| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 8, December 2019, pages 58-65
Flavanones common to citrus fruits activate the interferon-stimulated response element by stimulating expression of IRF7
David J. Fasta, *, Nathan P. Sterna, Jennifer Chuangb, Yingqin Lib, Jeffrey D. Scholtena, Chun Hub
aAmway Corporation, 7575 Fulton St. E., Ada, MI 49355, USA
bNutrilite Health Institute, 5600 Beach Boulevard, Buena Park, CA 90621, USA
*Corresponding author: David J. Fast, Amway Corporation, 7575 Fulton St. E., Ada, MI 49355, USA. Tel: 1-616-787-8604; E-mail: David.Fast@amway.com
DOI: 10.31665/JFB.2019.8207
Received: November 20, 2019
Revised received & accepted: December 30, 2019
| Abstract | ▴Top |
Citrus fruits are a rich source of vitamin C and phytochemicals and can be an important part of a healthy diet. Citrus is believed to prevent the occurrence or shorten the duration of symptoms of the common cold and influenza, but meta-analysis of vitamin C clinical trial data is inconclusive. We examined whether citrus flavonoids activated antiviral pathways that might explain the perceived efficacy against the common cold and influenza. We found that a citrus bioflavonoid blend augmented NFκB activation in the presence of imiquimod. In addition, the citrus bioflavonoid blend, as well as individual flavonoids found in the blend, activated the interferon-stimulated response element (ISRE). The ability to activate the ISRE appeared to due to the flavonoids’ ability to upregulate expression of the transcription factor interferon regulatory factor 7 (IRF7). Our results suggest that flavonoids from citrus may stimulate antiviral pathways due to their ability to activate the ISRE.
Keywords: Antiviral; Citrus; Hesperetin; Interferon; Naringenin; IRF7
| 1. Introduction | ▴Top |
Citrus fruits are a rich source of flavonoids, particularly from their peel and seeds (Tripoli et al., 2007). Flavanones present in citrus include aglycones hesperetin and naringenin. Other classes of flavonoids identified in citrus include flavones (apigenin, luteolin, diosmetin, quercetin, kaempferol) and their respective glycosides; flavanone neohesperidoside (naringin, neohesperidin, poncirin, neoeriocitrin), flavanone rutinoside (narirutin, hesperidin, didymin, eriocitrin, diosmin), and polymethylated flavones (scutellarein, sinensetin, tangeretin, hexamethoxylflavone, nobiletin, and heptamethoxyflavone) (Tripoli et al., 2007).
As a group, flavonoids are strongly implicated as having an important effect on human health (Cassidy et al., 2012; Croft, 1998; Havsteen, 2002). For example, the glycosides hesperidin and naringin are mainly responsible for the purported antioxidant activity of citrus peel extracts in vitro (Kanaze et al., 2009). Likewise, supplementation with hesperidin may affect antioxidant status in animals undergoing physiological challenges. In rats, hesperidin (200 mg/kg/day) for 10 days significantly increased levels of superoxide dismutase (SOD), glutathione–S–transferase (GST), and GSH after benzo[α]pyrene-induced oxidative stress (Arafa et al., 2009), while the same dosage of hesperidin did not attenuate antioxidant concentrations in healthy rats. Naringin increased levels of SOD, CAT, and vitamin E in New Zealand White rabbits fed a high cholesterol diet compared to those without the naringin (0.5 g/kg diet) supplement (Jeon et al., 2002). Perche et al found that orange juice or hesperidin, its major flavanone glycoside, did not induce modulation of cellular immune function in healthy well-nourished human subjects (Perche et al., 2014), but antioxidant markers and vascular function were improved in mild hypercholesterolemia subjects when drinking orange juice (Constans et al., 2015) . Therefore, supplementation of flavanone glycosides found in citrus peel extract may be beneficial only when the body is under severe oxidative stress, and when supplementation is given over time and not as a single administration. In addition to potential antioxidant benefits, citrus flavonoids such as narirutin and hesperidin have been reported in rodent models to have hyporglycemic, hypolipedmic, antihypetenisive, antihrombotic, hepatoprotective, and weight management activities (Alam et al., 2014; Hugel et al., 2016; Liu et al., 2008).
Citrus flavonoids may function through multiple mechanisms of action. Interaction with MAPK, PI3 kinase/Akt, NFκB, and PKC signaling pathways may explain their effects on inflammatory markers such as TNF-α, IL-6, and MCP-1 (Choi and Ahn, 2008; Choi and Lee, 2010; Vafeiadou et al., 2009). For example, naringenin exhibits anti-inflammatory activity through inhibiting iNOS expression by attenuating p38MAPK and STAT-1 phosphorylation/activation (Vafeiadou et al., 2009). In addition, the effects of flavonoids on different aspects of metabolism may be due to their ability to up-regulate energy regulators AMPK, PPARs, and PGC-1α (Alam et al., 2014).
