| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 9, March 2020, pages 52-57
Phenethylamine in hot water extract of Chlorella pyrenoidosa expands lifespan of SOD1 mutant adults of Drosophila melanogaster at very low dose
Yifeng Zhenga, Yoshihiro H. Inoueb, Nagi Kohnob, Masaki Fujishimac, Eri Okumurac, Kenji Satoa*
aDivision of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa-oiwake-cho, Kyoto 606-8502, Japan
bDepartment of Insect Biomedical Research, Center for Advanced Insect Research Promotion, Kyoto Institute of Technology, Matsugasaki, Kyoto, 606-0962, Japan
cResearch & Development Group, Sun Chlorella Co., Ltd., Osaka-cho, Kyoto, 600-8177, Japan
*Corresponding author: Kenji Sato, Division of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa-oiwake-cho, Kyoto 606-8502, Japan. Tel: +81 75 753 6444; Fax: +81 753 6400; E-mail: kensato@kais.kyoto-u.ac.jp
DOI: 10.31665/JFB.2020.9218
Received: March 17, 2020
Revised received & accepted: March 31, 2020
| Abstract | ▴Top |
Hot water extract of chlorella (WEC) increased the lifespan of superoxide dismutase (SOD1)mutant adults of Drosophila melanogaster in a dose dependent manner (200–800 µg/mL). Compounds in WEC were successively fractionated by solid phase extraction using a Sep-Pak C18 cartridge and size exclusion chromatography (SEC). Amino compounds in SEC fractions were derivatized with 6-aminoquinolyl-N-hydroxylsuccinimidyl carbamate and analyzed by reversed phased-liquid chromatography-tandem mass spectrometry. Phenylalanine, phenethylamine, isopentylamine, and 2-methylbutylamine were identified in the SEC fraction, which increased the lifespan of the D. melanogaster mutant adults. Phenethylamine, at very low doses (6–60 ng/g of diet) that roughly corresponded to those of phenethylamine in WEC (200–800 μg/mL), increased the lifespan of the D. melanogaster adults, while isopentylamine did not exert the lifespan elongation activity. Since phenethylamine did not show SOD-like activity, it did not increase lifespan by direct antioxidant activity.
Keywords: Chlorella; Lifespan; Superoxide dismutase-1; Drosophila melanogaster; Phenethylamine
| 1. Introduction | ▴Top |
Chlorella is a fresh water unicellular green alga. It is rich in vitamins, minerals, dietary fiber, nucleic acids, amino acids, and other bioactive substances (Merchant and Andre, 2001). Dried chlorella, Chlorella pyrenoidosa and Chlorella vulgaris, has a long history of being used as a food supplement. Beneficial effects of ingestion of dried C. pyrenoidosa have been demonstrated. C. pyrenoidosa ameliorated high fat diet-induced dyslipidemia in rats (Cherng and Shih, 2005), and reduced the risk of anemia, proteinuria, and edema in pregnant women (Nakano et al., 2010). In addition to the dried form of C. pyrenoidosa, hot water extract of C. pyrenoidosa (WEC) was prepared to concentrate potential bioactive compounds, which has been referred as chlorella growth factor (CGF) in some articles (Merchant and Andre, 2001). WEC was demonstrated to suppress ovariectomy-induced body weight gain and dyslipidemia in rats (Hidaka et al., 2004). WEC extended the lifespan of tumor-bearing mice possibly by exhibiting immunomodulatory activity (Miyazawa et al., 1988). These facts suggested that some water-soluble components of C. pyrenoidosa were responsible for these beneficial effects. In vitro experiments also demonstrated that WEC stimulated cytokine production in human peripheral blood mononuclear cells (Ewart et al., 2007). These beneficial activities of WEC could not solely be due to its conventional nutrients, even though it contained relatively high contents of vitamins, amino acids, and other nutrients. There is limited information on other active compounds, beyond conventional nutrients in WEC.
The objective of the present study was to identify active compounds responsible for the beneficial effects of WEC. However, it was difficult to detect active compounds in chlorella using in vitro assay, because the mechanism for the reported beneficial effects of chlorella was unknown. Animal experiments using rodents required a relatively large sample of fraction for evaluation of activity. Therefore, it was also difficult to identify active compounds by animal experiments. On the contrary, the superoxide dismutase (SOD1) mutant adults of Drosophilia melanogaster showed markedly short lifespan (10–20 days) (Reveillaud et al., 1994; Oka et al., 2015; Le et al., 2019). It was demonstrated that some food compounds extended the lifespan of the D. melanogaster adults, and this lifespan-elongation activity was associated with the health promoting activity of these food compounds (Jafari, 2011; Zuo et al., 2013; Lee and Min, 2015; Le et al., 2019). This assay compared to the one using rodents, was relatively shorter and required a smaller fraction. In the present study, a compound, at a very low dose, with lifespan-elongation activity towards Sod1 mutant adults of D. melanogaster was identified in WEC.
