United States
bInstitute of Food and Nutraceutical Science, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China
cBeijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology & Business University, Beijing 100048, China
dFood Composition and Methods Development Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, United States Department of Agriculture, Beltsville, MD 20705, United States
†These authors contributed equally to the work.
*Corresponding author: Monica Whent, Department of Nutrition and Food Science, University of Maryland, College Park, MD 20742, USA. Tel: +1 301 4054521; E-mail: mwhent@umd.edu
DOI: 10.31665/JFB.2020.10230
Received: June 8, 2020
Revised received & accepted: June 24, 2020
| Abstract | ▴Top |
The phytochemicals in broccoli, carrot, and milk thistle seed flours were extracted with ethanol. Their chemical composition, free radical (ABTS+ and DPPH•) scavenging, and anti-proliferative capacity were evaluated. Silychristin, glucoraphanin, and kaempferol-3-O-rutinoside were the primary components in milk thistle, broccoli, and carrot seed flour extract, respectively. The total phenolic contents of milk thistle, broccoli, and carrot seed flour extracts were 16.9, 8.4, and 1.8 mg gallic acid equivalent/g seed flour, respectively. The seed flour extracts demonstrated antioxidant activity against DPPH• and ABTS+. Milk thistle extract demonstrated antiproliferative activity against colon cancer cells. These findings could promote the utilization of the seed flours to add health value to foods.
Keywords: LC-MS; Carrot seed; Milk thistle seed; Broccoli seed; Functional food
| 1. Introduction | ▴Top |
Vegetable and herb seed flours are the by-products of seed oil production. Typically, the seed flours are discarded after the oil has been extracted. If seed flours contain health-beneficial properties, these flours can be valuable ingredients in nutraceuticals and functional foods. In this scenario, the seed oil production companies can benefit from increased use of the flour and waste can be reduced (Balasundram et al., 2006).
Previous studies have demonstrated that vegetable seed flours contain various antioxidant phytochemicals. Phenolic compounds have been found in broccoli, carrot, and milk thistle seed flours, including quercetin-3-glucoside, luteolin, and silymarin, respectively (Choe et al., 2018; Omar et al., 2012). Phenolic compounds possess the ability to donate hydrogen atoms and chelate metal ions, which gives them antioxidant activity (Balasundram et al., 2006). It is well known that oxidation reactions can cause damage to different tissues in human bodies, such as DNA, proteins, and lipids (Lobo et al., 2010). This damage may eventually lead to chronic disease, including cancer, liver disease, cardiovascular disease, and diabetes (Higashi et al., 2009; Paz-Elizur, et al., 2008; Rains and Jain, 2011). Antioxidant compounds can inhibit the oxidation reaction by interrupting the free-radical chain reaction in lipid oxidation or scavenging singlet oxygen. (Damodaran et al., 2008). Therefore, foods that contain antioxidant components may help to prevent some chronic diseases (Lobo et al., 2010).
The components of vegetable seed flours have previously demonstrated health enhancing properties. Glucoraphanin is one major glucosinolate found both in the broccoli and its seed flour (Choe et al., 2018; Moreno et al., 2006). It can be converted to sulforaphane by myrosinases in the plant or by other myrosinases and microbials in the colon (Juge et al., 2007). Sulforaphane has been shown to inhibit the growth of Helicobactor pylori, a bacteria that increases the risk of gastritis and gastric cancer. Sulforaphane was also shown to prevent tumor growth induced by benzo[a]pyrene and inhibit breast cancer and prostate cancer cells (Fahey et al. 2002; Y. Li et al., 2010; S. V. Singh, et al., 2005). Milk thistle seed flour is rich in silymarin which is a class of compounds including silychristin, silydianin, silybin A & B, and isosilybin A & B (Wallace et al., 2005). It has been reported that silymarin has hepatoprotective activity, and anticancer activity against skin, prostate, and lung cancers (Deep and Agarwal, 2007; Mateen et al., 2010). Carrot seeds have been shown to have antioxidant, hepatoprotective, and anti-inflammatory activity (Singh et al., 2012; Vasudevan et al., 2006). Luteolin is a component of carrot seed flour that has demonstrated anti-cancer properties (Imran et al., 2019).
While some health promoting properties of seed flours have been identified, the complete chemical composition of seed flours has not yet been studied extensively. The research on milk thistle seed flower has focused on silymarins, with few studies of the other compounds in milk thistle seed flour. Previous studies usually evaluated the whole seeds instead of seed flours after oil extraction. Acetone and methanol aqueous solutions have most often been selected as the extraction solvents. (McWalter et al., 2004; Choe et al., 2018).
