| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 1, March 2018, pages 143-152
Juglone down-regulates the Akt-HIF-1α and VEGF signaling pathways and inhibits angiogenesis in MIA Paca-2 pancreatic cancer in vitro
Namrata Karkia, Sita Aggarwalb, c, Frank Greenwayb, Roger A. Lained, Jack N. Lossoa, *
aSchool of Nutrition and Food Sciences, Louisiana State University, Baton Rouge, LA 70808
bPennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808
cCurrent; Southeastern Louisiana University, Hammond, LA 70401
dDepartment of Biological Sciences, Louisiana State University, Baton Rouge, LA 70808
*Corresponding author: Jack N. Losso, School of Nutrition and Food Sciences, Louisiana State University, Baton Rouge, LA 70808
DOI: 10.31665/JFB.2018.1133
Received: January 25, 2018
Revised received & accepted: March 5, 2018
| Abstract | ▴Top |
Hypoxia-inducible factor -1 alpha (HIF-1α), vascular endothelial growth factor (VEGF), and phosphorylated Akt (p-Akt) are critical in pancreatic cancer cell growth, angiogenesis and metastasis. The ability of MIA Paca-2 pancreatic cancer cells to migrate and invade after treatment with juglone (5 hydroxy-1, 4-naphthoquinone) was analyzed by a transwell invasion and migration assay, a wound healing assay, and an in vitro tube formation assay using human umbilical vein endothelial cells (HUVEC). ELISA was used to determine the levels of HIF1-α. Western blot analysis was used to determine the level of VEGF, p-Akt, and carbonic anhydrase IX expression. Juglone down-regulated the expression of HIF-1α, VEGF, and significantly suppressed the phosphorylation of Akt. Juglone dose-dependently inhibited the metastatic potential of pancreatic cancer cells by inhibiting cell migration, invasion and wound healing assays. Juglone attenuated the aggressiveness of pancreatic cancer cells in vitro.
Keywords: Juglone; angiogenesis; pancreatic cancer; HIF-1α; VEGF; p-AkT
| 1. Introduction | ▴Top |
Pancreatic cancer accounts for 3% of all cancers in the United States and still is the fourth leading cause of cancer related deaths in the US. The estimated number of new cases in the year 2015 was 48,960 and number of death was 40,560 (Siegel et al., 2015). The five- year survival rate for patients with pancreatic cancer is 6% with median survival time of 3–6 months (Sun et al., 2014). Surgical resection, chemotherapy or radiotherapy are the current treatment approaches for early stage pancreatic cancer; however there is no curative option for advanced pancreatic cancer, which is usually metastatic in nature. Better treatment approaches are needed to target pancreatic cancer metastasis.
The metastatic ability of malignant tumors plays an important role in tumor progression. Cancer metastasis is accountable for more than 90% of cancer related deaths (Gupta and Massague, 2006; Steeg, 2006; Valastyan and Weinberg, 2011). The principal sites of metastasis for pancreatic cancer cells are the liver and lungs (Nguyen et al., 2009). Metastasis involves a series of four sequential steps: detachment, migration, invasion and adhesion described as the “invasion-metastasis cascade” (Fidler, 2003; Klein, 2008). The adhesion and detachment ability of cells, interaction with other cells and with the extracellular matrix defines the extent of invasiveness of tumor cells. Angiogenesis, or the formation of new blood vessels, is an essential process of tumor metastasis (Folkman, 2002).
Tumor cells produce growth factors like vascular endothelial growth factor (VEGF), which regulates angiogenesis and stimulates blood vessel formation. The newly formed blood vessels are a primary route for tumor cells to escape the primary site and enter into circulation (Zetter, 1998). Hypoxia is a characteristic of solid tumors including pancreatic cancer. Hypoxia activates the hypoxia-inducible factor-1 alpha (HIF-1α) which in turn regulates the expression of angiogenic factors such as VEGF. It is suggested that targeting HIF-1α can inhibit angiogenesis, invasion, migration, and metastasis of pancreatic cancer.
Several anti-angiogenic therapeutic approaches have been developed. Several natural products, phytochemicals and dietary agents such as gallic acid, rhein, luteolin, and quercetin have been extensively studied for their anti-angiogenic potential as a therapy against cancer (Wasundara et al., 2015).
Juglone (5-hydroxy-1,4- naphthoquinone) is a natural quinone isolated from plants of the Juglandaceae family. Juglone is commonly found in black walnut, English walnut, butternut and others (Aithal et al., 2009). It is a component of ancient medicine for ringworm, fungal, bacterial and viral infections (Thakur, 2011), and limited studies have been conducted to understand the anti-tumor properties of juglone (Aithal et al., 2009; Avci et al., 2016; Filaet al., 2008; Sugie et al., 1998; Wu et al., 2017). Juglone at concentration of 5–10 μM is not toxic to normal cells (Costantino et al., 2016; Wahedi et al., 2016). The present study was designed to determine juglone inhibitory activities against the migration and invasion of human pancreatic cancer MIA Paca-2 cells in vitro.
| 2. Materials and methods | ▴Top |
2.1. Reagents
Juglone was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary and secondary antibodies against vascular endothelial growth factor (VEGF, #sc-7269) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); antibodies against β-actin (#4967), Akt (#2966) and p-Akt (#2531s) were purchased from Cell Signaling Technology (Danvers, MA, USA); antibody against carbonic anhydrase IX (F48969) was obtained from NSJ Bioreagents (San Diego, CA, USA).