| 2. Materials and methods | ▴Top |
2.1. Materials
Dried C. pyrenoidosa, and WEC were prepared and supplied by Sun Chlorella (Kyoto, Japan). Isopentylamine, pentylamine, 2-methylbutylamine, and phenethylamine were purchased from Nacalai Tesque (Kyoto, Japan) and Tokyo Chemical Industry (Tokyo, Japan), and 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AccQ) was purchased from Toronto Research Chemicals (Toronto, ON, Canada). Acetonitrile (HPLC grade) was purchased from Nacalai Tesque. WST-1 SOD assay kit was purchased from Dojindo laboratory (Kumamoto, Japan).
2.2. Chromatographic fractionation
Sep-Pak C18 35 cc Vac Cartridge (Waters, Miliford, MA) was successively pretreated with acetonitrile (50 mL) and water (50 mL). WEC was dissolved in distilled water to obtain 5% (w/v) solution. After centrifugation of the solution at 3,000 g for 10 min, the supernatant was collected. The supernatant (300 mL) was loaded onto the Sep-Pak C18 35 cc Vac Cartridge, and the unabsorbed fraction was collected. The cartridge was then washed with distilled water (50 mL). The compounds absorbed by the column were successively eluted using 10, 30, 100% (v/v) of acetonitrile, acetone, and hexan (50 mL). The unabsorbed fraction and fractions eluted with 10% and 30% acetonitrile were referred as the UA, 10ACN, and 30ACN fractions, respectively. These fractions were freeze-dried. Effluents with acetonitrile, acetone, and hexan were combined and dried in a rotary evaporator, and the resultant fraction was referred as the AAH fraction. These fractions were then evaluated for lifespan-elongation activity towards Sod1n1 mutant adults of D. melanogaster.
Based on the results of the first Sep-Pak C18 fractionation, compounds in WEC were re-fractionated. WEC (1% water solution, 100 mL) was loaded onto the Sep-Pak C18 35 cc Vac Cartridge. After the UA fraction was eluted, the cartridge was washed with distilled water (100 mL). The compounds absorbed by the column were eluted by 30% (v/v) acetonitrile (100 mL). The effluent was freeze-dried and dissolved in distilled water to obtain a 1% (w/v) solution. The solution was filtered through a Cosmonice filter W (0.45 μm, Nacalai Tesque). Aliquot (200 μL) of the filtrate was loaded onto the size exclusion chromatography (SEC) column (Superdex Peptide, 10/300, GE Healthcare, Buckinghamshire, UK), which was equilibrated with 30% acetonitrile containing 0.1% formic acid. Elution was performed at 0.5 mL/min. The fractions were collected every minute, and these steps were repeated 10 times.
2.3. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses
Compounds in the SEC fraction were resolved by reversed phase-high performance liquid chromatography (RP-HPLC) and detected by electrospray ionization-mass spectrometry (ESI-MS) in positive and negative modes. However, only a few peaks of nucleoside and amino acids were detected (data not shown). To improve resolution and detection of compounds with primary and secondary amines, derivatization with AccQ, followed by LC-MS/MS, was performed (Ejima et al., 2018). Aliquots (200 μL) of SEC fractions were dried under vacuum. The residue was dissolved in distilled water (20 μL), and 0.3% AccQ acetonitrile solution (20 μL) and 50 mM sodium borate buffer (60 μL) (pH 8.8) were added. The resultant solution was kept at 50 °C for 10 min. The reactant was clarified by passing it through a Cosmonice filter W. The filtrate (20 μL) was analyzed by LC-MS at precursor ion scan mode with an LCMS 8040 (Shimadzu, Kyoto, Japan) equipped with RP-HPLC column (Cosmosil 5C18 MS-II, Nacalai Tesque), targeting the AccQ-derived product ion (m/z 171.1) at collision energy −35 eV. A binary linear gradient, with 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B), was used at a flow rate of 0.2 mL/min. The gradient program was as follows: 0–15 min, 0–50% B; 15–20 min, 50–100% B; 20–25 min, 100% B; 25–25.1 min, 100–0% B; 25.1–35 min, 0% B. The column was maintained at 40 °C.