Citrus, in particular orange juice, has popularly been used for self-medication for treatment of the common cold and flu. The basis for this folklore is unknown but may due in part to the popularization of vitamin C by Linus Pauling. Multiple clinical studies have been done to evaluate the ability of vitamin C to prevent or treat cold/flu, and meta-analysis of these studies suggests that vitamin C probably does not prevent infection by rhinoviruses or influenza viruses, but may shorten the duration of disease (Hemilä and Chalker, 2013). Citrus bioflavonoids such as those mentioned above may facilitate vitamin C absorption (Vinson and Bose, 1988). In Vinson’s work on vitamin C absorption, citrus extract contained 19.0% naringin, 0.34% naringenin and 0.17% hesperidin, and promotion of vitamin C absorption was demonstrated with a 2-gram citrus extract dose containing 500 mg ascorbic acid per oral dose with 380 mg naringin, 6. 8 mg naringenin and 3.4 mg hesperidin. Commercially available citrus bioflavonoid blends include various combinations of citrus fruit extracts. The citrus bioflavonoid blend used as the starting point for this study were standardized to 5.7–6.2% flavonoids, with profile of three primary flavonoids naringin: narirutin: hesperidin at ratio of 50%: 15%: 35% (structures are seen below).
| 2. Materials and methods | ▴Top |
2.1. Citrus bioflavonoid blend
Citrus bioflavonoid blend is a mixture of extracts of grapefruit (Citrus × paradisi), lemon (Citrus limon), mandarin orange (Citrus reticulata) and sweet orange (Citrus sinensis) in a ratio of 20:5:5:70 respectively. These citrus extracts were blended and standardized to 5.7–6.2% flavonoids, with the three primary flavonoids naringin: narirutin: hesperidin at ratio of 50%: 15%: 35% (Figure 1a). Chemical structures for flavonoids of interest are shown in Figure 1b. Citrus flavonoids were analyzed by HPLC using the following method. Samples were prepared for analysis by weighing 250 mg of citrus bioflavonoid blend and adding 10 mL of 4/1 Methanol/DMSO. The solution was vortexed briefly to disperse, sonicated for 15 minutes, and then filtered with 0.22 µm PVDF prior to analysis on a Waters H-class UPLC system with a PDA detector. The A mobile phases for UPLC analysis was 0.2% OPA in water, the B mobile phase was 100% MeOH, and the C mobile phase was 100% acetonitrile. A seven μl sample of each extract was injected into a Waters UPLC HSS 100 mm analytical column. UPLC separation was carried out as shown below in Table 1.
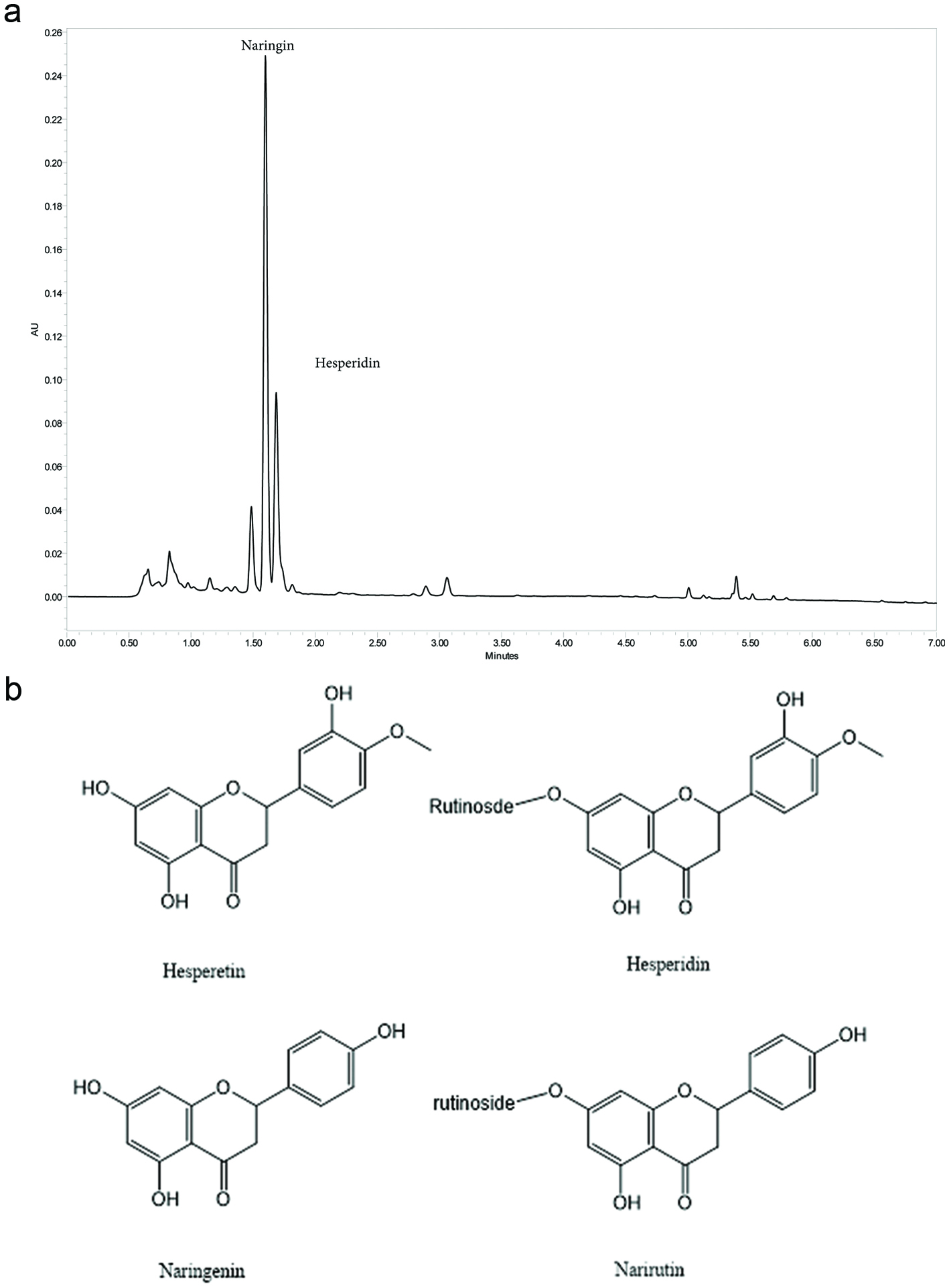 Click for large image | Figure 1. Chemistry of Citrus Bioflavonoid Blend. (a) Representative UHPLC Chromatogram of Proprietary Citrus bioflavonoid blend Extract at 280 nm. Major component peaks identified and labeled as Hesperidin and Naringin. (b) Structures of hesperetin, hesperidin, naringenin and narirutin. |
 Click to view | Table 1. Instrument Conditions |
2.2. Reagents
Naringenin, Narirutin, Hesperetin, Hesperidin, and Imiquimod were purchased from MilliporeSigma (St. Louis, MO). A549 cells (CCL-185) and U-2 OS (HTB-96) were purchased from ATCC (Manassas, VA). McCoy’s 5A and Ham’s F12K media, amphotericin B, and penicillin/streptomycin were purchased from Mediatech (Manassa, VA). OptiPro serum-free media was purchased from ThermoFisher (Waltham, MA). Fetal bovine serum was purchased from HyClone (Logan, UT). Luciferase reagents were purchased from Biotium (Hayward, CA). RNeasy kit, QIA shredder columns, and SYBR green labeled primers for IRF1, IRF3, IRF7, and IRF9 were purchased from Qiagen (Valencia, CA). iScript cDNA synthesis kit and Ssofast Evagreen supermix were purchased from BioRad (Hercules, CA).