2.2. Cell culture
Human umbilical vein endothelial Cells (HUVEC) and pancreatic cancer MIA Paca-2 cells were purchased from American Type Cell Culture (Manassas, MD, USA). Cells were cultured in a humidified incubator containing 5% CO2 at 37 °C. HUVEC were maintained in Medium 200 (Invitrogen) supplemented with Low Serum Growth Supplement (LSGS kit, Invitrogen, Carlsbad, CA, USA). MIA Paca-2 cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10 % fetal bovine serum, penicillin and streptomycin and both cell lines were sub-cultured after reaching 70–80% confluency.
2.3. In-vitro endothelial cells tube formation assay
This assay was performed as suggested by the manufacturer using the in vitro angiogenesis assay kit from MilliporeTM. Briefly, 50 μL of matrigel were added to the wells of a 96-well plate at 4 °C and allowed to polymerize at 37 °C for at least 1 h. Human umbilical vein endothelial cells (HUVEC) were seeded at a density of 1 × 103 cells per well on the surface of the matrix. Cells were treated with juglone at concentrations ranging from 100 nm to 10 μM. In a different set, HUVEC were also treated with conditioned media collected from MIA Paca-2 cells treated with juglone. Cells were allowed to incubate at 37 °C for at least 6 h and tube formation was analyzed and photographed using a Leitz phase-contrast inverted microscope at 10× magnification. Lengths of the capillaries were measured by using Image-Pro software (Media Cybernetics, Inc, Warrendale, PA, USA).
2.4. Wound healing migration assay
MIA Paca-2 cells were plated in 96-well plates overnight to achieve a confluent cell layer. A linear wound was created with micropipette tips and cell layers were washed twice with serum free media to remove the floating cells. Cells were then treated with juglone (0, 1, 5 or 10 μM). The effect of juglone on tumor cell migration was observed after 24 h incubation. Each well of the plate was photographed at the same site at 0 h and 24 h and compared. The width of the wound at 0 h and 24 h was measured using Image-Pro Software. Results are expressed as the ratio of wound width at 0 and 24 h after treatment (Moutasim et al., 2011).
2.5. Transwell migration assay
Transwell Migration is also referred to as Boyden chamber assay. MIA Paca-2 cells at 70% confluence were treated with juglone (0, 1, 5 or 10 µM)) for 6 h and after treatment viable cells were harvested with 0.25% trypsin EDTA (Invitrogen, Carlsbad, CA, USA) and plated at a density of 5 × 104 MIA Paca-2 cells in 200 μL serum-free growth media per insert in the apical chamber of Transwell with 8 μM pore size (Corning). The basal chamber was filled with 500 μL of medium with 20% FBS as a chemoattractant. After 72 h of incubation, media from the apical and basal chambers of transwell were removed and non-migrated cells in the apical chamber were scraped off by moist cotton swab. Cells in the undersurface of the transwell inserts were washed with phosphate buffer saline (PBS) and then fixed with 70% ethanol for 15 min at room temperature. Fixed cells were then stained with 0.25% crystal violet (CV). After 30 min of incubation with CV, excess dye was removed and the inserts were washed in running water. Images of stained cells were captured with Leitz phase-contrast inverted microscope. Retained CV in cells were dissolved with 33% acetic acid and optical density was read at 595 nm (Benchmark Plus, BioRad, Hercules, CA, USA).
2.6. Matrigel invasion assay
Matrigel invasion assay is used to evaluate chemotaxis and the ability of tumor cells to invade the extracellular matix (Kramer et al., 2013; Moutasim et al., 2011). The matrigel invasion assay is similar to the transwell migration assay however; the difference in the set-up is that the inserts in the transwell insert are first coated with ECM matrix (matrigel). Matrigel (BD Biosciences) was thawed overnight at 4 °C, diluted with serum-free media in the ratio of 1:1 and 50 μL of matrigel were added to each transwell insert. Transwell inserts with matrigel were placed in the incubator at 37 °C for at least 1 h to form a thin layer gel. The rest of the protocol was similar to the one described in the migration assay.
2.7. Expression of HIF-1α and VEGF
MIA Paca-2 cells were plated in a black 96 well plate (R&D Systems, Minneapolis, MN) at a cell density of 1 × 104 cells per well. After allowing cells to attach overnight, cells were treated with juglone (1, 5 or 10 µM) for 6 h. After required incubation, media were removed and cells were washed with PBS. Immediately, cells were fixed with 4% formaldehyde for 15 min. After 15 min, cells were washed with PBS and endogenous peroxidase activity of cells was blocked by incubation with 30% hydrogen peroxide for 15 min. Cells were then incubated with blocking buffer for 1 h, washed with PBS at least 3 times and incubated overnight with HIF-1α antibody.
VEGF was analyzed in cell lysates by Western Blot. In brief, cells were plated at a cell density 2.5 × 105 cells/well in a six well plate. After seeding overnight, cells were treated with juglone for 6 h. After 6 h, media were removed and cells washed with phosphate buffer saline. Whole cell lysate was prepared by using cell lysis buffer from Cell Signaling Technology (Danvers, MA, USA). Protein concentrations of the samples were determined using the BCA assay. Fifty micrograms of protein was loaded in SDS polyacrylamide gel; 4–12% Bis-Tris (Invitrogen). Prepared samples were separated by gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen). Membranes were blocked with 5% BSA in TBS containing 0.05% Tween 20 (TBS-Tween) for 1 h. The PVDF membranes were then incubated with primary antibody in TBS-Tween containing 5% BSA overnight at 4 °C with gentle agitation. Visualization of the bound primary antibody was done by probing the membrane with horseradish peroxidase–conjugated secondary antibodies and exposure to chemiluminescent substrate (Thermo Fisher Scientific, Hampton, NH, USA). Chemiluminescence was recorded by BioRad Imaging system. For quantification, densitometric analysis of the bands was done by Quantity One 1-D analysis software (BioRad).