2.4. Determination of monoamines in WEC and crude chlorella
Monoamines were dissolved in distilled water or ethanol to obtain 10 μM solutions, which were used as standard solutions. Crude chlorella and WEC powders were suspended in distilled water to obtain 1% (w/v) solutions, which were vigorously stirred and then centrifuged at 15,000 g for 10 min. The supernatants were collected. Monoamines in standard and sample solutions were derivatized with AccQ, as described above. The AccQ-derivatives were determined by LC-MS/MS at multiple-reaction monitoring (MRM) mode. The elution conditions were the same as described above. MRM conditions for the AccQ-derivatives of standard monoamines were optimized using LabSolutions Version 5.65 (Shimadzu, Kyoto, Japan).
2.5. Survival assay using Sod1 mutant D. melanogaster flies
The Sod1n1 mutant flies were prepared by a method as described previously (Le et al., 2019). This mutant showed a considerably declined SOD1 activity due to an amino acid substitution without the reduced mRNA level (Oka et al., 2015). The young adult mutant males, homozygous for Sod1n1 were collected within 24 hrs after eclosion and 20 flies were added into the tube (100 mm in height and 15 mm in diameter) with medium containing sample at suitable contents and incubated at 25 °C and 50–55% humidity. The assay was repeated three to five times (using 60–100 Sod1 mutant flies). Dead adults in each vial were counted every 12 h for the Sod1 mutants. Food vials were changed every three days.
2.6. SOD-like activity assay
Phenethylamine was dissolved in water to obtain the resultant solution (0.6–600 ng/mL). SOD-like activity was examined by using a WST SOD assay kit. In addition, wild D. melanogaster was homogenized with phosphate buffer saline (PBS) (10 μL/individual). The homogenates were mixed with the same volume of water or phenethylamine above and incubated at 25 °C for 30 min. The mixture was then centrifuged at 15,000 g for 10 min. The supernatants were collected, and SOD-like activity was examined.
2.7. Statistics
A survival curve was calculated based on Kaplan-Meier survival estimation and analyzed by log-rank test between the drug-treated and control groups. For comparison of the two groups, we used the Student’s t-test. One-way ANOVA with post-hoc Tukey’s test was applied to assess the differences among the groups. Data were considered significant at p-values < 0.05.
| 3. Results | ▴Top |