In this study, ethanol extracts of broccoli, carrot, and milk thistle seed flours after cold pressing were investigated for their chemical composition, antioxidant, and anti-proliferative activity. Ethanol with a concentration over 95% (v/v) is easier to recover than other solvents during oil extraction. Hence, the extraction solvent in this study is more practical for seed production companies.
| 2. Materials and methods | ▴Top |
2.1. Seed flours
Broccoli, carrot, and milk thistle seed flour samples were provided by Botanic Oil Innovation (Spooner, WI, USA). The seed flours were the remains from the cold-pressing process.
2.2. Chemicals
(±)-6-Hydroxy-2,5,7,8-tetramethyl-chromane-2-carboxylic acid (Trolox), fluorescein (FL), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid and Folin & Ciocalteu’s phenol reagent (FC) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethanol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Fisher Scientific (Pittsburgh, PA, USA). Dulbecco’s Modified Eagle Medium (DMEM) was purchased from Corning Inc. (Corning, NY, USA). Fetal bovine serum was purchased from Hyclone Laboratories Inc. (Logan, UT, USA).
2.3. Sample preparation
Each ground seed flour sample (1.5 g) was accurately weighed, extracted with 15 ml of 100% ethanol using a Soxhlet extractor for 3 hours, and filtered through Whatman No.1 filter paper. The filtrate was transferred to a 25 ml volumetric flask, diluted with 100% ethanol to volume, and mixed. All experiments were performed in triplicate.
2.4. Total phenolic contents
The ethanol extracts of seed flour were analyzed for their total phenolic content using Folin-Ciocalteu (FC) reagent with a previously described procedure (Stevanato et al., 2004). The final reaction mixture consisted of 250 μl FC reagent, 750 μl 20% sodium carbonate, 50 μl seed flour extract (or standard, or blank solvent control), and 3.0 ml ultrapure water. The absorbance at 765 nm was measured after 2 hours of reaction at ambient temperature. Gallic acid was used as the standard in concentrations of 50, 100, 200, 400, and 800 μg/mL. Experiments were carried out in triplicate.
2.5. ABTS+ scavenging capacity
The scavenging capacities of the seed flour extracts were measured against ABTS+ generated using a chemical method according to a published protocol (Moore et al., 2006). The working solution of ABTS+ was prepared by oxidizing the solution of ABTS with manganese dioxide in ambient temperature for 30 min and then diluted to 0.700 ± 0.005 at 734 nm. The final reaction mixture contained 1.0 ml of ABTS+ working solution and 100% ethanol as control or 80 μl of the tested sample or standard solution (Trolox). After vortexing for 30 s, the mixture was measured for its absorbance at 734 nm after 90 s of reaction. The standard curve was set using concentrations of 0.01, 0.02, 0.04, 0.06, and 0.08 µmol Trolox/mL. Experiments were conducted in triplicate.
2.6. Relative DPPH• scavenging capacity
The relative DPPH• scavenging capacities (RDSC) of the seed flour extracts were evaluated using an assay described by Cheng and others (Cheng et al., 2006). The procedure of this assay involved a Victor3 multilabel plate reader. To start the reaction, 100 μl of 0.2 mmol/L DPPH was mixed with the Trolox standard, blank solvent control, or the samples. The absorbance was read at 515 nm every minute for 1.5 hours. The DPPH• scavenging capacities were derived from the areas under the curve. Experiments were conducted in triplicate.
2.7. Ultra high-performance liquid chromatography photo diode array high-resolution multi-stage mass spectrometry (UHPLC-PDA-ESI/HRMSn)
The UHPLC-HRMS analysis was performed by an LTQ Orbitrap XL mass spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with an Agilent 1290 Infinity liquid chromatography linked with a DAD detector as it was reported by Choe et al. (2018). The scanning range of the UV-vis spectrum was 190–600 nm. The separations were conducted on a 4.6 mm i.d. × 250 mm, 5 μm particle size Luna C18 column. A gradient mobile phase of HPLC-grade water (solvent A) and acetonitrile (solvent B) both containing 0.1% formic acid (v/v) has the flow rate of 1.0 ml/min. The gradient elution started with 5% of solvent B. Then solvent B was increased to 13% at 5 min through a linear gradient followed by being increased to 20% at 10 min. After that solvent B was increased to 27% at 25 min, followed by increasing to 33% at 40 min. Then solvent B was increased to 50% at 45 min and increased to 90% at 46 min. 90% of solvent B was kept until 51 min and then the 10 min post-run time was carried out for re-equilibration. The injection volume was set as 5 μl. The oven temperature was 40 °C. The compounds were analyzed under a negative ionization mode. The heated capillary temperature was set at 325 °C, capillary voltage at −50 V, spray voltage at 4.5 kV, then tub lens offset voltage at −120 V. The full scan covered the range from m/z 100 to 2,000 with the resolution of 30,000. The data was then postprocessed using QualBrowser part of Thermo Scientific Xcalibur 2.2 software.