2.8. Statistical analysis
All the experiments were conducted in triplicates. Results are expressed as means ± SD of experiments. Analysis of variance (ANOVA) was conducted to examine the differences between treatments followed by Tukey’s analysis using SAS. A P-value of <0.05 was considered to be statistically significant.
| 3. Results | ▴Top |
3.1. Juglone inhibits in-vitro tube forming ability of endothelial cells
Highly organized, extensively aligned capillary-like structures were formed in untreated HUVEC. Similarly, highly organized and extensively aligned capillary-like structure were formed when HUVEC were treated with conditioned media from MIA Paca-2 cells. Juglone dose-dependently inhibited endothelial cell alignment (Fig. 1 and 2). The tube formation in HUVEC was higher when media from cancer cells was used when compared to regular media used in culturing HUVEC (Fig. 2), which might be due to the increased level of growth factors released by cancer cells in culture. At a concentration as low as 250 nM, juglone reduced tube length and at concentrations of 5 and 10 µM the network was completely disrupted and was unreadable (NR).
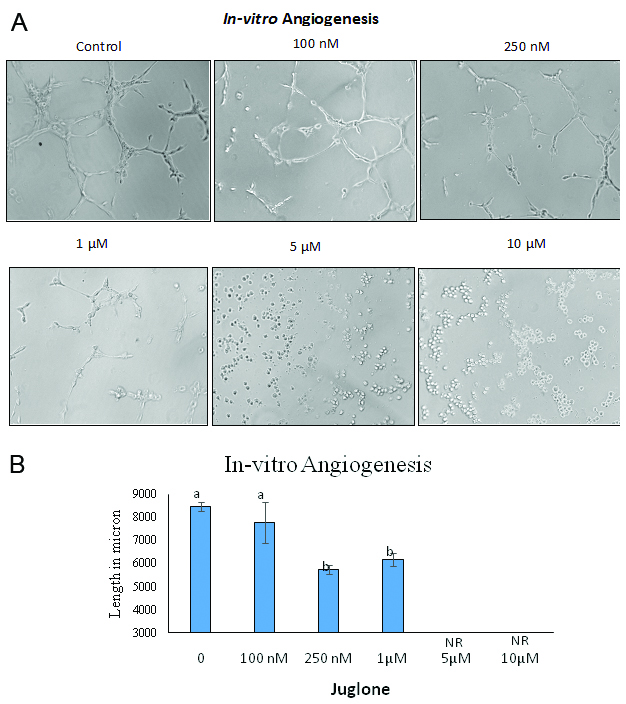 Click for large image | Figure 1. Effect of juglone on HUVEC tube formation assay |
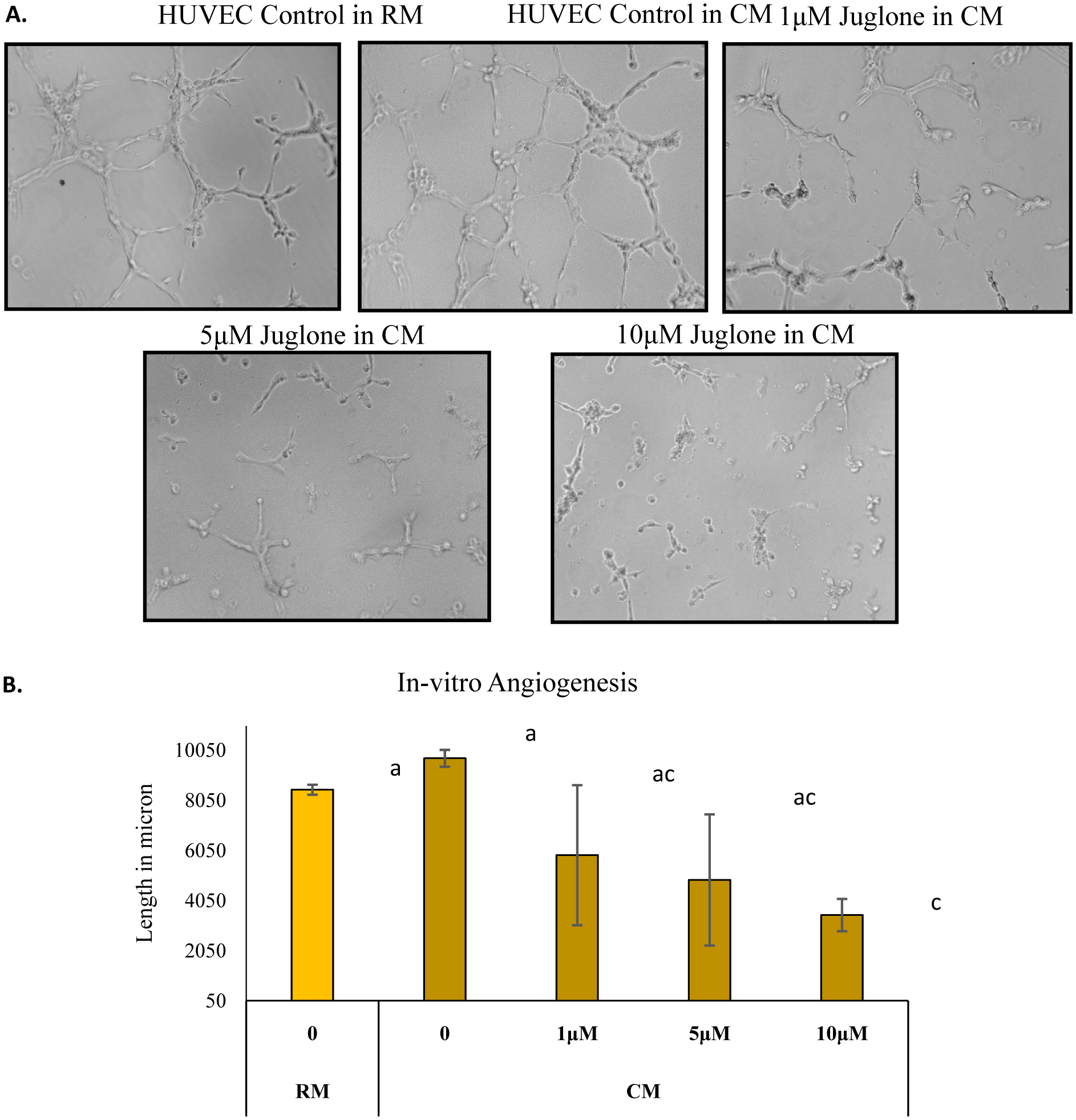 Click for large image | Figure 2. Effect of juglone on HUVEC tube formation using conditioned media (CM) from MIA Paca-2 cells |
3.2. Juglone suppresses pancreatic MIA Paca-2 cells migration
The wound scratch assay was performed for analyzing the effect of juglone in inhibiting migration. The width of the wound was measured after 24 h of treatment and the ratio of wound size at 0 and 24 h was calculated (Fig. 3A). All the treatments with juglone resulted in inhibition of wound closure. In untreated cells, the ratio was 1.3. In cells treated with 1, 5 and 10 μM juglone, the ratio dropped to 1.24, 1.11, and 0.91, respectively. These ratios are presented in terms of percentage of untreated cells in Fig. 3 B. A similar pattern was observed in the transwell chamber migration assay where juglone treatment significantly decreased the number of migrated cells at 5 and 10 μM (Fig. 4).