2.3. Luciferase reporter cell line generation
For NFκB, a pNFκB-Luc plasmid was purchased from Clontech (Mountain View, CA). A 200 bp fragment containing four copies of the NFκB binding consensus sequence and a TATA-like promoter region from the Herpes simplex virus thymidine kinase promoter was excised with NheI and HindIII and gel purified. The fragment was ligated into pGL4.17[luc2/Neo] (Promega, Madison, WI). Plasmid DNA was transfected into human A549 cells using Fugene 6 (Roche, Indianapolis, IN). Cells were incubated in the presence of selective media containing G418 (400 μg/mL) for 10 days and colonies of surviving cells were cloned by limiting dilution. For the ISRE, a pGL4.45[luc2P/ISRE/Hygro] vector (Promega, Madison, WI) was transfected into human osteosarcoma U-2 OS cells using Fugene 6, and cells were incubated in selective media containing hygromycin (500 μg/mL). Resulting clones were tested for optimal responsiveness to IFNα.
2.4. NFkB luciferase reporter assay
NFkB-luc cells were plated at 4 × 104/well in white, 96 well, clear bottom microtiter plates and were incubated overnight in OptiPro SFM. The following day, the cells were exposed to samples, imiquimod (20 μM), and IL-1β (50 pg/mL). Six hours after addition of IL-1β, the cells were lysed and the relative amount of luciferase was assessed using luciferin reagents from Biotium (Hayward, CA). Data are expressed as per cent control in which the relative luminescence units elicited by control cells exposed only to IL-1β is set at 100%.
2.5. ISRE luciferase reporter assay
ISRE-luc cells were plated at 2 × 104/well in white, 96 well, clear bottom microtiter plates and were incubated overnight in McCoy’s 5A-10% FBS. The following day, cells were exposed to samples. Recombinant human IFNα (PBL Sciences, Tarrytown, NJ) was used as a positive control (1,000 IU/mL). The cells were incubated overnight, and the relative amount of luciferase activity was assessed the following morning using luciferin reagents from Biotium.
2.6. Real-time quantitative PCR analysis
U-2 OS cells were plated in 12-well plates (5 × 105 cells/well) and grown in McCoy’s 5a media supplemented with 10% FBS and incubated 24 h in a humidified, 37 °C, 5% CO2 incubator. The following day, flavonoid sample stock solutions were prepared with DMSO which were then diluted in growth media prior to adding to the cells. The cells were then incubated for an additional 18 h. The media was aspirated, and RNA harvested using Qiashredder columns and an RNeasy kit per the manufacturer’s specifications. Total RNA was quantified by A260/A280 ratio, diluted to 1 µg per reaction, and reverse transcribed using an iScript cDNA synthesis kit (BioRad). Real-time qPCR reactions were performed using SsoFast EvaGreen qPCR mix on a CFX96 Real-Time Thermocycler (Bio-Rad). The reaction conditions were as follows: 95 °C for 30 sec; 45 cycles of 58 °C for 5 sec; and 95 °C for 5 sec. Fluorescent detection was measured following completion of each cycle. Cycle times of the IRF genes were normalized to β-actin, a housekeeping gene, prior to comparisons with control samples.
2.7. Statistical analysis
The results of most experiments described here are expressed as mean ± SD values and are representative of three independent experiments. Statistical analysis was carried out by student’s t test using PRISM version 7.01 statistical analysis software (GraphPad Software, Inc., San Diego).
| 3. Results | ▴Top |
3.1. Citrus bioflavonoid blend augments imiquimod and IL-1β stimulated activation of NFκB
While screening botanical extracts for an ability to influence IL-1β-stimulated activation of an NFκB -linked luciferase reporter, we observed that several citrus extracts augmented activation of NFκB (data not shown). As the TLR7 ligand imiquimod also augments activation of NFκB in our model, we speculated whether citrus flavonoids and imiquimod together might augment activation of NFκB to a degree greater than either sample alone. The data in Figure 2 demonstrate that in the presence of imiquimod, the citrus bioflavonoid blend did augment activation of NFκB, in a dose dependent manner, above the degree to which IMQD alone activates NFκB.
