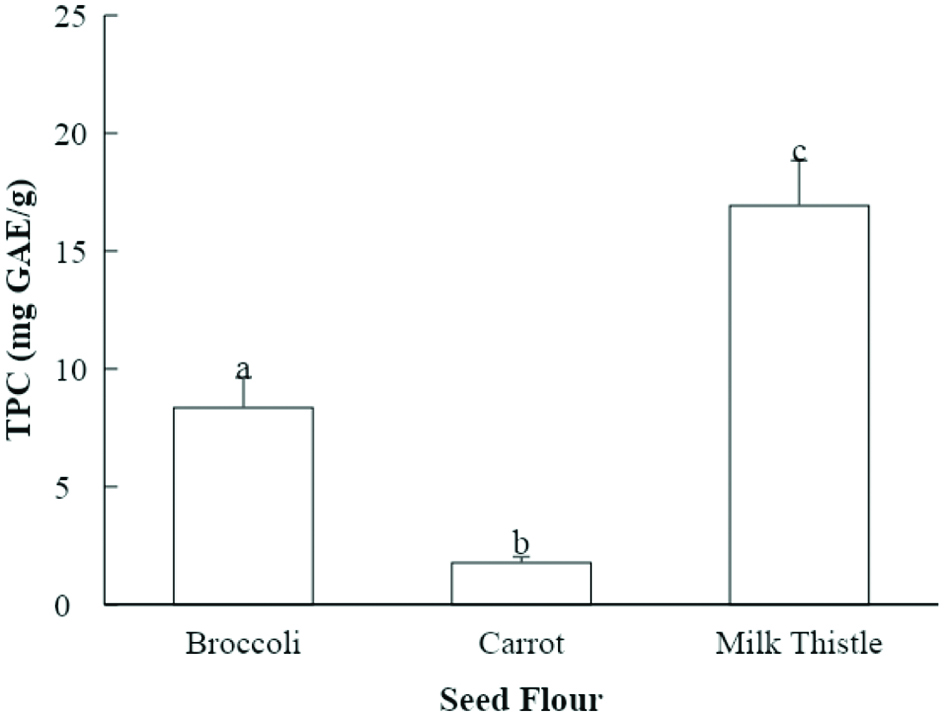
Figure 1. Total phenolic content (TPC) of seed flour samples (mg GAE/g seed flour). Values marked by different letters are significantly different (P < 0.05).
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 10, June 2020, pages 77-85
The chemical composition, antioxidant activity, and antiproliferative activity of selected seed flours
Figures

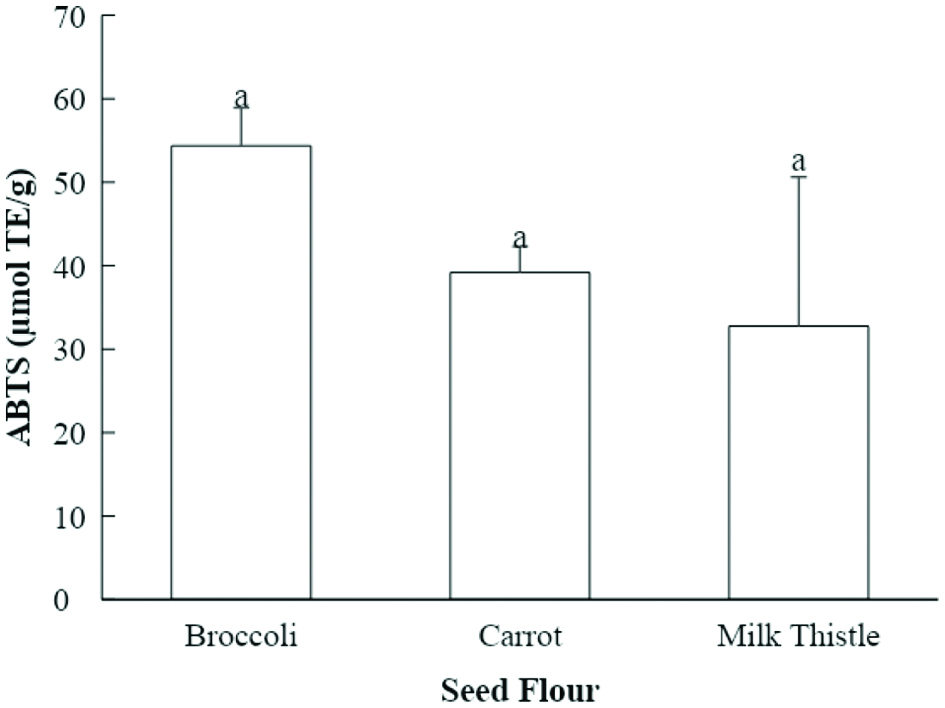
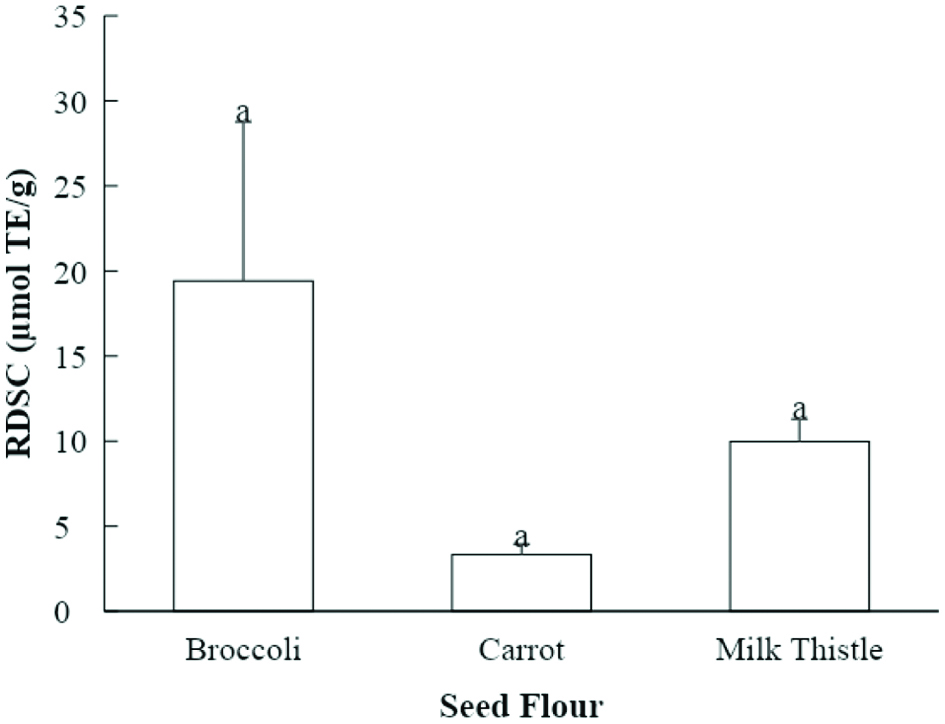
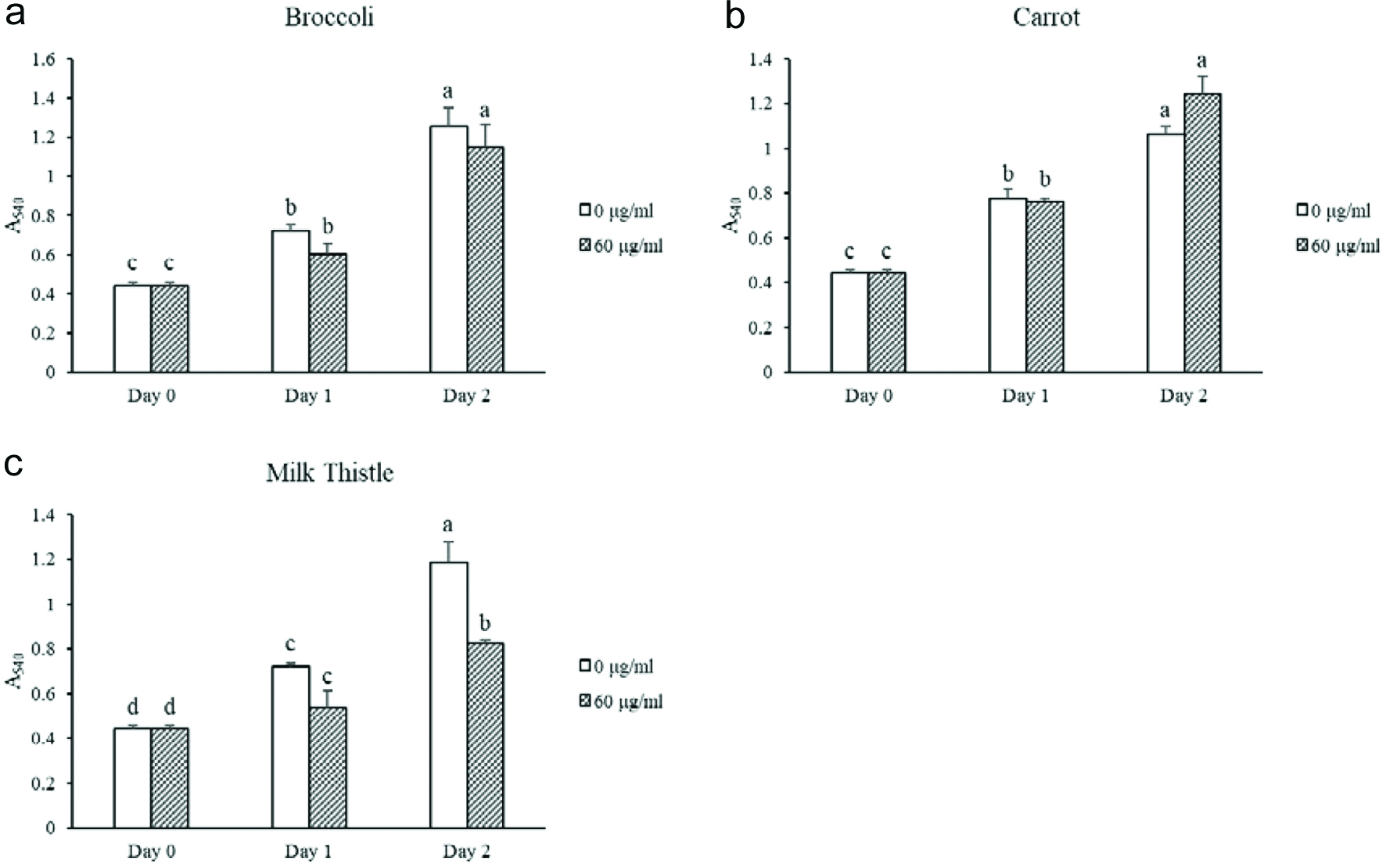
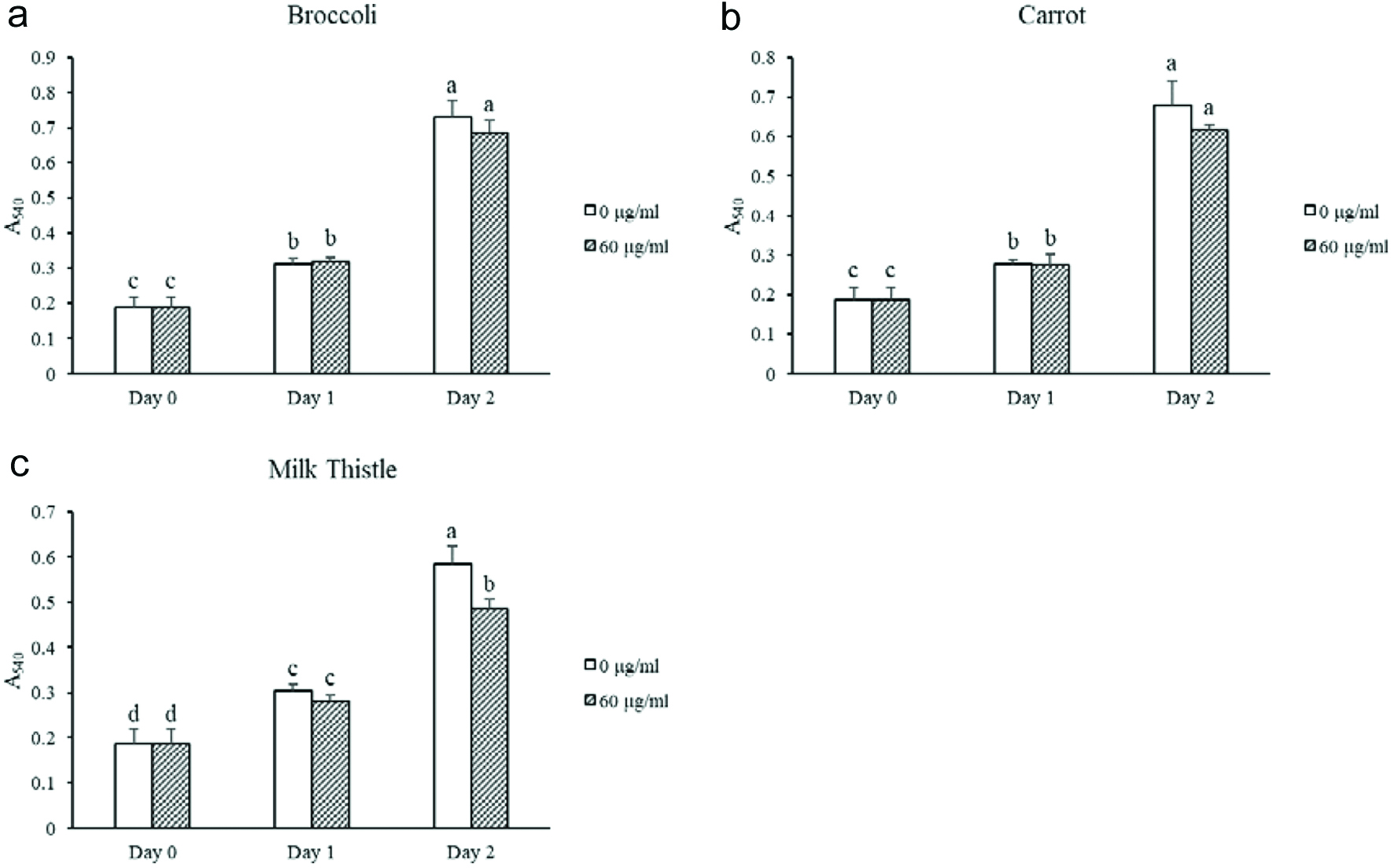
Tables
| Peak ID | tR (min) | Chemical Formula | Tentatively Identification | Theoretical [M-H]− | Experimental [M-H]− | MS2 Ions | References |
|---|---|---|---|---|---|---|---|
| 1,2,3Represented the three greatest peaks based on the typical UHPLC chromatogram peak area. | |||||||
| 1 | 3.62 | C12H23NO10S3 | Glucoraphanin isomer | 436.0406 | 436.0407 | 372.0422, 259.0999 | Rochfort et al., 2008 |
| 2 | 3.99 | C12H23NO10S3 | Glucoraphanin isomer | 436.0406 | 436.0408 | 372.0489, 259.1865 | |