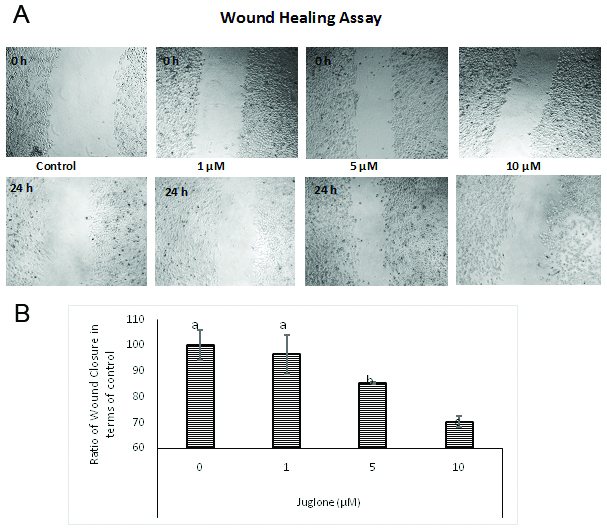 Click for large image | Figure 3. Effect of juglone on wound closure. |
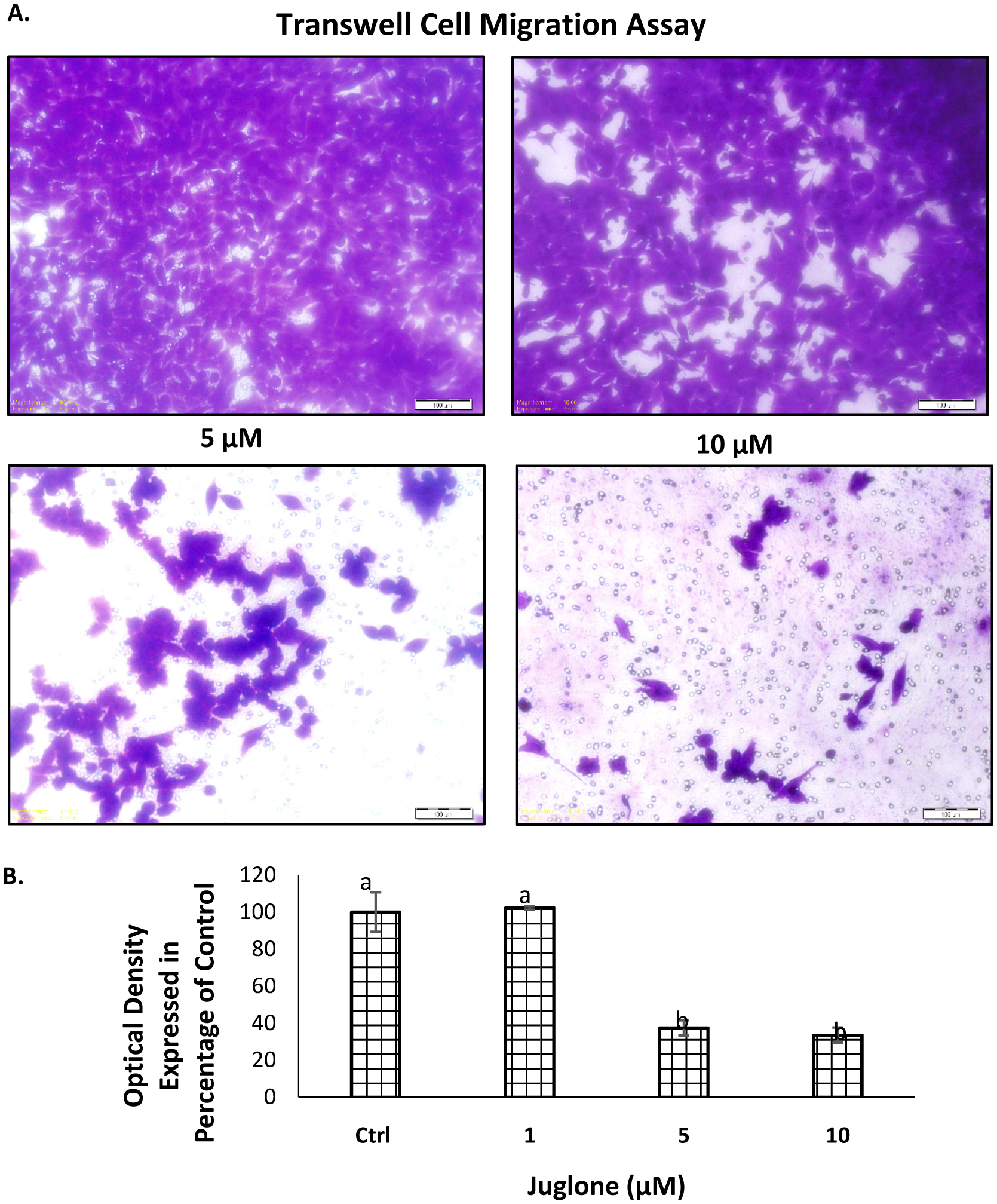 Click for large image | Figure 4. Effect of juglone on the migration of MIA Paca-2 cells. |
3.3. Juglone inhibits pancreatic cancer MIA Paca-2 cell invasion
The Boyden Chamber assay was used to evaluate the effect of juglone on MIA Paca-2 cell invasion. To rule out the cytotoxic effect of juglone on migration and invasion, cells were treated with juglone (1, 5 or 10 µM) for 6 h and only viable cells were collected and utilized for both migration and invasion assay. Results in Fig. 5 A show that juglone dose-dependently inhibited the invading potential of MIA Paca-2 cells. Treatment with juglone at 1, 5 and 10 μM allows invasion in matrigel of MIA Paca-2 cells by 65.2, 71.1, and 99.7 %, respectively. (Fig. 5).