2.8. Antiproliferative activity
The selected seed flour extracts were evaluated for their anti-proliferative capacity using colorectal cancer (CRC) cells (HCT116, SW480) and 3T3-L1 preadipocytes. CRC cells (5 × 103 cells per well) were cultured at 37 °C under 5% carbon dioxide in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. After 18 h, the cells were treated with media containing the seed flour extract final concentration of 60 µg flour equivalent per ml in the culture mixture for 24 and 48 h. After that, the supernatant was removed and the mixture of MTT and serum-free media (1:5, v/v) was added. After 3 h, DMSO was added and the absorbance at 540 nm was measured. Experiments were carried out in triplicate.
2.9. Statistical analysis
Data were reported as mean ± standard deviation (SD) for each point. A one-way analysis for variation (ANOVA) with Tukey’s post-hoc test was performed with IBM SPSS Statistics (Version Rel. 22.0.0.0, IBM Inc., Armonk, NY) to identify the significant differences among means. Statistical significance was declared at P < 0.05.
| 3. Results and discussion | ▴Top |
3.1. Total phenolic content
The total phenolic content (TPC) of milk thistle seed flour extract was 16.9 mg GAE/g of seed flour and that of broccoli seed flour extract was 8.4 mg gallic acid equivalent (GAE)/g of seed flour. Carrot seed extract exhibited TPC levels of 1.8 of seed flour (Fig. 1). Parry and others reported that the TPC of 50% acetone extract of milk thistle seed flour was 25.2 mg GAE/g seed flour (Parry et al., 2008), which is higher than that found in the current study.
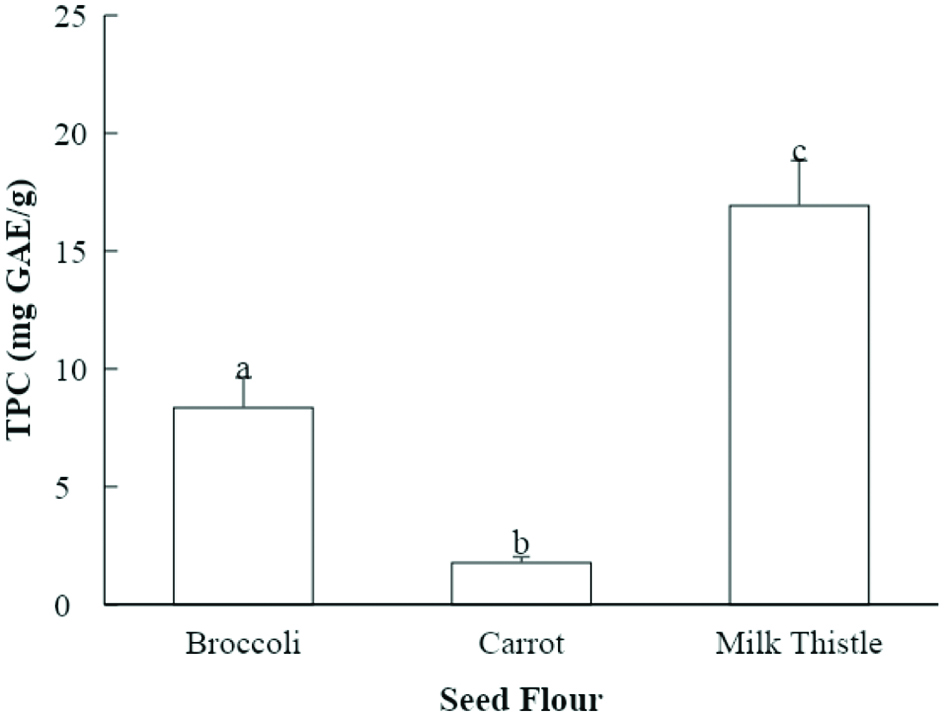 Click for large image | Figure 1. Total phenolic content (TPC) of seed flour samples (mg GAE/g seed flour). Values marked by different letters are significantly different (P < 0.05). |
The evaluation of phenolic content using Folin-Ciocalteau reagent is commonly used for plant extracts. This method may be less accurate when samples have a high content of ascorbic acid or if there are biological impurities in the extract (Sánchez-Rangel et al., 2013). However, it remains an accepted method to estimate phenolic content. The TPC of milk thistle seed flour extract in the current study may be lower than that in the study by Parry et al. due to differences in the extraction solvents or in the growing conditions of the plants.