3.1.. Lifespan-elongation activity of WEC
As shown in Figure 1a, WEC extended the survival period of the Sod1 mutant flies in a dose dependent manner to 800 μg/mL in diet. WEC (3,200 μg/mL), however, showed a survival period similar to that of WEC (200 µg/mL) and shorter than that of WEC (800 μg/mL).
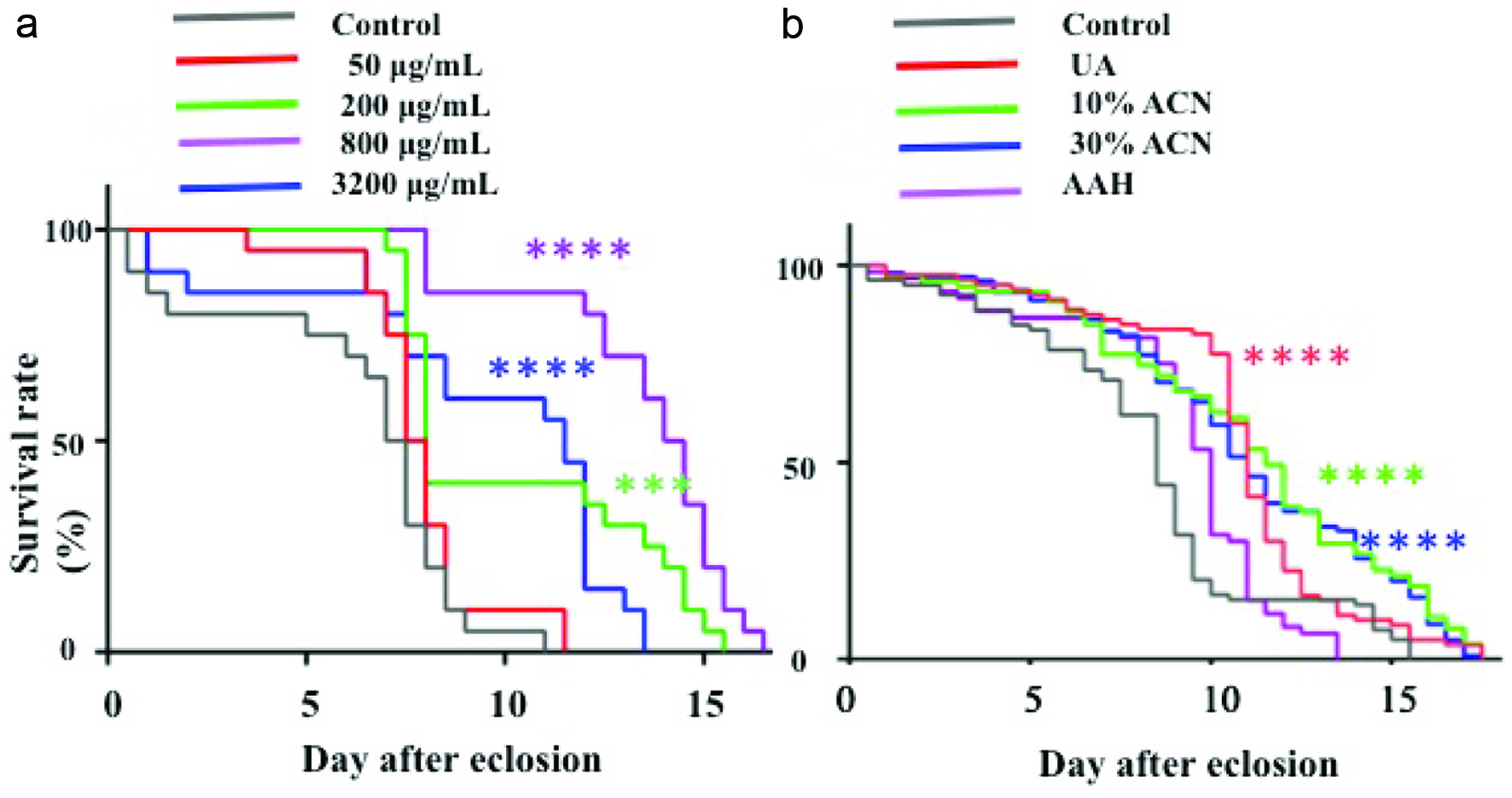 Click for large image | Figure 1. Effect of different concentration of WEC (a) and its Sep-Pak C18 fractions (200 μg/g) (b) on survival rate of Sod1 mutant flies. |
3.2. Identification of compounds with life span elongation activity
WEC was first fractionated by solid phase extraction using a Sep-Pak C18. As shown in Table 1, the UA fraction accounted for approximately 70% (w/w) of the initial materials. The 10ACN and 30ACN fractions approximately accounted for 4% of the initial materials, respectively, while only small amounts of compounds were recovered into the AAH fraction. As shown in Figure 1b, until day 9, survival rates of all groups treated with Sep-Pak C18 fractions were higher than that of the control group. Thereafter, survival rate of the AAH group drastically decreased. After day 10, survival rate of the UA group also decreased. On the contrary, the 10ACN and 30ACN groups showed survival curves similar to that of crude WEC. Based on these results, active compounds in WEC were eluted with 30% acetonitrile from the solid phase column after washing the cartilage with water. The active fraction was further fractionated using SEC. As shown in Figure 2a, fractions 16–35, 36–40, 41–44, 45–50, and 51–70 were collected and referred as SEC Fr. I, II, III, IV, and V, respectively. Lifespan-elongation activity of these fractions was evaluated. As shown in Figure 2b, SEC Fr. III and V (200 μg/g)-administered fractions, compared to the control, showed a decreased survival rate after day 7. SEC Fr. II-administered group, compared to the control, showed a reduced survival rate between days 2 and 7. Before day 8, SEC Fr. I and IV-administered fractions showed survival curves similar to each other and survival rates higher than that of the control. However, after day 8, survival of D. melanogaster of the SEC Fr. I group decreased. SEC Fr. IV-administered group, compared to the control, showed an increased survival rate and lifespan of D. melanogaster throughout the experiment (P < 0.001). Thus, SEC Fr. IV was used as the active fraction for the following experiments.
