| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 7, September 2019, pages 43-47
Antioxidant activity of soybean peptides on human hepatic HepG2 cells
Carmen Lammi*, Carlotta Bollati, Anna Arnoldi
Department of Pharmaceutical Sciences, University of Milan, 20133 Milan, Italy
*Corresponding author: Carmen Lammi, Department of Pharmaceutical Sciences, University of Milan, via Mangiagalli 25, 20133 Milan, Italy. Tel: +39 02-50319372; E-mail: carmen.lammi@unimi.it
DOI: 10.31665/JFB.2019.7197
Received: September 28, 2019
Revised received & accepted: September 28, 2019
| Abstract | ▴Top |
Soybean is an interesting source of bioactive peptides useful for the development of functional foods and nutraceuticals. In this study, the antioxidant activity of peptic (P) and tryptic (T) soybean hydrolysates was characterized. Results suggest that both hydrolysates are able to scavenge DPPH radical. Moreover, after induction of oxidative stress by using H2O2, either Soybean P or T pre-treatment reduces the ROS, lipid peroxidation, and NO levels in human hepatic HepG2 cells. HepG2 cells, exposed to H2O2 alone, produce a significant augmentation of intracellular reactive oxygen species (ROS) levels by 29.5%, with the consequence of an augmentation of cellular lipid peroxidation levels up to 112.4 ± 0.5%. The pre-treatment with soybean hydrolysates restores the basal level of ROS and induces a reduction of cellular lipid peroxidation. The antioxidant ability of Soybean P and T are also confirmed by their ability to reduce the H2O2-induced NO levels in HepG2 cells.
Keywords: Antioxidant; Bioactive peptides; Food bioactive peptide; Hydrolysates; Ros; Soybean
| 1. Introduction | ▴Top |
Cardiovascular disease (CVD) is a leading cause of death worldwide. Many risk factors are responsible for the development of this multifactorial disease, with a prevalence of those related to atherosclerosis, which is strictly connected with oxidative stress and inflammatory processes (Wu, Xia, Kalionis, Wan, & Sun, 2014).
Although reactive oxygen species (ROS) are produced by living organisms as a result of normal cellular metabolism, at high concentrations they produce adverse effects on cell components, such as lipids, proteins, and DNA. Oxidative stress, which refers to the shift in balance between oxidants/antioxidants in favour of oxidants, contributes to many pathological conditions (Dhalla, Temsah, & Netticadan, 2000). Aerobic organisms have integrated antioxidant systems, which include enzymatic and nonenzymatic antioxidants, that are usually effective in blocking harmful effects of ROS. However, in pathological conditions, the antioxidant systems can be destroyed and the use of food-derived antioxidant agents could be a good strategy to impair the progression of disease related to oxidative stress (Lorenzo et al., 2018). For instance, egg, milk, meat, and fish have been identified as a good sources of peptides with interesting antioxidant activity (Ibrahim, Isono, & Miyata, 2018; R. Liu, Xing, Fu, Zhou, & Zhang, 2016; Nazeer, Kumar, & Jai Ganesh, 2012; Zambrowicz et al., 2015).
Digestion or suitable technological treatments can deliver from food proteins bioactive peptides, some of which showing a multifunctional behaviour (Lammi, Aiello, Boschin, & Arnoldi, 2019). In particular, some anti-diabetic, hypotensive, and hypocholesterolemic peptides display also antioxidant activity (Girgih et al., 2014; Iqbal & Hussain, 2009; Siow & Gan, 2013; Zambrowicz et al., 2015).
Numerous clinical studies have associated soy food consumption with a reduced risk of developing some chronic diseases, such as obesity, hypercholesterolemia, and insulin-resistance/type II diabetes (Velasquez & Bhathena, 2007). As for the active substance in soy foods, the protein plays a role in cholesterol reduction (Fukui et al., 2002; Liu et al., 2014) and some hypocholesterolemic and anti-diabetic peptides have been already identified in the sequences of glycinin and β-conglycinin (Lammi, Zanoni, & Arnoldi, 2015a; Lammi, Zanoni, & Arnoldi, 2015b). Soybean represents thus a promising source of protein hydrolysates with a multifunctional behaviour that has been recently investigated. In particular, it has been demonstrated that peptic (P) and tryptic (T) hydrolysates from soybean protein show an in vitro hypocholesterolemic activity targeting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCoAR). Through the inhibition of this enzyme, both hydrolysates lead to an augmentation of the low-density lipoprotein (LDL) receptor (LDLR) protein levels producing an increased ability of hepatic HepG2 cells to clear LDL from the extracellular space(Lammi, Arnoldi, & Aiello, 2019). Moreover, the same hydrolysates are able of inhibiting dipeptidyl peptidase-IV (DPP-IV) in vitro on the human recombinant enzyme as well as in human intestinal Caco-2 cells expressing DPP-IV, suggesting a potential anti-diabetic effect.