3.2. Antioxidant activity
The ABTS+ scavenging capacities of the seed flour samples ranged from 32.8 to 54.4 μmol TE per g of seed flour (Fig. 2). Broccoli seed flour extract had the highest value for ABTS+ scavenging capacity (54.4 μmol TE/g of seed flour) among the evaluated seed flours. Carrot seed flour extract had the second highest ABTS+ scavenging capacity (39.2 μmol TE/g of seed flour), followed by the milk thistle seed flour extract (32.8 μmol TE/g of seed flour) (Fig. 2). The seed flour extracts did not have statistically significant differences in ABTS+ scavenging capacity. Previously, the ABTS+ scavenging capacity of 50% acetone extracts of broccoli, carrot, and milk thistle seed flour was measured by Choe et al., and the values were 175.9, 250.0, and 116.2 μmol TE/g (Choe et al., 2018; Choe et al., 2019). The stronger ABTS+ scavenging capacity found by Choe et al. could be related to differences in extraction solvents, as mentioned above.
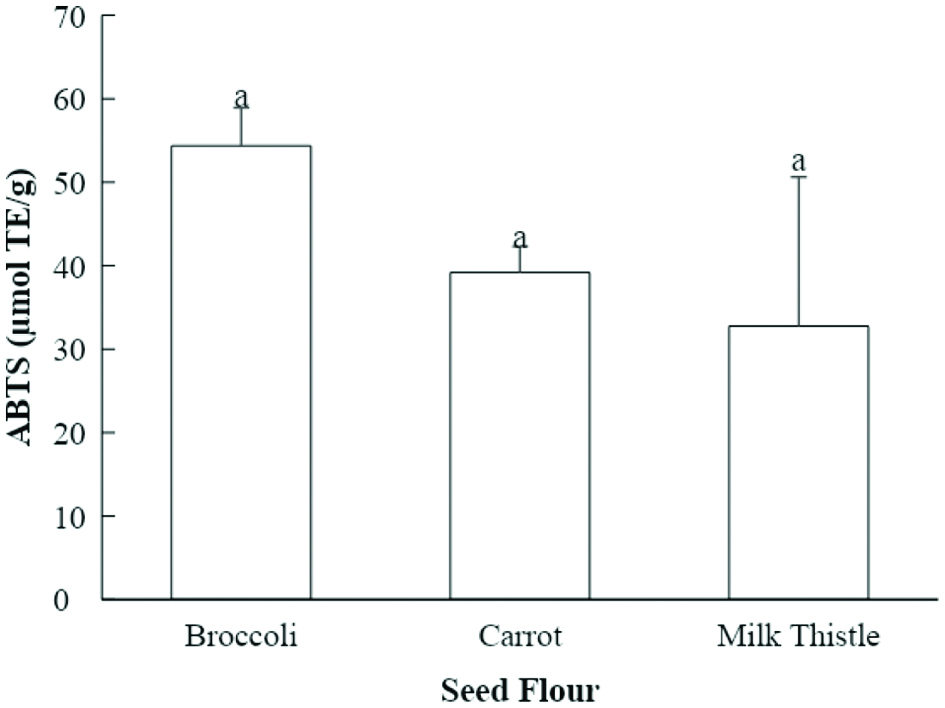 Click for large image | Figure 2. ABTS+ scavenging capacities of seed flour samples (µmol TE/g seed flour). Values marked by different letters are significantly different (P < 0.05). |
The seed flour samples in the current study all exhibited relative DPPH• scavenging capacity (RDSC) (Fig. 3). The RDSC method evaluates the scavenging of DPPH• compared to the tocopherol analogue Trolox. Broccoli seed flour extract had the highest RDSC value of 19.4 μmol Trolox equivalent (TE)/g of seed flour, followed by milk thistle seed flour extract (10.0 μmol TE/g of seed flour). Carrot seed flour extract exhibited lower RDSC value of 3.3 μmol TE/g of seed flour. However, the differences in these values did not show statistical significance. Previous studies showed that 50% acetone (v/v) extracts of broccoli, carrot, and milk thistle seed flour possessed the RDSC values of 84.8, 16.0, and 48.6 μmol TE/g, respectively (Choe et al., 2018; Choe et al., 2019). Parry and others extracted the milk thistle seed flour with 50% acetone (v/v) as well and reported the RDSC level to be 61.1 μmol TE/g (Parry et al., 2008). The values acquired by Choe et al. and Parry et al. were both higher than those in the current study. The RDSC of pumpkin and parsley seed flour extracts evaluated by Parry and others was 2.2 and 18.1 µmol TE/g, respectively (Parry et al., 2008). Parsley seed flour extract from that study had similar RDSC value to that of broccoli seed flour in the current study. Parry and others evaluated the acetone extracts of mullein and cardamom seed flours, and found that the RDSC was 21.2–24.0 and 19.5 µmol TE/g (Parry et al., 2008). The DPPH• scavenging capacities of these two seed flours were stronger than that of milk thistle seed flour from the current study.