| 3 | 4.22 | C12H23NO10S3 | Glucoraphanin isomer | 436.0406 | 436.0400 | 372.0485, 291.1234, 259.1592 | |
| 4 | 9.87 | C12H23NO9S3 | Glucoerucin | 420.0457 | 420.0457 | 340.2986, 275.0135, 259.0399, 242.0890 | Rochfort et al., 2008 |
| 5 | 11.61 | C17H22O10 | Sinapoylhexose | 385.1135 | 385.1136 | 223.0611, 205.0510 | Shao et al., 2014 |
| 6 | 21.38 | C34H42O19 | Disinapoylgentiobiose1 | 753.2242 | 753.2244 | 529.1563, 223.0612 | Shao et al., 2014 |
| 7 | 25.89 | C28H32O14 | 1,2-Disinapoylglucoside3 | 591.1714 | 591.1708 | 367.1031, 223.0611, 205.0509 | Shao et al., 2014 |
| 8 | 26.86 | C45 H52 O23 | 1,2,2′-Trisinapoylgentiobioside2 | 959.2821 | 959.2809 | 735.1401, 529.1462 | Velasco et al., 2011 |
| Peak ID | tR (min) | Chemical Formula | Tentatively Identification | Theoretical [M-H]− | Experimental [M-H]− | MS2 Ions | References |
|---|---|---|---|---|---|---|---|
| 1,2,3Represented the three greatest peaks based on the typical UHPLC chromatogram peak area. | |||||||
| 1 | 7.60 | C21H28O14 | Caffeoyldihexoside | 503.1401 | 503.1400 | 341.1647, 179.0352, | Oszmiański et al., 2018 |