Considering that both diabetes and hypercholesterolemia are correlated to oxidative stress, this study was aimed at characterizing the antioxidant properties of the same soybean hydrolysates. This was done, initially, by evaluating in vitro the antioxidant activity employing the 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) reagent, then by pre-treating HepG2 cells with the hydrolysates after the induction of oxidative stress using H2O2 and assessing their ability to reduce the ROS, lipid peroxidation, and nitric oxide (NO).
| 2. Material and methods | ▴Top |
2.1. Materials and cell cultures
All chemicals and reagents were of analytical grade. DPPH, ROS, lipid peroxidation and Nitrite/Nitrate assays were from Sigma-Aldrich (St. Louis, MO, USA). The HepG2 cell line was bought from ATCC (HB-8065, ATCC from LGC Standards, Milan, Italy) and was cultured following the conditions previously described (Lammi et al., 2015b).
2.2. Production of Soybean P and T hydrolysates
Soybean P and Soybean T hydrolysates were obtained by extracting the proteins from 2 g of defatted soybean flour and by hydrolysing them with pepsin or trypsin. Their production and analysis of these materials are described elsewhere (Lammi et al., 2019).
2.3. DPPH assay
The DPPH assay to determine the antioxidant activity in vitro was performed by a standard method with slight modifications. The DPPH solution (0.0125 mM in methanol, 45 μL) was added to 15 μL of the Soybean P and Soybean T hydrolysates at different concentrations (0.5–5.0 mg/mL). The reaction for scavenging DPPH radicals was performed in the dark at room temperature and the absorbance was measured at 520 nm after 30 min incubation.
2.4. Cell culture
HepG2 cell line was bought from ATCC (HB-8065, ATCC from LGC Standards, Milan, Italy) and was cultured following the conditions previously described (Lammi et al., 2015b).
2.5. Nitrite/nitrate assay
A total of 3 × 104 HepG2 cells/well were seeded on a 96-well plate. The next day, cells were treated with Soybean P and Soybean T at different concentrations (0.5 and 1.0 mg/mL) overnight and then 0.5 mM H2O2 was added in each well for 30 min at 37 °C. After incubation, cells were centrifuged at 1,000 g for 15 min to remove insoluble material. The supernatant was transferred in a 96-well plate, then 10 µL of nitrate reductase solution and 10 µL of the enzyme co-factors solution were added to the samples and the plate was incubated at 25 °C for 2 h. Afterward, 50 µL of Griess Reagent A was added to each well and, after 5 min, 50 µL of Griess Reagent B was added for 10 min. For the detection step, the absorbance at 540 nm was measured using a Synergy H1 microplate reader.
2.6. Fluorometric intracellular ROS assay
For cells preparation, 3 × 104 HepG2 cells/well were seeded on a 96-well plate overnight in growth medium. The day after, the medium was removed, 50 μL/well of Master Reaction Mix was added and the cells were incubated at 5% CO2, 37 °C for 1 h in the dark. Then, cells were treated with 5 μL of 12× Soybean P and Soybean T to reach the final concentrations of 0.5 mg/mL and 1.0 mg/mL and incubated at 37 °C for 1 h in the dark. To induce ROS, cells were treated with H2O2 at a final concentration of 0.5 mM for 30 min a 37 °C in the dark and fluorescence signals (ex./em. 490/525 nm) were recorded using a Synergy H1 microplate reader.
2.7. Lipid peroxidation (MDA) assay
HepG2 cells (5 × 105 cells/well ) were seeded in a 6 well plate and, the following day, they were treated with 0.5–1.0 mg/mL of Soybean P and T for 24 h at 37 °C under 5% CO2 atmosphere. The day after, cells were incubated with H2O2 1mM or vehicle (H20) for 30 min, than collected and homogenized in 300 µL ice-cold MDA lysis buffer containing 3 µL of BHT (100×). Samples were centrifuged at 13,000 × g for 10 min, then they were filtered through a 0.2 µm filter to remove insoluble material. To form the MDA-TBA adduct, 300 µL of the TBA solution were added into each vial containing samples and incubated at 95 °C for 60 min, then cooled to RT for 10 min in an ice bath. For analysis, 100 µL of each reaction mixture were pipetted into a 96 well plate and the absorbance was measured at 532 nm using the Synergy H1 fluorescent plate reader from Biotek. To normalize the data, total proteins for each sample were quantified by Bradford method.