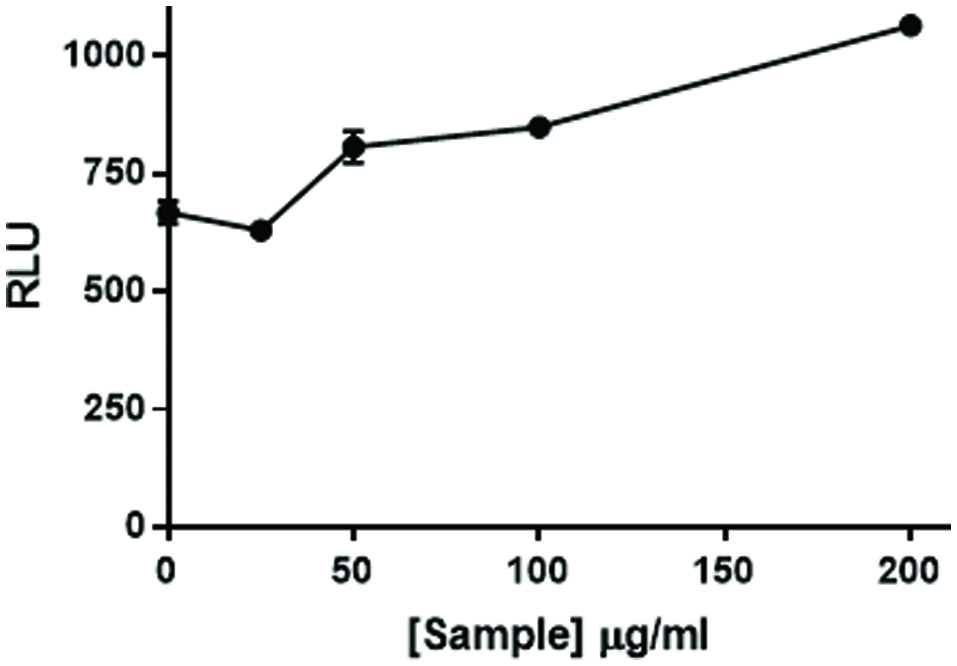 Click for large image | Figure 2. Citrus bioflavonoid blend flavonoid mixture augments NFκB activation. NF49 cells stably transfected with an NFκB response element-luciferase construct were treated with increasing levels of a mixture of citrus flavonoids prior to NFκB activation with imiquimod and human IL-1β (50 pg/mL). The cells were incubated for 6 hr. at which time luciferase activity was measured on a Spectramax M5 plate reader. Data from a representative experiment are expressed as RLU compared to positive control cells treated with IMQD and IL-1β only. |
To further investigate this observation, we tested several of the individual flavonoids known to be present in the citrus bioflavonoid blend mixture. The data in Figure 3 demonstrate that the response to hesperetin, its glycoside hesperidin, and naringenin all augmented the NFkB response although only the response to hesperetin was significant. Narirutin, the glycoside of naringenin however, had no activity.
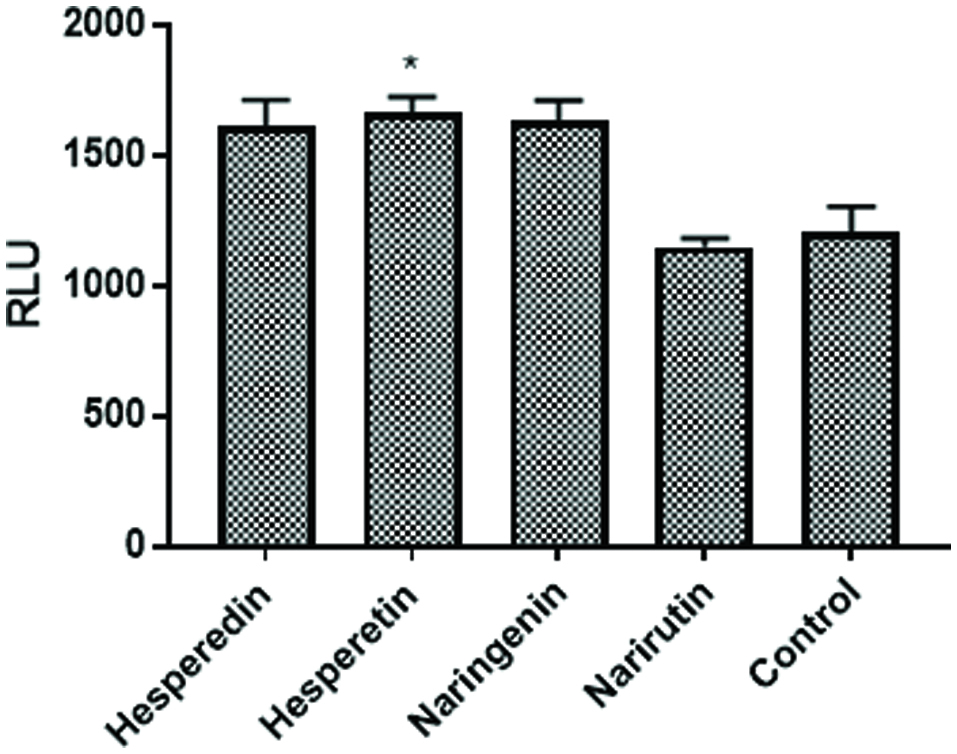 Click for large image | Figure 3. Individual Flavonoids augment NFκB activation. NF49 cells were treated with flavonoids (20 μM) know to be present in the citrus mixture prior to NFκB activation with IMQD and human IL-1β (50 pg/mL). The cells were incubated for 6 hr. at which time luciferase activity was measured on a Spectramax M5 plate reader. Data from a representative experiment are expressed as RLU compared to positive control cells treated with IMQD and IL-1β only. |
3.2. Citrus bioflavonoids activate the ISRE
The fact that the citrus bioflavonoid blend augmented NFκB activation by IMQD and IL-1β suggested that compounds found in the citrus bioflavonoid blend might augment anti-viral activity as IMQD is a TLR7 agonist. In addition to activation of NFkB, binding of TLR7 ligands stimulates gene expression of interferon via activation of the ISRE. Therefore, we speculated whether the citrus bioflavonoid blend might also influence activation of a pathway(s) under