| 2 | 7.80 | C21H28O13 | Cistanoside F | 487.1452 | 487.1452 | 325.1151, 179.0351 | Xiao-Long et al., 2019 |
| 3 | 8.00 | C22H30O14 | Lycibarbarphenylpropanoid C | 517.1557 | 517.1558 | 355.2340, 193.0508 | Li et al., 2020; Xiao-Long et al., 2019 |
| 4 | 11.38 | C27H30O15 | Kaempferol-3-O-rutinoside isomer | 593.1506 | 593.1505 | 447.1625, 285.1403 | Inbaraj et al., 2010 |
| 5 | 15.17 | C27H30O15 | Kaempferol-3-O-rutinoside isomer1 | 593.1506 | 593.1501 | ||
| 6 | 16.27 | C21H20O11 | Kaempferol-3-O-glucoside isomer3 | 447.0927 | 447.0932 | 285.0400, 284.0326 | Kelebek, 2016 |
| 7 | 18.08 | C27H30O14 | Apigenin-7-O-β-D-rutinoside | 577.1557 | 577.1558 | 269.0451, 227.1987 | Wang et al., 2017 |
| 8 | 18.95 | C28H32O15 | Diosmetin-7-rutinoside | 607.1663 | 607.1661 | 299.1501 | Gironés-Vilaplana et al., 2014 |
| 9 | 19.90 | C21H20O11 | Kaempferol-3-O-glucoside isomer | 447.0927 | 447.0940 | 285.0403 | Kelebek, 2016 |
| 10 | 30.26 | C15H10O6 | Luteolin2 | 285.0399 | 285.0402 | 217.1554, 199.0589, 175.0652, 133.0716 | De la Torre-Carbot et al., 2005 |