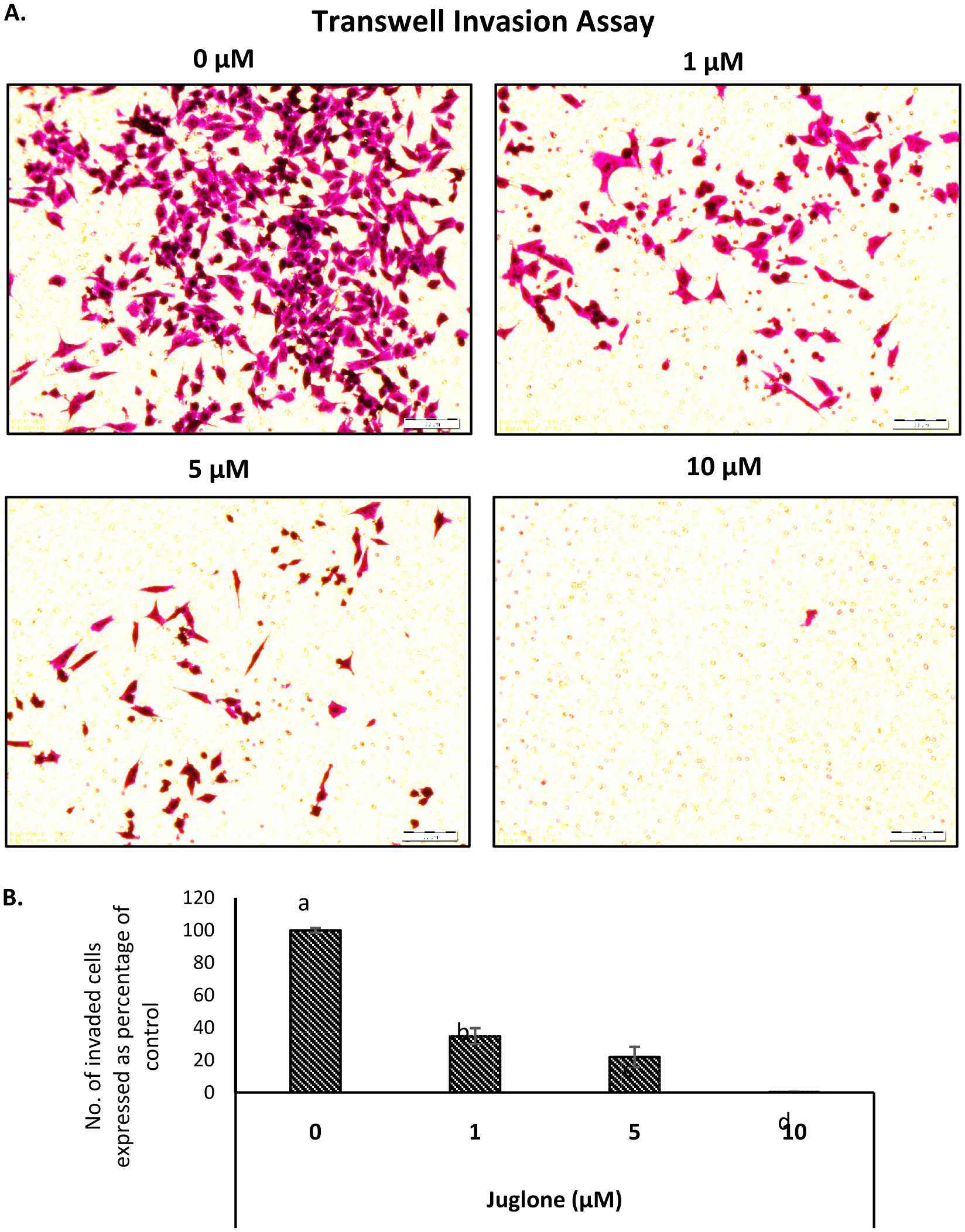 Click for large image | Figure 5. Effect of juglone on the invasion ability of MIA Paca-2 cells. |
3.4. Juglone inhibits expression of proteins associated with cell migration and invasion in vitro
The level of HIF-1α in MIA Paca-2 cells after treatment of juglone was measured by cell based ELISA and is reported in Fig. 6. Juglone dose-dependently inhibited HIF-1α, VEGF, and p-Akt compared to untreated cells (Fig. 7 and 8). Carbonic anhydrase IX was analyzed by Western blot and results in Fig. 10 show that high expression of carbonic anhydrase IX was observed in MiaPaca-2 control and MiaPaca-2 incubated with CoCl2 only. In juglone-treated MiaPaca-2 cells, juglone dose-dependently inhibited the level of the enzyme.