control of the ISRE. To test this hypothesis, we exposed a stable ISRE-luciferase cell line to the citrus bioflavonoid blend as well as several flavonoids found in the mixture. The data in Figure 4a demonstrate that the citrus bioflavonoid blend, hesperetin, and naringenin induced expression of luciferase while hesperidin and narirutin did not. One possible mechanism by which flavonoids may activate the ISRE is by inducing production of type I interferon which might act in an autocrine manner. To investigate this possibility, we treated cells with hesperetin, naringenin, and IFNα in the presence or absence of an anti-IFNα antibody. The data in Figure 4b show that under the conditions tested, the antibody inhibited the response to the IFNα control by approximately 80% but had little effect on the response induced by the two flavonoids. These results suggest that naringenin and hesperetin activate the ISRE by a mechanism other that simply inducing expression of IFNα. As such, the possibility existed that combining naringenin or hesperetin with a low concentration of interferon might activate the ISRE to a level greater than could be stimulated by either alone. The data in Figure 5 demonstrate that naringenin and hesperetin both enhanced the response to a suboptimal concentration of IFNα in a dose dependent manner. At all concentrations of flavonoid tested, the combined response was greater than would be expected if the response was additive. In our hands the response of these cells plateaus when exposed to IFNα at a concentration of 1,000 IU/mL (unpublished observation). As the response to flavonoids and low dose IFNα is approximately three-fold higher than the maximum response stimulated by IFNα alone, these results suggest that flavonoids interact with whatever the rate limiting step is in the response of the cells to IFNα.