| Peak ID | tR (min) | Chemical Formula | Tentatively Identification | Theoretical [M-H]− | Experimental [M-H]− | MS2 Ions | References |
|---|---|---|---|---|---|---|---|
| 1,2,3Represented the three greatest peaks based on the typical UHPLC chromatogram peak area. *CE stands for a chlorogenic acid equivalent. SE stands for a silibinin equivalent. | |||||||
| 1 | 8.24 | C16H18O9 | Chlorogenic acid isomer | 353.0873 | 353.0875 | 191.0563, 179.0351, 135.0455, 111.0900 | Willems et al., 2016 |
| 2 | 10.15 | C16H18O9 | Chlorogenic acid isomer | 353.0873 | 353.0876 | 191.0563, 173.0940, 127.0063, 111.1268 | |
| 3 | 13.90 | C31H34O16 | 5-p-(6-caffeoyl-glucopyranosyl)-coumaroylquinic acid isomer | 661.1769 | 661.1760 | 499.1453, 337.1499, 173.1541, 163.0531 | Choe et al., 2019 |
| 4 | 15.32 | C31H34O16 | 5-p-(6-caffeoyl-glucopyranosyl)-coumaroylquinic acid isomer | 661.1769 | 661.1764 | 499.1456, 337.1122, 173.0676, 163.1128 | |