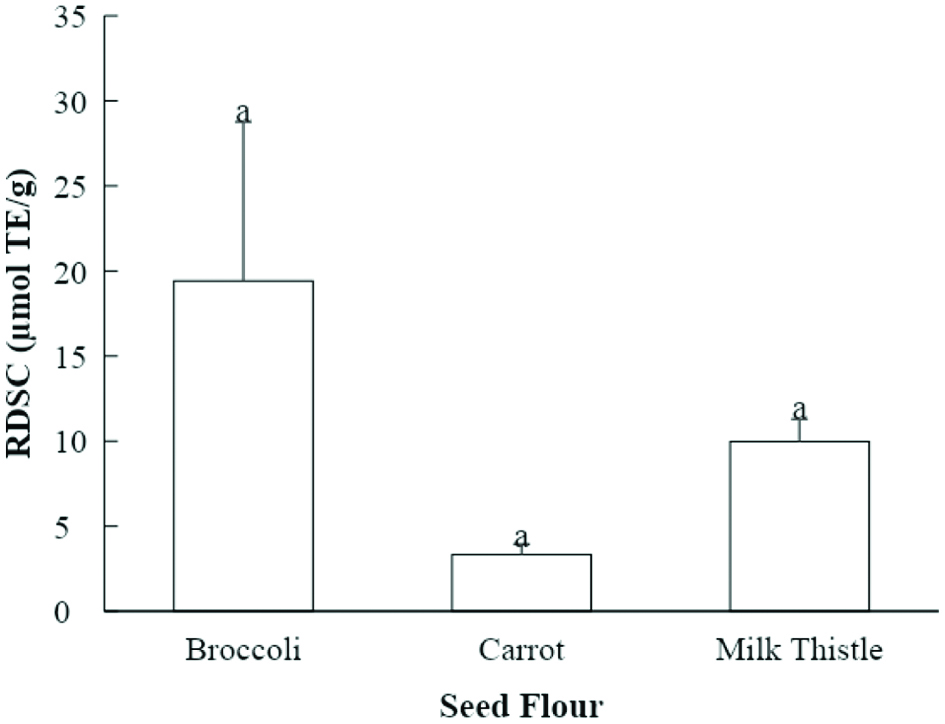 Click for large image | Figure 3. Relative DPPH• scavenging capacities (RDSC) of seed flour samples (µmol TE/g seed flour). Values marked by different letters are significantly different (P < 0.05). |
Apart from the differences in cultivars and growing conditions, an important factor affecting the varying antioxidant capacity might be the extraction solvent. Ayoub and others found that when acetone was applied as a solvent, the extracted phenolic yield was higher compared to methanol used as the extraction solvent (Ayoub et al., 2016). Because ethanol is similar in structure and properties to methanol, it is reasonable to assume ethanol also has less extraction ability than acetone. Hence, the change of extracting solvent may alter the values obtained from antioxidant assay and explain why the RDSC values from the current study were lower than those in other studies.
It was reported in previous studies that there was a significant correlation between the total phenolic content and antioxidant assays including ABTS+ scavenging capacity and relative DPPH• scavenging capacity (da Silva, et al., 2016; de Camargo et al., 2015). In the current study, the milk thistle seed flour extract had the highest total phenolic content. Broccoli seed flour showed the highest antioxidant capacity using the selected methods but did not show a statistically significant difference from the milk thistle seed flour.
3.3. Chemical composition
Eight chemical compounds were provisionally identified in the broccoli seed flour, which included glucoraphanin isomers, glucoerucin, sinapoylhexose, disinapoylgentiobiose, 1,2-disinapoylglucoside, and 1,2,2′-trisinapoylgentiobiose (Table 1 and Fig. S1). In a previous study, McWalter et al. analyzed the glucosinolates in defatted broccoli seed extracted with methanol. Sinigrin, gluconapin, progoitrin, glucoiberin, glucoraphanin, glucoalyssin, and gluconasturtiin were detected in the extract of broccoli seed flour (McWalter et al., 2004). Choe et al. analyzed 50% acetone extract of broccoli seed flour. A total of nine chemical compounds were detected in the extract, eight of which were the same as reported in this study. Choe et al. (2018) detected one additional chemical, quercetin-3-glucoside. The difference in the glucosinolates composition of the broccoli seed flour in this study compared to the others could be due to the different extracting solvents and extraction methods, and different cultivars and growing conditions.