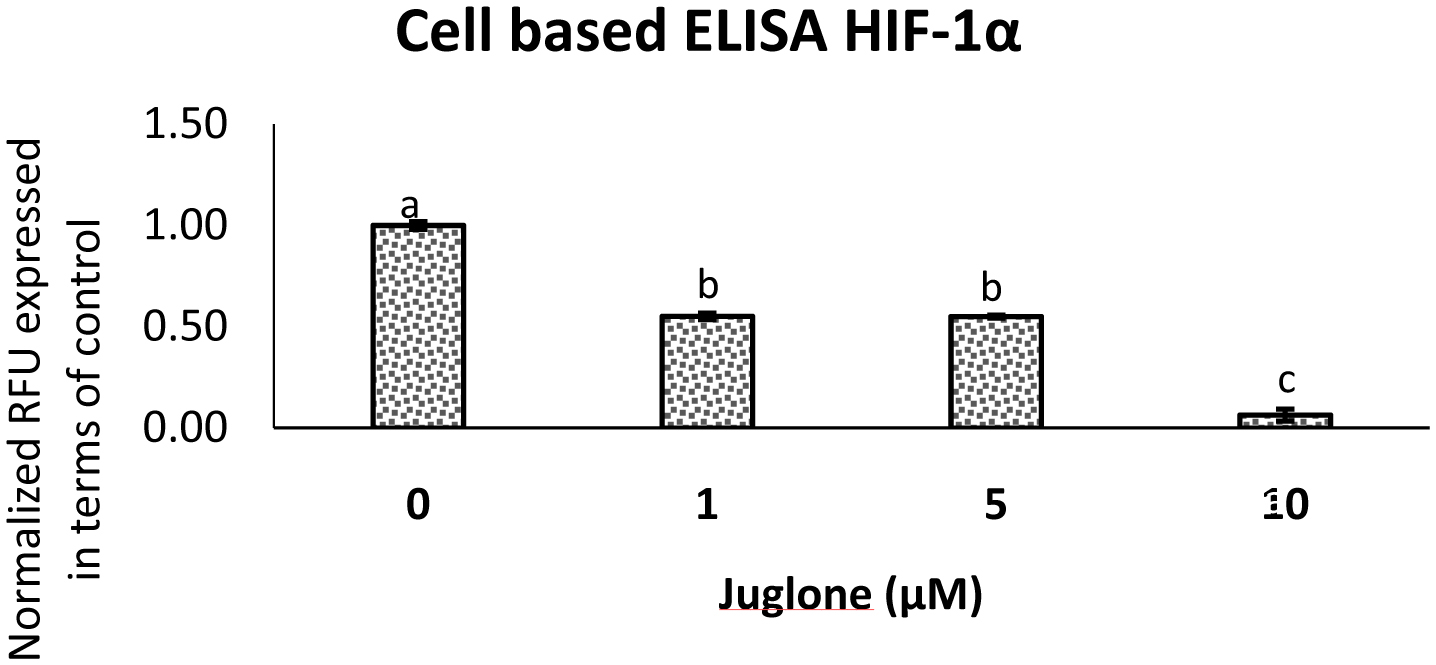 Click for large image | Figure 6. Expression of HIF-1α in MIA Paca-2 cells treated with juglone as measured by a cell based ELISA assay. |
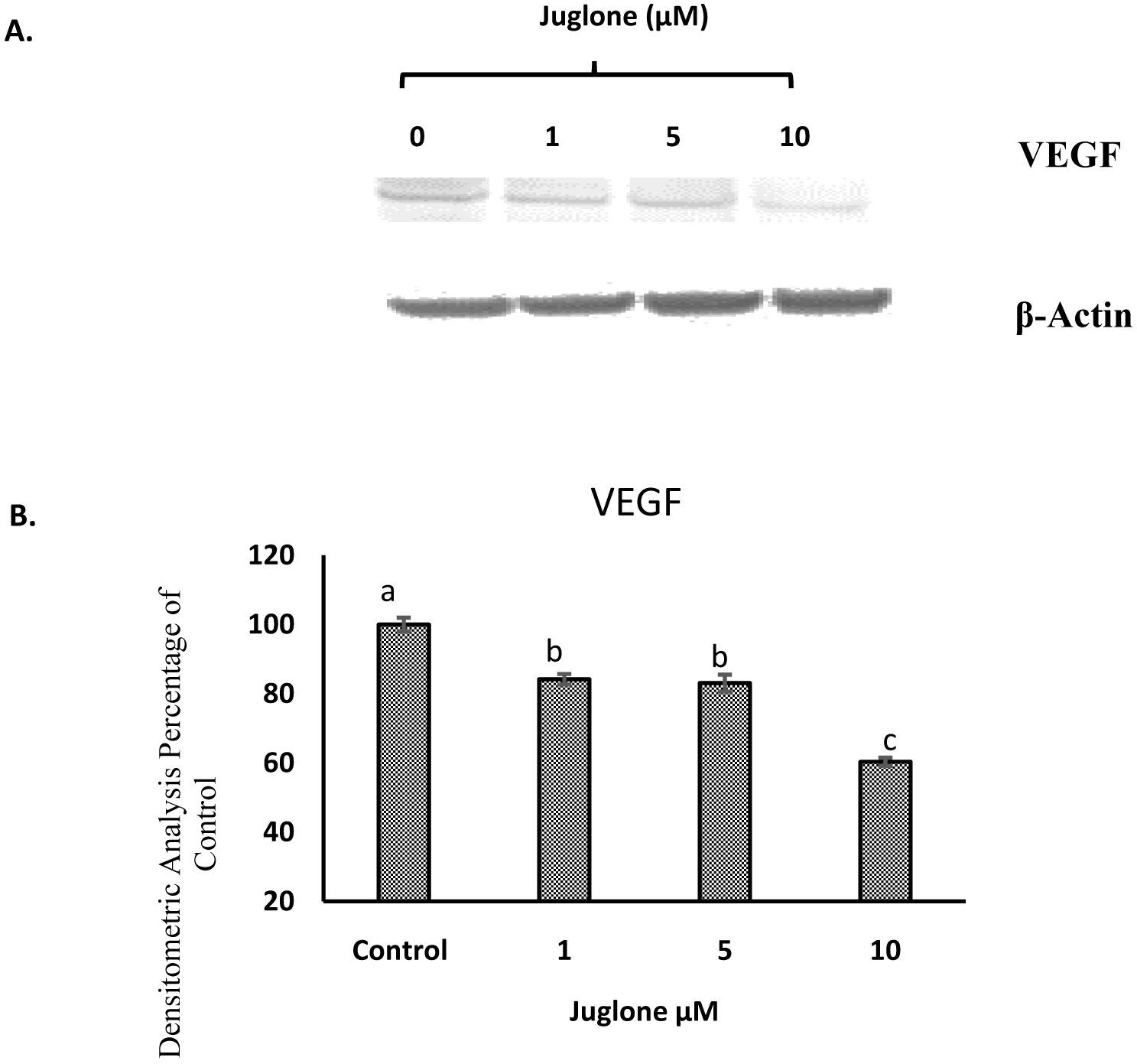 Click for large image | Figure 7. Western Blot analysis of VEGF expression in MIA Paca-2 cells. |
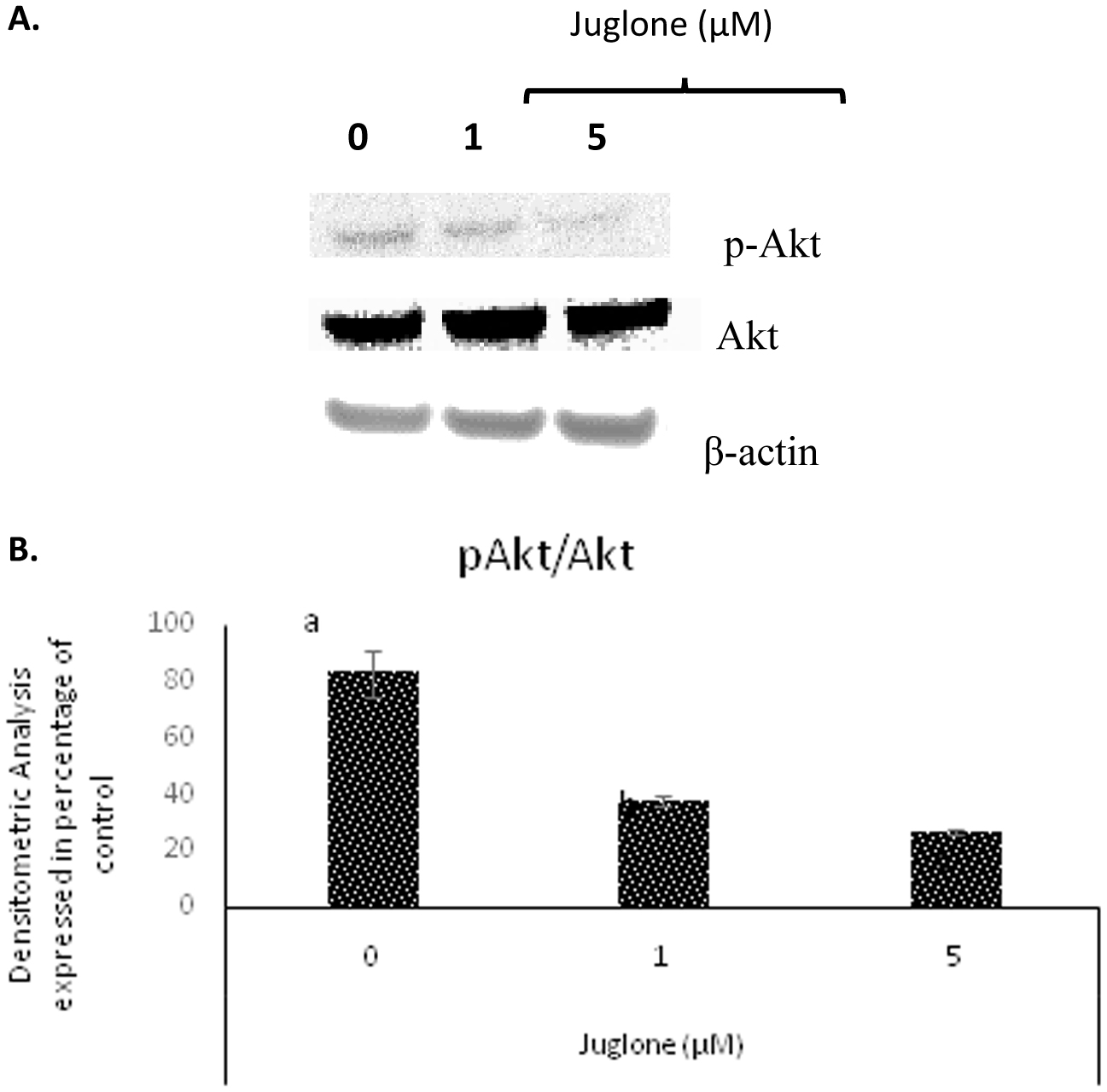 Click for large image | Figure 8. Western Blot analysis of Akt and p-Akt expression in MIA Paca-2 cells. |
| 4. Discussion | ▴Top |
Studies have shown that juglone at a concentration of 10 μM is not cytotoxic to normal cells. Also, normal pancreatic cells are very expensive and difficult to grow to very high number of cells. We therefore did not use normal pancreatic cells for comparative studies. Several studies suggest that juglone possesses numerous anti-cancer properties. The effect of juglone as an anti-cancer agent against ovarian cancer cells was reported (Fang et al., 2015). The inhibitory activities of juglone against cervical cancer cells have been demonstrated (Zhang et al., 2012). However, there are no reports on the effect of juglone in pancreatic cancer cells. This study investigated the inhibitory activity of juglone against the migration and invasion of human pancreatic cancer MIA Paca-2 cells in vitro.