| 5 | 15.62 | C32H36O17 | Methyl 5-(6-Caffeoyl-glucopyranosyl)-caffeoylquinic acid | 691.1874 | 691.1886 | 661.1766, 499.1458, 173.0309 | Choe et al., 2019 |
| 6 | 17.47 | C15H12O7 | Taxifolin | 303.0505 | 303.0511 | 285.0483, 177.0815, 124.9742 | Álvarez-Ferná́ndez et al., 2016 |
| 7 | 22.83 | C25H22O10 | Silychristin isomer | 481.1135 | 481.1141 | 463.1035, 453.1453, 445.1099, 179.0201 | Shibano et al., 2007 |
| 8 | 24.74 | C25H22O10 | Silychristin isomer | 481.1135 | 481.1142 | 463.1968, 453.1838, 419.2541, 355.1195, 179.0223 | |
| 9 | 25.33 | C25H22O10 | Silychristin isomer2 | 481.1135 | 481.1141 | 463.0804, 453.0892, 355.0830, 178.9522 | |
| 10 | 26.04 | C25H22O10 | Silychristin isomer1 | 481.1135 | 481.1139 | 463.1879, 453.1561, 327.0796, 179.0533 | |
| 11 | 36.87 | C25H22O10 | Silybin A | 481.1135 | 481.1142 | 463.0794, 453.1011, 355.1670, 300.9895 | Shibano et al., 2007 |
| 12 | 37.35 | C25H22O10 | Silybin B | 481.1135 | 481.1141 | 463.1127, 453.1870, 355.1905, 300.9849 | |
| 13 | 40.31 | C25H22O10 | Isosilybin3 | 481.1135 | 481.1137 | 463.2560, 453.1837, 301.1072, 283.0059 | Shibano et al., 2007 |