 Click to view | Table 1. Characterization of chemical compounds present in the broccoli seed flour extract using UHPLC-MS |
Ten chemical compounds were tentatively identified in the carrot seed flour, including caffeoyldihexoside, cistanoside F, lycibarbarphenylpropanoid C, kaempferol-3-O-rutinoside isomers, apigenin-7-O-β-D-rutinoside, diosmetin-7-rutinoside, kaempferol-3-O-glucoside isomer, and luteolin. Kaempferol-3-O-rutinoside and luteolin were two primary chemicals identified (Table 2 and Fig. S2).
 Click to view | Table 2. Characterization of chemical compounds present in the carrot seed flour extract using UHPLC-MS |
Luteolin, luteolin 3′-O-β-D-glucopyranoside, and luteolin 4′-O-β-D-glucopyranoside were identified in 60% methanol (v/v) extract of the carrot seed by Kumarasamy and others (Kumarasamy et al., 2005). The compounds identified were similar to those in the current study, because luteolin was one of the greatest peaks in the UHPLC chromatogram of the carrot seed extract. A previous study by Choe et al. (2018) used 50% acetone (v/v) to extract carrot seed flour. They identified the same compounds as those in this study. The comparison with the previous findings shows that ethanol is an adequate extraction solvent to identify the chemical components of carrot seed flour.
A total of 13 chemical compounds were tentatively identified in the milk thistle seed flour, including chlorogenic acid and its isomers, 5-p-(6-caffeoyl-glucopyranosyl)-coumaroylquinic acid isomers, methyl 5-(6-Caffeoyl-glucopyranosyl)-caffeoylquinic acid, taxifolin, silychristin isomers, silybin A, silybin B, and isosilybin (Table 3 and Fig. S3). Wallace and others extracted defatted milk thistle seed flour with boiling ethanol (79 °C) for 10 h to achieve maximum yields. They detected silymarin, taxifolin, silychristin, silydianin, silybinin A and silybinin B with HPLC. (Wallace, et al., 2005). The chemical compositions of two sources of milk thistle seeds were extracted with 100% methanol and analyzed by Mudge and others. Silychristin, silydianin, silybin A, silybin B, isosilybin A, and isosilybin B were detected (Mudge et al., 2015). Choe et al. identified several major chemicals in milk thistle seed flour as well. The seed flour was extracted with 50% acetone (v/v) using sonication. The major compounds identified were silychristin, silybin A, silybin B, and isosilybin A & B (Choe et al., 2019). The silymarin composition identified in the ethanol extracts of the current study is consistent with the major components identified in previous studies. The presence of chlorogenic acid and its derivatives is also consistent with the findings of Choe (Choe et al., 2019).
 Click to view | Table 3. Characterization of chemical compounds present in the milk thistle seed flour extract using UHPLC-MS |
The components identified in the seed flours have previously been shown to have potential effects on human health. Glucophoranin from broccoli seed is converted to sulphorophane, which has demonstrated effects against cancer growth (Fahey et al., 2002; Li et al., 2010). Luteolin from carrot seed and silymarin from milk thistle seed have also shown anti-cancer activity in previous studies (Imran et al., 2019; Deep and Agarwal, 2007; Mateen et al., 2010). The identification of these components shows the potential for the seed flours to add health value in foods. Further studies on the feasibility of using these flours in functional foods would be beneficial.
3.4. Antiproliferative activity
The cell toxicity of broccoli, carrot, and milk thistle seed flours were tested on 3T3-L1 preadipocytes, to measure potential anti-obesity effects of the extracts. Of the tested seed flour extracts, only the milk thistle seed flour showed cell toxicity at the concentration of 60 µg seed flour equivalent per ml (Fig. 4). A higher concentration of seed flour extract may be needed to show effects from the other extracts.