Juglone at 1μM significantly inhibited the migration and invasion capabilities of pancreatic cancer MIA Paca-2 cells. Cancer cells grow rapidly and are efficient in migrating and closing the artificial scratch wound. Decrease in the ratio or the size of the wound with time and dose of juglone suggests that cancer cell migration can be significantly reduced with juglone treatment. VEGF overexpression is regulated by HIF-1α (Forsythe et al., 1996; Rey and Semenza, 2010) and is associated with significant metastasis, angiogenesis and poor prognosis of PC (Hafeez et al., 2012). Angiogenesis inhibitors are beneficial in reducing tumor growth and metastasis. Many naphthoquinone derivatives like vitamin K2 (Yoshiji et al., 2005) have been reported to act as inhibitors of angiogenesis. In this study, the anti-angiogenesis mechanism of juglone, a naphthoquinone derivative was studied in functions of HUVEC and results demonstrate that juglone significantly inhibits the formation of capillary like network as shown in Fig. 1. Our results agree with the findings from a study where 1, 4-naphthoquinone was used as a drug of interest and inhibited HUVEC tube formation at a similar concentration as juglone (Kayashima et al., 2009). Even in this set of experiments, juglone containing conditioned media inhibited tube formation. These results of tube formation assays taken together suggest juglone as a strong anti-angiogenesis agent. Juglone inhibited pancreatic cancer cells wound closure, migration and invasion (Fig. 3–5) and one of the underlying molecular mechanisms may involve reduced expression of VEGF.
The transcription factor HIF-1α is very closely related with the regulation of numerous genes associated with angiogenesis (Semenza, 2002), erythropoiesis, glycolysis, iron metabolism, and cell survival (Wenger, 2000). Besides, the level of oxygen tension there are other oxygen independent factors like nitric oxide (Sandau et al., 2001), cytokines, serum, insulin, and insulin-like growth factors (Zelzer et al., 1998) that can enhance the activity of HIF-1α. Overexpression of HIF-1α in human tumors is connected to metabolic pathways that might lead to the activation of tumor angiogenesis, invasion, and metastasis (Kimura et al., 2004; Koukourakis et al., 2002). Our results demonstrated that juglone significantly decreased the level of HIF-1α compared to the untreated control (Fig. 6). Previous studies with anthraquinones such as emodin or rhein (Fernand et al., 2011) have shown its efficacy in inhibiting migration and invasion of cancer cells with down regulation of associated proteins like HIF-1α and VEGF (Lu et al., 2009), which is in agreement with our data (Fig. 6 and 7).
One of the downstream signaling molecules of HIF-1α is VEGF, which is considered as an important factor for regulating angiogenesis and is related to growth and metastasis of pancreatic adenocarcinoma (Itakura et al., 1997; Rey and Semenza, 2010). VEGF is highly expressed in physiological cases such as wound healing and embryonic development and in pathological cases like cancer. A recent study has demonstrated that juglone inhibited tumor proliferation, angiogenesis and migration of breast cancer MCF7 cells via downregulation of VEGF and cyclin E proteins (Hu et al., 2015). In our study, juglone significantly inhibited VEGF expression in MIA Paca-2 cells (Fig. 7).
Akt plays a central role in regulating angiogenesis as it transmits signals received from cytokines and growth factors to upregulate HIF-1α and VEGF expression in cancer cells. There exists an autocrine loop between Akt and VEGF expression and Akt activation is a requirement for HIF-1α and VEGF expression (Jiang and Liu, 2008). Various natural compounds e.g. resveratrol from grapes, capsaicin from pepper have shown inhibitory effect on HIF-1α and VEGF by inhibiting the Akt pathway (Min et al., 2004). Juglone at 20 μM in combination with ascorbate (1 mM) was recently shown to inhibit p-Akt in MCF-7 breast cancer cells (Ourique et al., 2015). Our results indicate that juglone by itself is very effective in inhibiting p-Akt at 5μM in pancreatic cancer MIA Paca-2 cells (Fig. 8).
In summary, juglone appears to have the ability to inhibit angiogenesis and metastasis of pancreatic cancer MIA Paca-2 cells. While we have demonstrated that juglone appears to be a promising anti-cancer agent and inhibits metastasis and angiogenesis of pancreatic cancer cells in vitro. Future pre-clinical animal studies to establish efficacy of juglone in in vivo models for the treatment of pancreatic cancer needs to be investigated
Acknowledgments
This research was funded through Jack Losso’s Hatch Project at Louisiana State University Agricultural Center.
| References | ▴Top |
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi
doi