 Click to view | Table 1. Recovery of solid phase extraction fractions using Sep-Pac C18 cartilage |
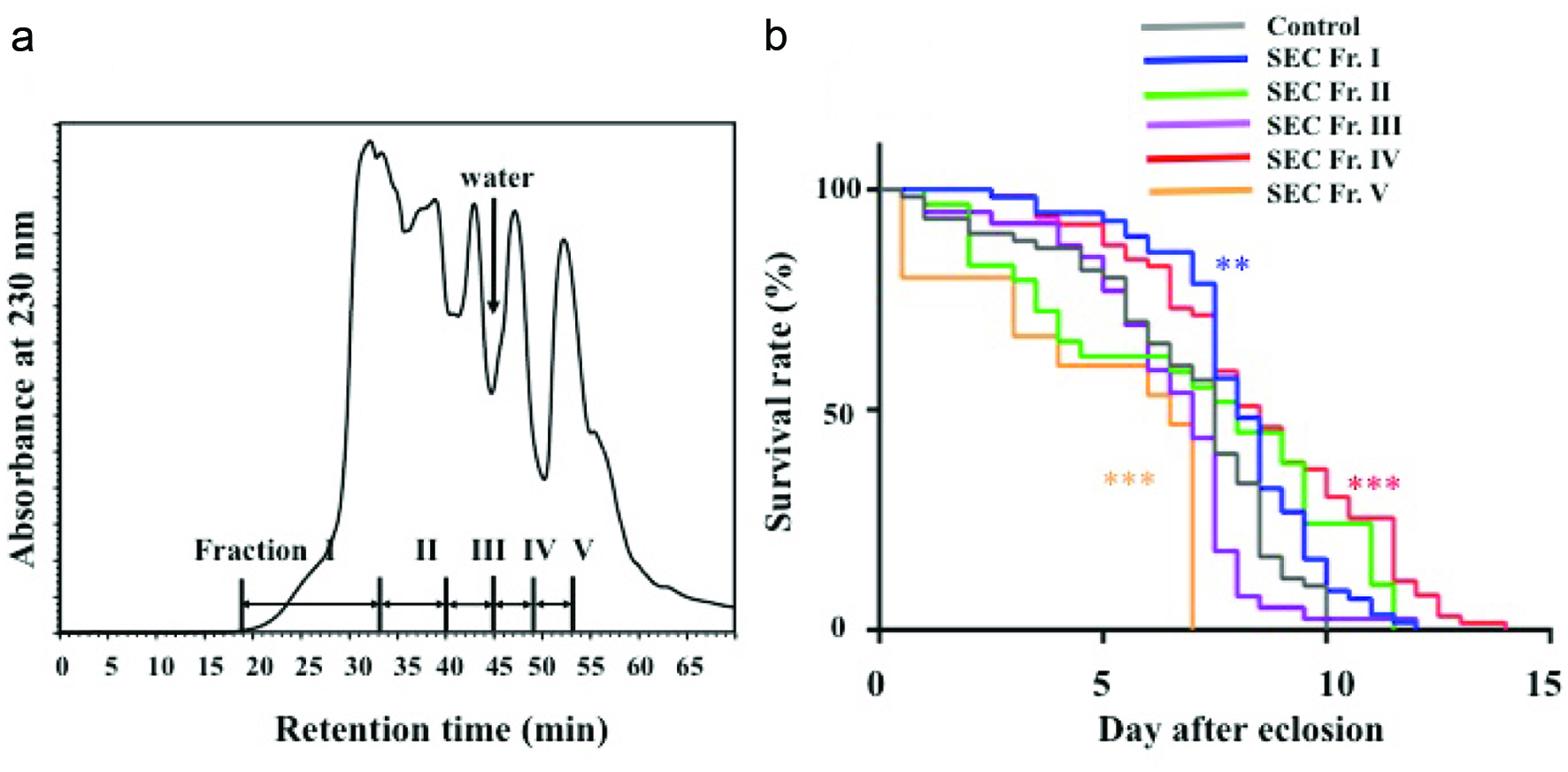 Click for large image | Figure 2. Size exclusion chromatography (SEC) elution pattern of compounds in Sep-Pak C18 30% acetonitrile fraction (a) and effect of SEC fractions (I–V) (200 μg/g) on survival rate of Sod1 mutant flies (b). |
Amino compounds in SEC Fr. III and IV were detected by derivatization with AccQ, followed by LC-MS/MS at precursor scan mode targeting product ions from AccQ (m/z = 171.1). As shown in Figure 3, some peaks marked with asterisk (*) appeared in both SEC Fr. III (inactive fraction) and SEC Fr. IV (active fraction). On the other hand, four peaks indicated as 1–4 that appeared in SEC Fr. IV were not present in SEC Fr. III. The mass to charge ratio (m/z) of compounds in peaks 1–4 were 336, 258, 258, and 292, respectively. The m/z 336 and 292 corresponded to protonated AccQ derivatives of phenylalanine and its decarboxylated form, phenethylamine, respectively. The m/z 258 corresponded to decarboxylated isoleucine (2-methylbutylamine) and leucine (isopentylamine). Retention times of peaks 1–4 coincided with those of AccQ derivatives of authentic phenylalanine, 2-methylbutylamine, isopentylamine, and phenethylamine. Product ion patterns of these peaks by LC-MS/MS also coincided with those of the authentic amines, respectively. Therefore, peaks 1–4 were identified as phenylalanine, 2-methylbutylamine, isopentylamine, and phenethylamine. Contents of 2-methylbutylamine, isopentylamine, and phenethylamine in WEC were 2.5 ± 0.2, 10.7 ± 0.2, and 11.7 ± 0.4 μg/g, respectively, and those in dry chlorella powder were 0.0 ± 0.00, 0.5 ± 0.02, and 0.8 ± 0.04 μg/g, respectively. As shown in Figure 1a, WEC (800 μg/mL), which contained isopentylamine and phenethylamine at 8.6 ng/mL and 10 ng/mL, respectively, showed the best lifespan-elongation activity towards the Sod1 mutant flies. As shown in Figure 4b, administration of isopentylamine (8.6 ng/mL) did not significantly affect the survival rate. A higher dose (86 ng/mL) of isopentylamine showed survival rates higher than that of the control before day 5. However, after day 6, the survival rate drastically decreased, and maximum lifespan was shorter than that of the control group. Phenethylamine (60 ng/mL) showed a survival rate significantly higher (P < 0.001) than that of the control group and a maximum survival period approximately 150% of that of the control group. However, phenethylamine (600 ng/mL) showed a survival rate lower than that of the control group. On the other hand, pentylamine, a non-natural monoamine, exerted lifespan-elongation activity at doses of 20 and 200 ng/mL (p < 0.001 and 0.0001 respectively).