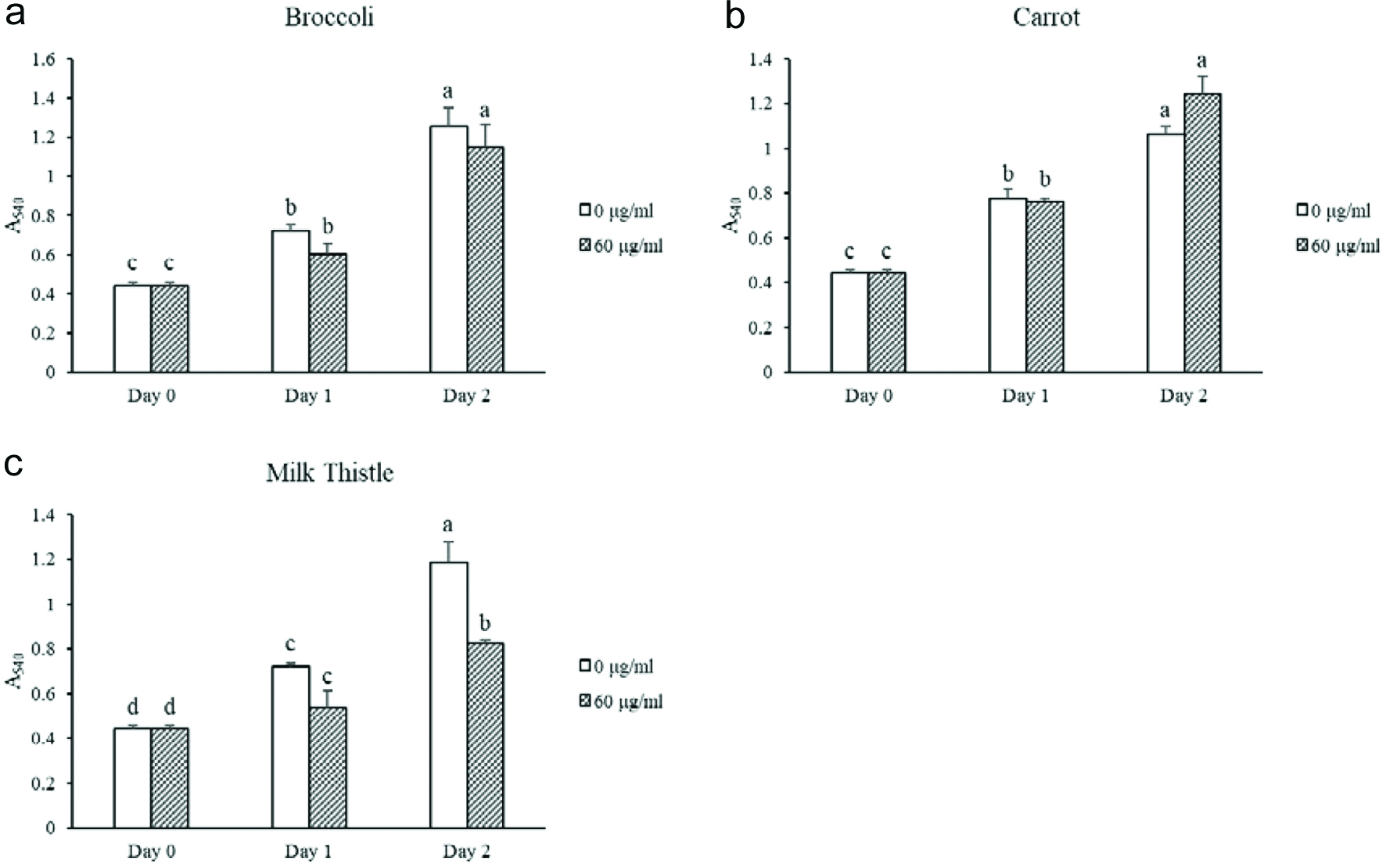 Click for large image | Figure 4. Cell toxicity in 3T3-L1 preadipocytes for selected seed flour extracts. Different letters within the same day represent significant difference (P < 0.05). |
As shown in Figure 5, broccoli and carrot seed flours had no antiproliferative activities in human colon cancer cells SW480 at the testing concentration in 24 and 48 h using 60 µg seed flour equivalent per ml. The extract of milk thistle seed flour inhibited the cell growth of SW480 by 17% in 48 h. Therefore, the milk thistle seed flour potentially possessed antiproliferative activity in SW480 colon cancer cells. Antiproliferative activity against HCT116 colon cancer cells was also tested but did not show significant activity at 48 h using 60 µg seed flour equivalent per ml (Fig. S4). Previous studies have shown antiproliferative effects from the components of these seed flours on different cancer cell types. (Singh et al., 2005; Deep and Agarwal, 2007; Imran et al., 2019). Changes in the concentration of the seed flour extracts may be needed to show more effects on the cells in this study.