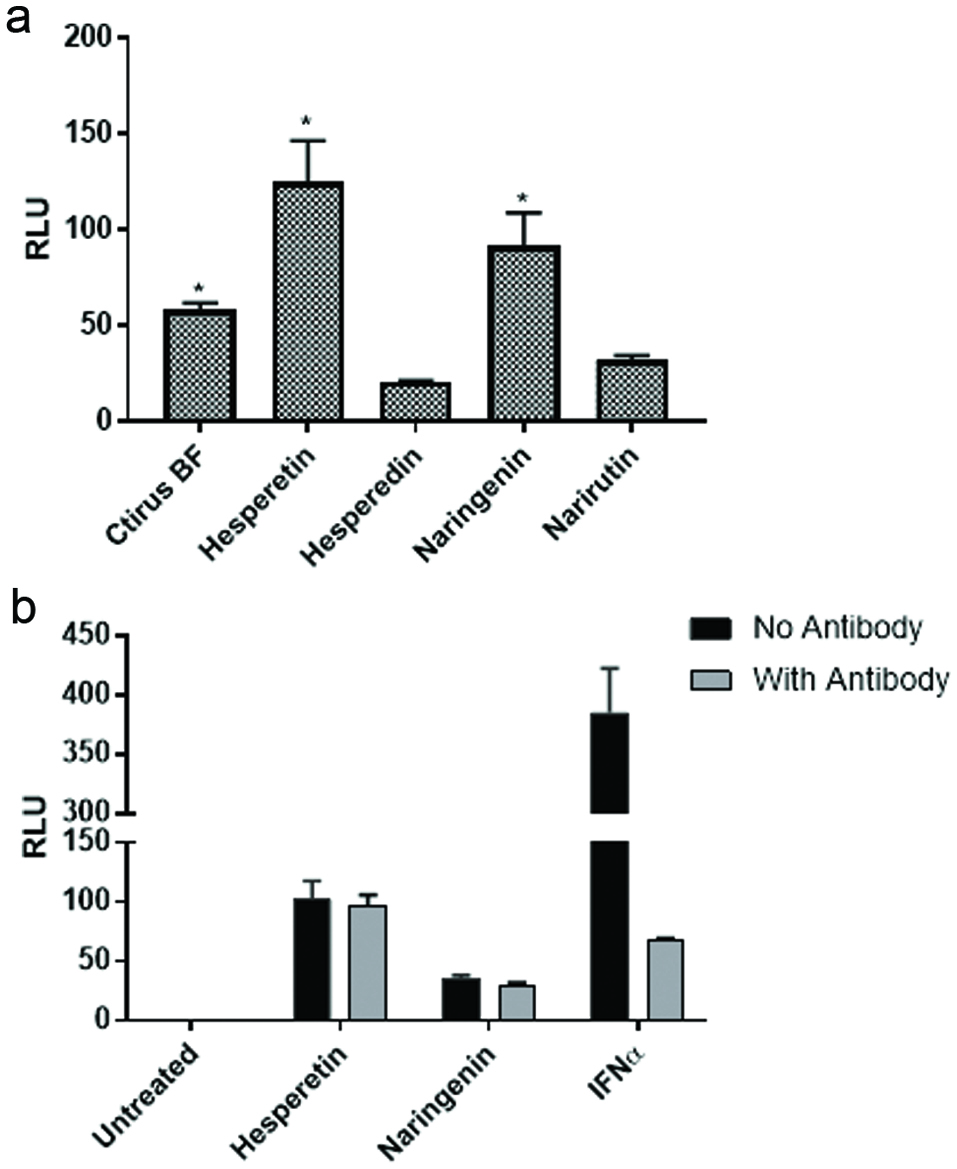 Click for large image | Figure 4. Aglycones but not glycosides activate the ISRE. (a) U2OS cells stably transfected with an ISRE-luciferase plasmid were treated with flavonoids (20 μM) known to be present in the citrus mixture. The cells were incubated for 18 hr. at which time luciferase activity was measured on a Spectramax M5 plate reader. Data from triplicate wells are expressed as RLU compared to untreated negative control cells. (b) U2OS cells stably transfected with an ISRE-luciferase plasmid were treated with flavonoids (20 μM) in the presence or absence of anti-IFNα antibody. Recombinant human universal type I IFN was used as a positive control. Data from triplicate wells are expressed as RLU compared to untreated negative control cells. |
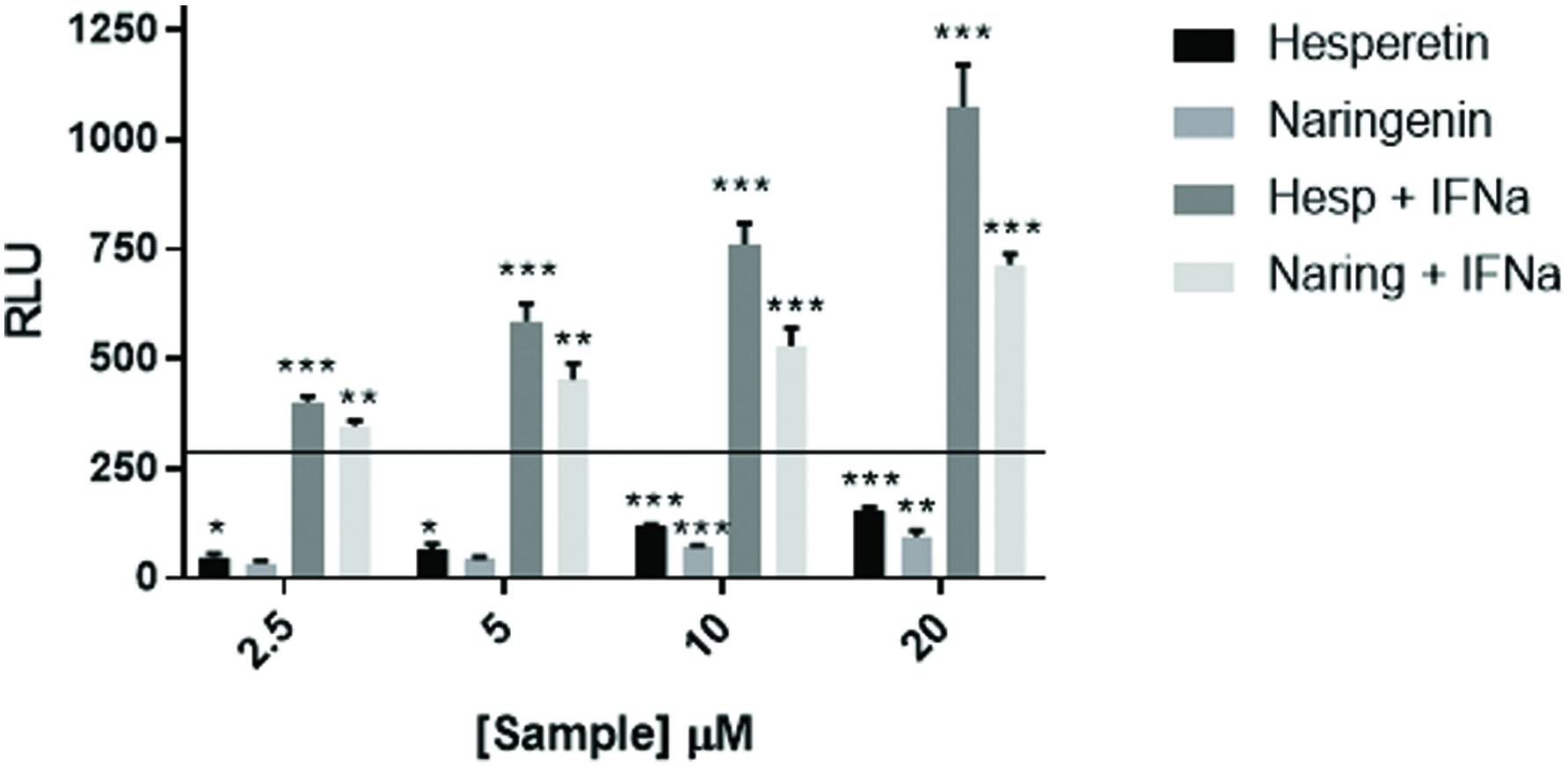 Click for large image | Figure 5. Flavonoids augment ISRE response to IFNα. U2OS cells stably transfected with an ISRE-luciferase plasmid were treated with hesperetin and naringenin at the concentrations shown and with IFNα at 200 IU/mL. The cells were incubated for 18 hr. at which time luciferase activity was measured on a Spectramax M5 plate reader. Data from triplicate wells are expressed as RLU compared to untreated negative control cells. Recombinant human universal type I IFN was used as a positive control. Data from triplicate wells are expressed as RLU compared to untreated negative control cells. |
3.3. Citrus flavonoids induce gene expression of IRF7
Another such mechanism by which flavonoids might activate the ISRE would be to augment expression of IRFs, the transcription factors that bind the ISRE. To test this possibility, U2OS cells were treated with flavonoids and gene expression of several IRFs was assessed by RT-qPCR. The data in Figure 6a show that expression of IRF7, but not of the other IRFs tested, was upregulated in response to naringenin by 30 minutes with maximal expression by 1 hr. The data in Figure 6b show that IRF7 expression increased in a dose dependent manner in cells treated with naringenin for 1 hr. Finally, as shown in Figure 6c, only IRF7 expression increased in a dose dependent manner in cells exposed to hesperetin for 1 hr.