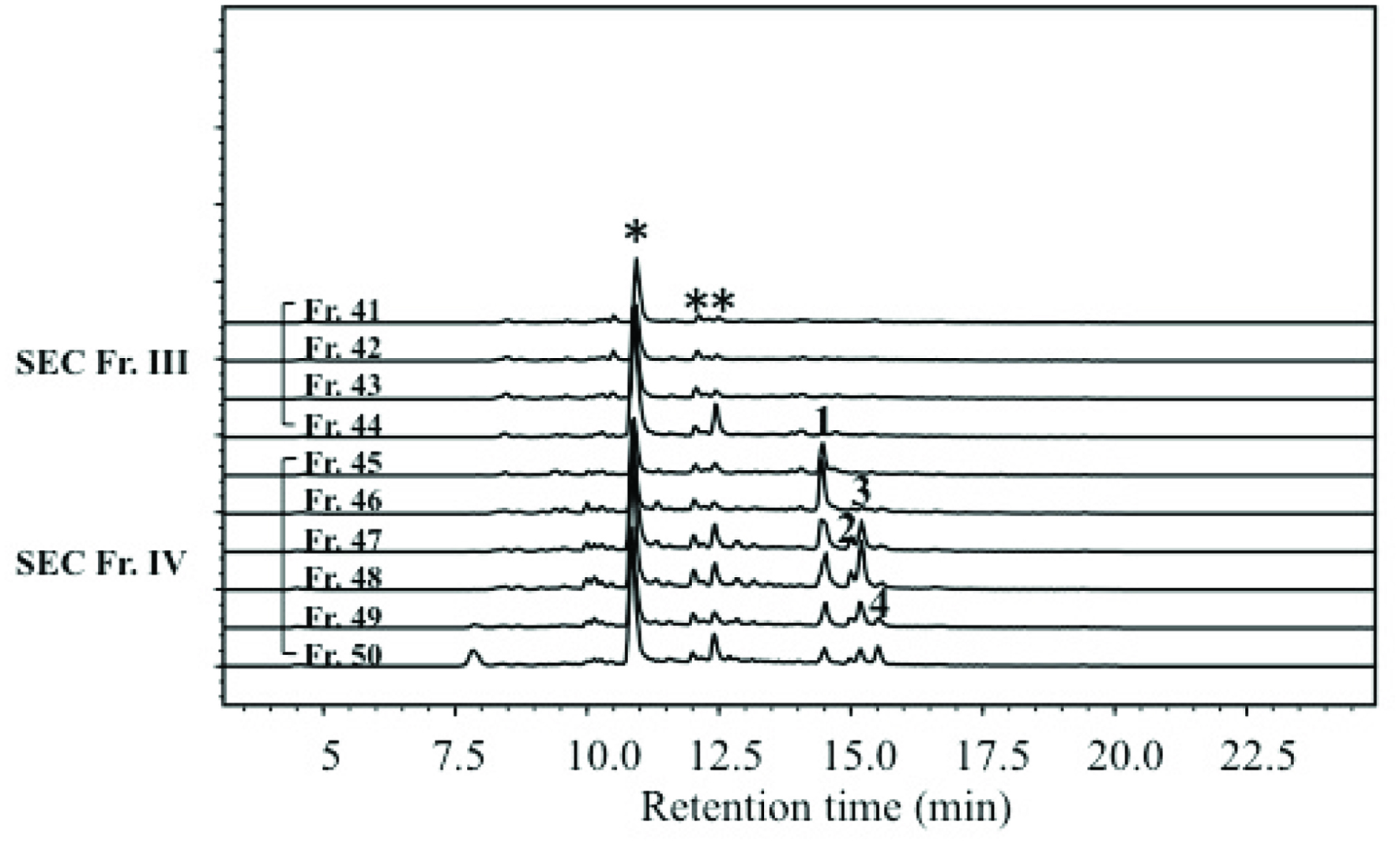 Click for large image | Figure 3. Precursor ion scan of AccQ derivatives in SEC fraction III and IV. *Shows reagent peak. |
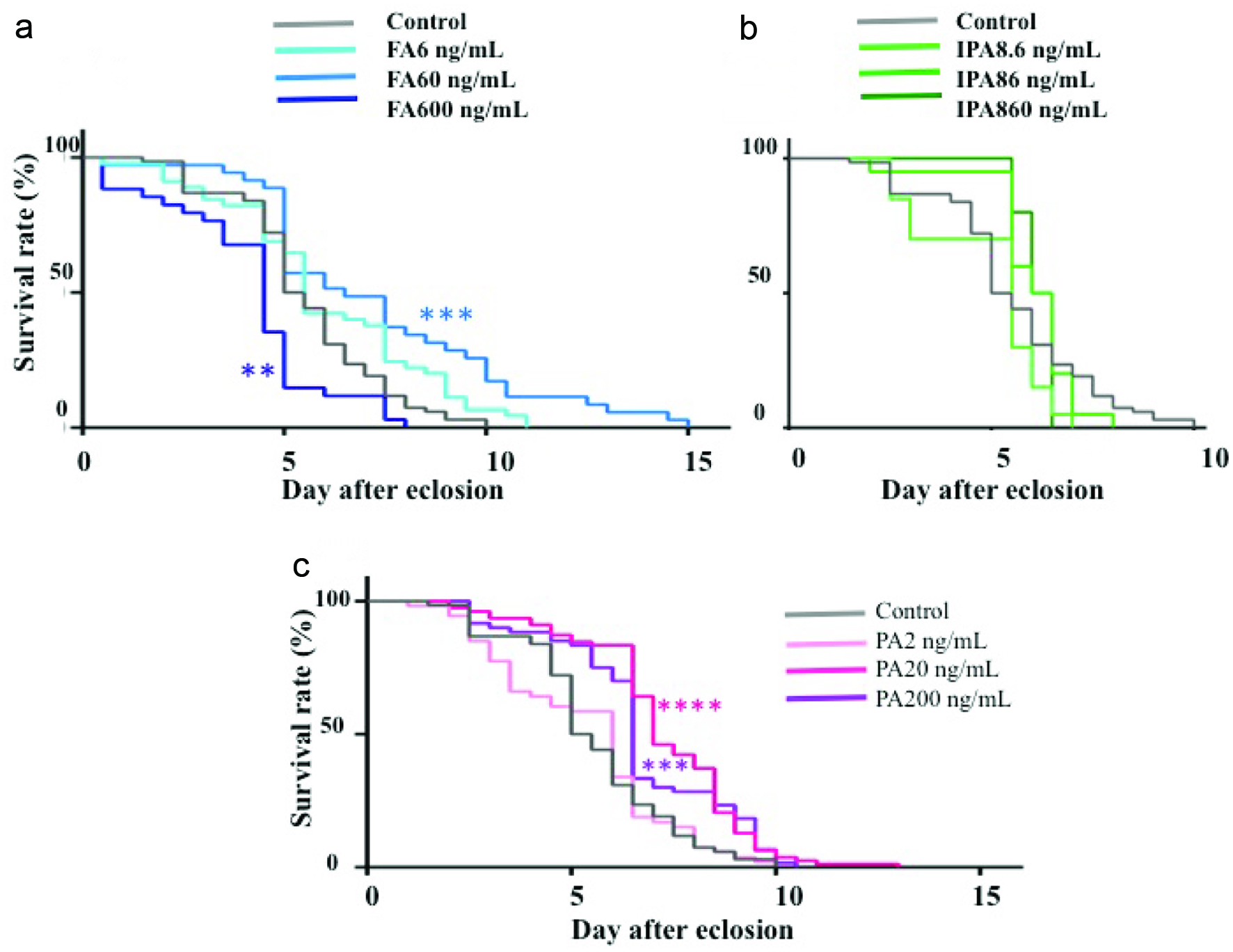 Click for large image | Figure 4. Effect of different concentration of phenethylamine (a), isopentylamine (b) and pentylamine (c) on survival rate of Sod1 mutant flies. |
| 4. Discussion | ▴Top |
Phenethylamine is an endogenous trace amine, and is contained in some food items such as cheeses (0–3 μg/g) (Novella-Rodriguez et al., 2003), a chocolate (2.6 μg/g) (Mayr and Schieberle, 2012), and wines (approximately 1 μg/mL) (Landete et al., 2005). The present study demonstrated that WEC contained phenethylamine (10 μg/g of dry matter). It has been reported that oral supplementation of phenethylamine (10–60 mg/day) with selegiline (monoamine oxidase-B inhibitor, 10 mg/day) relieved depression (Sabelli et al., 1996). On the other hand, high dose of phenethylamine (25–75 mg/kg body weight of mice) induced psychomotor dysfunction and decrease in striatal biogenic amines (Sengputa and Mohanakumar, 2010). However, the amount of phenethylamine (10–100 μg) obtained from consumption of a few grams of WEC was similar or less than that obtained from one serving of cheese, chocolate, or wine. Therefore, such low doses of phenethylamine did not exert any adverse effects on human health.