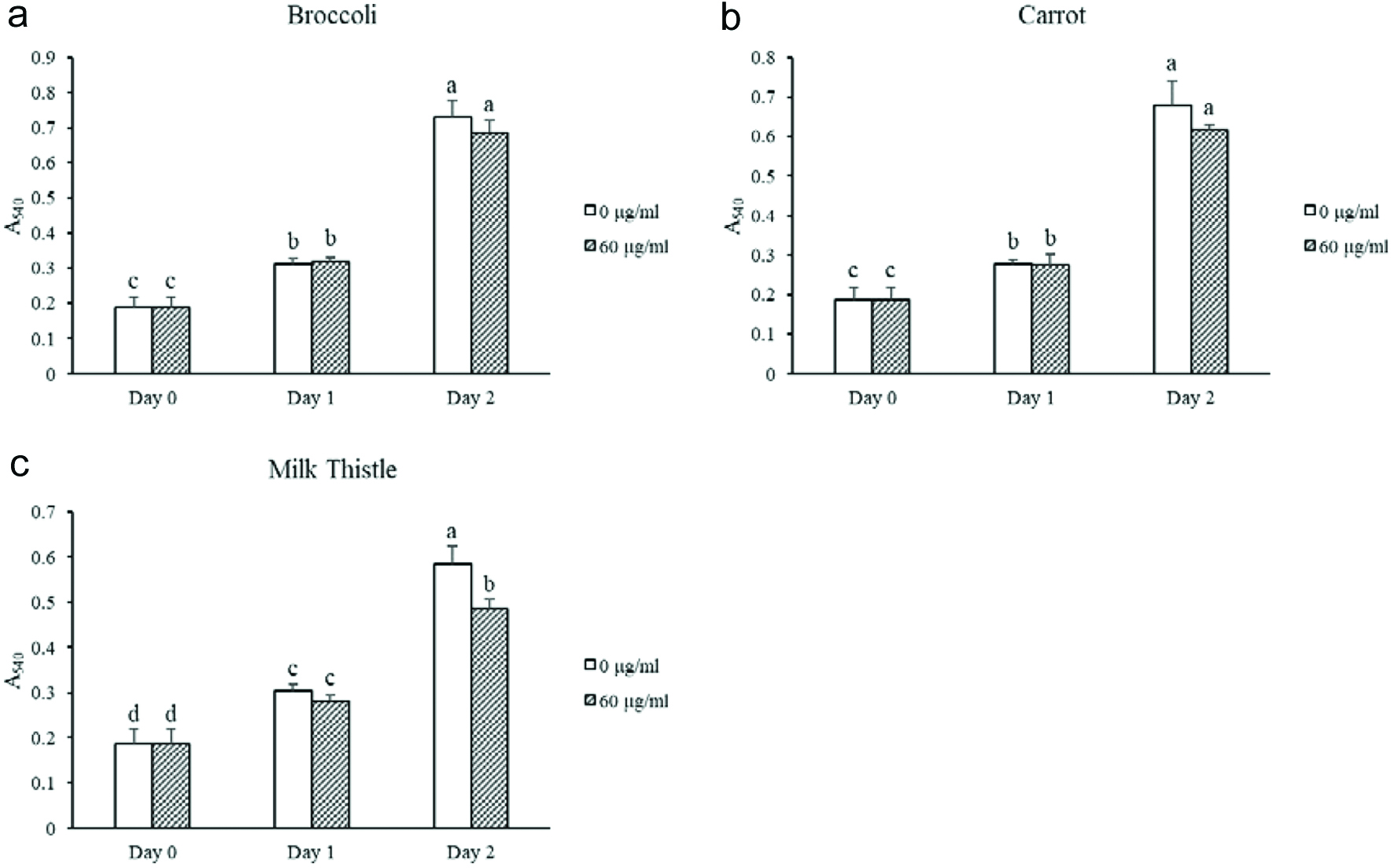 Click for large image | Figure 5. Antiproliferation of SW480 colon cancer cells treated with seed flour extracts. Different letters within the same day represent significant difference (P < 0.05). |
| 4. Conclusion | ▴Top |
This study identified chemicals in broccoli, carrot, and milk thistle seed flours that are recognized as bioactive compounds, such as glucoraphanin, luteolin, and silychristin, respectively. There were also compounds discovered that have been not been frequently identified, including 5-p-(6-caffeoyl-glucopyranosyl)-coumaroylquinic acid and methyl 5-(6-caffeoyl-glucopyranosyl)-caffeoylquinic acid. The results also showed that broccoli, carrot, and milk thistle seed flour all possessed antioxidant activity. Milk thistle seed flour demonstrated the capacity to inhibit the growth of SW480 colon cancer cells in vitro and demonstrated cell toxicity effects on 3T3-L1 preadipocytes. These properties demonstrate potential health benefits and confer value to seed flour in the nutraceuticals market. Further studies of the bioactive properties of seed flours would be useful, such as continued investigation into antiproliferative activity.
| Supplementary Material | ▴Top |
Figure S1. Typical UHPLC chromatogram of the broccoli seed flour extract detected at 348 nm.
Figure S2. Typical UHPLC chromatogram of the carrot seed flour extract detected at 348 nm.
Figure S3. Typical UHPLC chromatogram of milk thistle seed flour extract detected at 348 nm.
Figure S4. Antiproliferation of HCT116 colon cancer cells treated with seed flour extracts. Different letters within the same day represent significant difference (P < 0.05).
| References | ▴Top |