2.8. Statistically analysis
Statistical analyses were carried out by One-way ANOVA (Graphpad Prism 8) followed by Brown-Forsythe’s test. Values were expressed as means ± sd; P-values < 0.05 were considered to be significant.
| 3. Results and discussion | ▴Top |
3.1. In vitro radical scavenging activity of Soybean P and T hydrolysates
In order to evaluate the in vitro radical scavenging activity of Soybean P and T hydrolysates, the DPPH assay was employed. The hydrolysates were tested in the range from 0.5 to 5.0 mg/mL. The results clearly suggested that both hydrolysates have a modest ability to scavenge DPPH radical (Figure 1a–b). Soybean P reduces the DPPH radicals by 13.7%, 15.5, and 22.1% at 0.5, 1.0, and 5.0 mg/mL, respectively (Figure 1a), whereas Soybean T diminishes the DPPH radicals by 5.1%, 11.3%, and 18.4%, respectively (Figure 1b), indicating that the former hydrolysate has a better radical scavenging activity than the latter. This different behaviour may be explained considering the different physiochemical properties of these hydrolysates. Thus, Soybean P is predominantly characterized by peptides ranging from 1 to 1.2 kDa, whereas Soybean T contains mostly large amounts of medium and long peptides with a molecular weight larger than 2 kDa. Moreover, the average hydrophobicity of pepsin peptides is larger than that of trypsin peptides (Lammi et al., 2019). Instead, Soybean P contains 22.2% peptides with lengths ranging from 8 to10 amino acid residues and an average hydrophobicity of 48.1 kcal mol−1, 73.6% peptides with a length of 11–20 amino acid residues and an average hydrophobicity of 44.5 kcal mol−1, and 4.2% of peptides with a length of 20–21 amino acids and an average hydrophobicity of 50.7 kcal mol−1. On the contrary, Soybean T contains 6.2% peptides with a length of 9–10 amino acid residues and an average hydrophobicity of 32.2 kcal mol−1, 67.2% peptides with a length of 11–20 amino acid residues and an average hydrophobicity of 39.2 kcal mol−1, and 26.6% peptides with a length of 20–27 amino acids and an average hydrophobicity of 40.4 kcal mol−1.
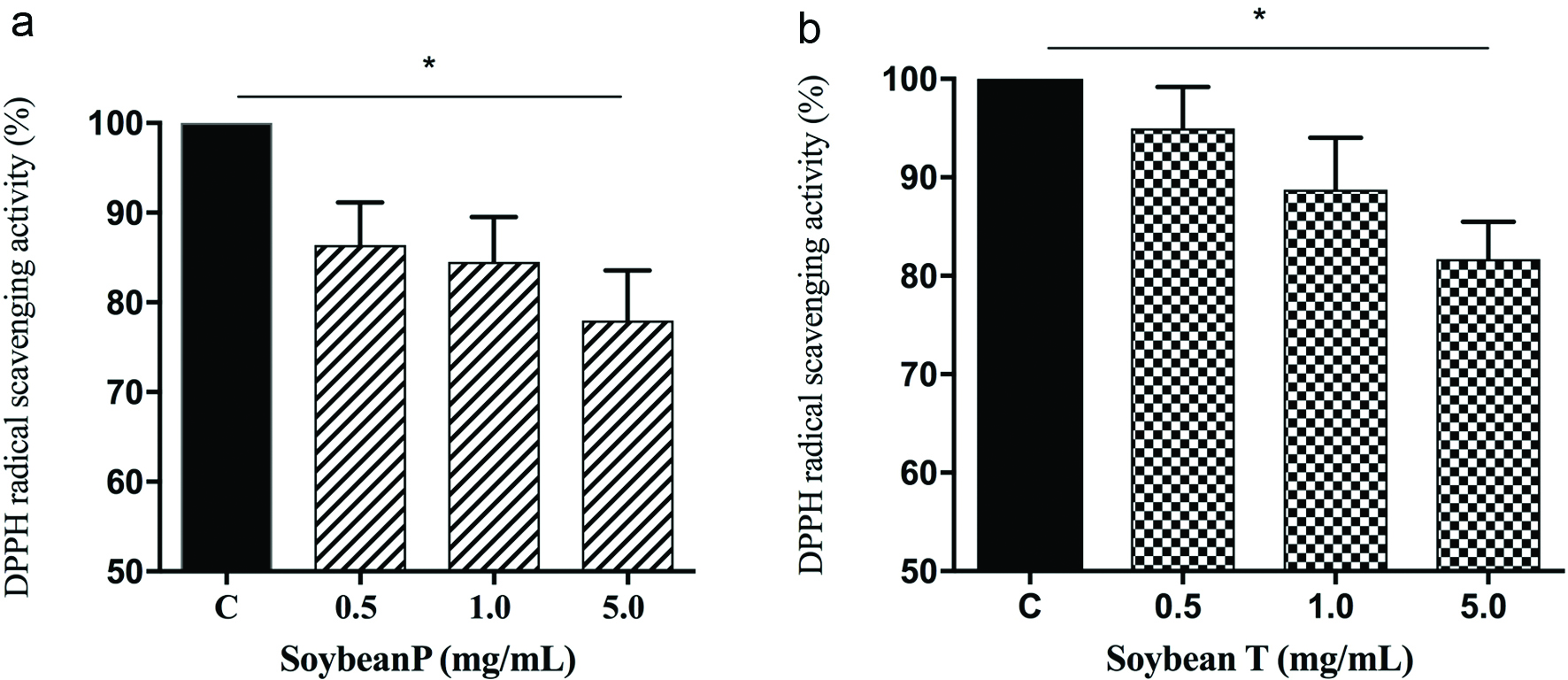 Click for large image | Figure 1. In vitro evaluation of the DPPH radical scavenger activity of Soybean P (a) and Soybean T (b) hydrolysates. The data points represent the averages ± sd of four independent experiments in duplicate. (*) p < 0.05. C: control sample. |