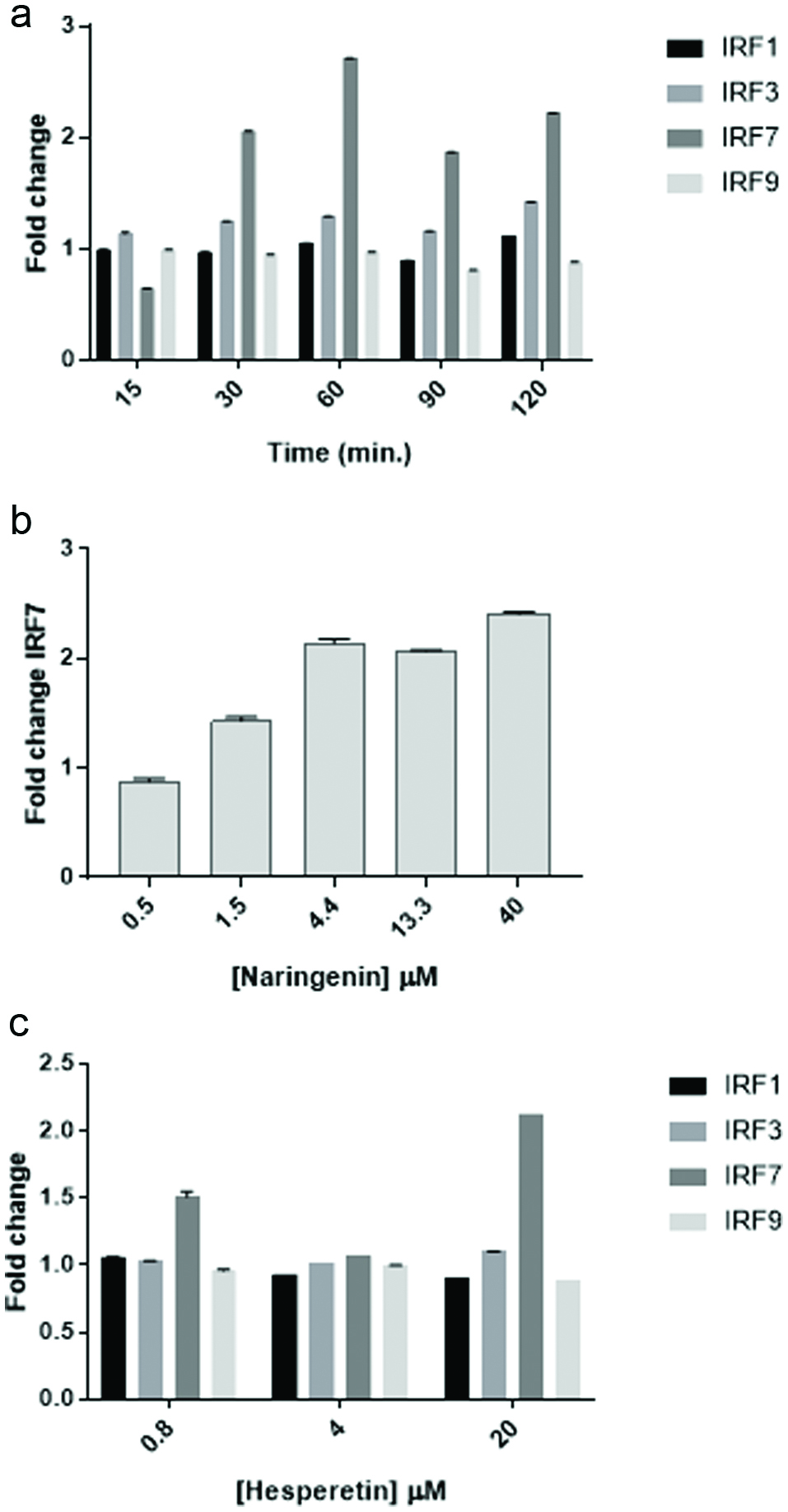 Click for large image | Figure 6. Naringenin and hesperetin stimulate IRF7 gene expression. (a) Time course of IRF expression following treatment of cells with naringenin. U2OS cells were treated with naringenin (20 μM) for the lengths of time indicated. RNA was collected using Qiagen RNEasy columns and 2 step PCR was performed using BioRad iScript and Evagreen SsoFast reagents and primers for IRF1, IRF3, IRF7, and IRF9. Data from quadruplicate wells are expressed as mean fold change ± SD compared to actin as a reference gene. (b) Dose response of IRF7 expression in cells treated with naringenin. U2OS cells were exposed to naringenin at the concentrations indicated for 1 hr. and expression of IRF7 was assessed by PCR as described above. Data from quadruplicate wells are expressed as mean fold change ± SD compared to actin as a reference gene. (c) Dose response of IRF expression in cells treated with hesperetin. U2OS cells were exposed to hesperetin at the concentrations indicated for 1 hr. and expression of IRF7 was assessed by PCR as described above. Data from quadruplicate wells are expressed as mean fold change ± SD compared to actin as a reference gene. |
| 4. Discussion | ▴Top |
Consumption of an abundance and variety of plant based foods is in general correlated with health benefits (Lampe, 1999), and consumption of citrus fruits accounts for a significant portion of overall fruit intake in the majority of geographic diet clusters (Murphy et al., 2014) . One of these benefits might be disease prevention through stimulation of antiviral activity as phytochemicals have been shown to impact several antiviral mechanisms (Kapoor et al., 2017; Saha et al., 2009). Direct effects of such phytochemicals include those with the ability to inhibit entry of viruses into cells as well as those with the ability to inhibit viral replication (Kapoor et al., 2017). In addition, phytochemicals can have indirect antiviral effects through stimulation of cellular antiviral mechanisms. These antiviral effects are mediated primarily by receptors of the innate immune system called toll-like receptors (TLR) that recognize viral nucleic acids to stimulate production of inflammatory cytokines and interferons important for elimination of viruses (Kawai and Akira, 2007)
Based on an unpublished observation that several citrus extracts augmented imiquimod and IL-1β stimulated activation of an NFκB-luciferase reporter cell line, the goal of this study was to investigate a potential role of citrus derived flavonoids in the initiation of antiviral activity. In this study, we observed that a citrus bioflavonoid blend augmented IL-1β and imiquimod stimulated activation of NFκB (Figure 2), and that this activity was also found in response to some of the individual flavonoids found in the blend (Figure 3). In contrast to these results, others have found that naringenin and hesperetin had anti-inflammatory effects (Choi and Lee, 2010; Vafeiadou et al., 2009). It is unknown why our results were different from these authors but could be due to different cell types and/or different stimuli (i.e. LPS/IFNγ) stimulating different toll-like receptors.