It has been demonstrated that some antioxidant vitamins and food compounds extended the lifespan of D. melanogaster and Caenorhabditis elegans. Vitamin C (20 mM), lutein (0.1 mg/g), and apple polyphenols (10 mg/g) in the diet extended the lifespan of D. melanogaster, respectively (Bahadorani et al., 2008; Zhang et al., 2014; Peng et al., 2011). Vitamin E (16.7 mg/g of diet) also extended the lifespan of the Sod1 mutant flies (Bahadorani et al., 2008). Coenzyme Q10 (50 μg/g of diet) elongated the lifespan of C. elegans (Ishii et al., 2004). A strong superoxide dismutase/catalase mimetic, EUK-134, at a very low dose of 50 μM (ca.20 μg/g) in the diet, extended the lifespan of C. elegans (Melov et al., 2000). The present study demonstrated that phenethylamine (6–60 ng/g of diet) expanded the lifespan of the Sod1 mutant flies, and these doses were far less than those reported in the previous studies. On the contrary, isopentylamine did not expand the lifespan of the Sod1 mutant flies; however, pentylamine, a linear chain monoamine, did, suggesting that all hydrophobic monoamines did not always exhibit lifespan-elongation activity and structure of the hydrophobic moiety contributed to the elongation activity. To our best knowledge, there is no food compound, except some trace metals such as selenium, which exerts beneficial activity in such low doses (Diplock, 1987).
Inhibition of SOD1 resulted in the accumulation of superoxide radicals (O2−) (Huang, 2000). Over production of O2− caused oxidation of some biomolecules, such as DNA, protein, and lipids (Ames et al., 1993), damaging cells. However, phenethylamine (6–6,000 ng/mL) did not show significant SOD-like activity. In addition, phenethylamine, which was treated with a homogenate of D. melanogaster, did not show significant SOD-like activity at the same dose. As mentioned above, the dose of phenethylamine for elongation of lifespan of the Sod1 mutant flies was far less than that of vitamins E and C, which are strong antioxidants. It was, therefore, unlikely that antioxidant activities of phenethylamine and its metabolites contributed to the lifespan extension of the Sod1 mutant flies. Although the mechanism of lifespan extension by phenethylamine remains unknown, it is possible that phenethylamine exerted this activity by binding to some cell surface receptors, intracellular transcription factors, or their regulators. It was demonstrated that some trace amines, such as tyramine and octopamine, exerted significant bioactivities via G protein-coupled receptors (Grandy, 2007). However, the specific receptor for phenethylamine was not identified. Now, further studies on target proteins of phenethylamine in D. melanogaster are being conducted.
Effects of oral administration of such low doses of trace amines, such as phenethylamine, on animal and human health have not been examined. Now, effects of low dose phenethylamine on high fat diet-induced dysfunction in mice are being examined, and this might shed light on the function of trace amines found in food.
| 5. Conclusion | ▴Top |
Oral administration of WEC (200 and 800 μg/g of diet) increased the lifespan of the Sod1 mutant flies in a dose dependent manner. By in vivo activity-guided fractionation, phenethylamine was demonstrated to exhibit lifespan-elongation activity. Phenethylamine, compared to compounds including antioxidant vitamins and phytochemicals, increased life span at an extensively lower dose (6–60 ng/g of diet). Since phenethylamine did not show SOD-like activity, it did not exert lifespan elongation activity towards the Sod1 mutant flies by only direct antioxidant activity. While there is no report on the beneficial effects of ingestion of trace amounts of monoamine, phenethylamine obtained from ingestion of chlorella and other foods might show some beneficial effects on human health. To examine the effects of trace amounts of phenethylamine, an animal experiment using NAFLD mouse model is being conducted.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing. This work was supported by Commissioned Research between Kyoto University and Sun Chlorella Co., Ltd. (Project number 150201000001).
| References | ▴Top |