In addition to augmenting NFκB activation, we observed that the citrus bioflavonoid blend activated an ISRE—luciferase reporter cell line, and that hesperetin and naringenin, but not their respective glycosides hesperidin and narirutin, appear to be responsible for that activity (Figure 4a). Thus, steric hindrance may play a role either in entry of the flavonoids into cells or in binding their molecular target(s). In addition, it appears that hesperetin and naringenin do not act by inducing IFNα production as an antibody to IFNα did not inhibit the response to either hesperetin or naringenin but did abolish the stimulatory effect of recombinant human IFNα on the ISRE reporter (Figure 4b). These results suggested that the citrus flavonoids MOA in the activation of the ISRE is different from the MOA of IFNα. To initially investigate this possibility, we tested whether hesperetin and naringenin combined with a suboptimal concentration of IFNα would induce a greater ISRE-luciferase response than either reagent alone. As a matter of fact, the response to hesperetin or naringenin with IFNα was much greater than the response that would be expected due to a simple additive response (Figure 5).
One possible explanation for the apparent synergistic ISRE activation by flavonoids and IFNα is that the flavonoids stimulated expression of an IRF. To initially test this possibility, U2-OS cells were exposed to naringenin for up to 2 hr. and expression of IRF1, IRF3, IRF7, and IRF9 was determined by RT-qPCR. Of these, only IRF7 was upregulated by naringenin with maximal expression occurring by 1 hr. of treatment. Likewise, the only IRF upregulated by hesperetin was IRF7. These results suggest that hesperetin and naringenin stimulate expression of IRF7. By themselves, this is sufficient to activate the ISRE, and in the presence of IFNα leads to significant activation of the ISRE.
Secondary metabolites from citrus possess a number of biological properties including anti-inflammatory effects (Lv et al., 2015). For example, naringenin has been shown to reduce production of pro-inflammatory cytokines in human blood cells (Bodet et al., 2008) while hesperidin reduced inflammation in a mouse model of LPS-induced lung inflammation (Yeh et al., 2007). This contrasts with what we observed in that hesperetin, hesperidin, and narirutin all augmented activation of NFκB in a luciferase reporter assay. We speculate that the difference between our results and others in the effect of flavonoids on inflammation is due to engagement of different toll-like receptors (TLR) (Medzhitov et al., 1998).The aforementioned studies made use of endotoxin, a TLR4 agonist. In contrast, we used imiquimod, a TLR7 agonist (Hemmi et al., 2002).
Of citrus derived flavonoids, approximately 95% are flavanones. These exist in both aglycone and glycoside forms in which the aglycones are linked to a sugar moiety (Lv et al., 2015). This is also reflected in our UPLC chromatogram on the citrus bioflavonoid blend in which naringin and hesperidin are the major components. Conversion of the glycoside forms of these flavonoids into their respective aglycone forms undoubtedly occurs in the presence of the gut microbiome (Manach and Donovan, 2004; Nielsen et al., 2006) to release naringenin and hesperetin so that they can act on their molecular targets. Both hesperetin and naringenin were found in plasma after their glycoside forms such as hesperidin and naringin rich citrus juice ingestion by human subject (Erlund et al., 2001; Nielsen et al., 2006). Kaul et al found hesperetin but not the intracellular replication of virus in a monolayer cell (Kaul et al., 1985). In summary, our results suggest that citrus flavonoids stimulate anti-viral activity mediated by upregulation of IRF7 gene expression. To our knowledge, this is a unique antiviral activity of citrus flavonoids.
| 5. Conclusion | ▴Top |
The results presented here suggest that aglycone forms of flavanones commonly found in citrus fruits have the potential to activate antiviral pathways under the control of the ISRE and interferonα Specifically, the flavanones tested upregulated expression of IRF7, a transcription factor important in defense against influenza virus infection.
Acknowledgments
The authors wish to thank Mark Proefke and Christine Hernandez for their support of this work.
D.F. and C.H. wrote the main manuscript; N.P. performed the UPLC analysis; D.F. performed the cell culture experiments and data analyses. C.H. revised the manuscript. All authors designed the study and reviewed the manuscript.
| References | ▴Top |