Even though, the radical scavenging activity of food protein hydrolysates is influenced by many factors (i.e. the proteases used for the generation of the hydrolysates, size and amino acid composition of the peptides, and the DPPH assay conditions), our findings are in line with previous studies (Aluko & Monu, 2003; Li, Jiang, Zhang, Mu, & Liu, 2008; Udenigwe, Lu, Han, Hou, & Aluko, 2009). In particular, soybean P and T hydrolysates resulted to be more active that a hempseed protein hydrolysate, obtained by co-digesting the proteins with pepsin and pancreatin, which has shown a poor scavenger of DPPH activity, i.e. about 4% (Girgih, Udenigwe, & Aluko, 2011). Instead, rice bran protein hydrolysates, obtained after the hydrolysis of the proteins with Alcalase, displayed a DPPH free radical scavenging activity of about 32% at 20 mg/mL (Wattanasiritham, 2015). Finally, fish and chicken bones hydrolysates, obtained using trypsin, have shown an antioxidant activity of about 15% and 10%, respectively, at 5.0 mg/mL (Centenaro, Mellado, & Prentice-Hernandez, 2011).
3.2. Soybean P and T hydrolysates modulate the H2O2-induced oxidative stress in human hepatic HepG2 cells
Excessive production of intracellular ROS leads to severe cellular damage, which may affect proteins, DNA and lipid stability. (Dhalla et al., 2000) For this reason, in order to evaluate whether Soybean P and T hydrolysates modulate the H2O2-induced ROS production, HepG2 cells were pre-treated with both hydrolysates (0.5 and 1.0 mg/mL) O/N at 37 °C. The following day, the same cells were treated with 0.5 mM H2O2 for 30 min at 37 °C. Results (Figure 2) clearly highlight that HepG2 cells, exposed to H2O2 alone, produce a significant augmentation of intracellular ROS levels by 29.5% vs control cells (p < 0.01), which was attenuated by the pre-treatment with Soybean P and T hydrolysates: Soybean P reduced the H2O2-induced intracellular ROS by 19.5% and 14.2% at 0.5 and 1.0 mg/mL, respectively, whereas Soybean T by 17.3% and 13.3% at 0.5 and 1.0 mg/mL, respectively (p < 0.05). These findings underline a dramatic increase of intracellular ROS, but the pre-treatment with Soybean P and T hydrolysates significantly protects the HepG2 cells restoring the ROS level to basal condition and confirming their good ability to act as natural antioxidant. As already underlined, high oxidative stress results in significant damage to human cells by altering proteins, lipids and DNA, leading to several simultaneous processes, which may culminate in pathological conditions involved in CVD progression.
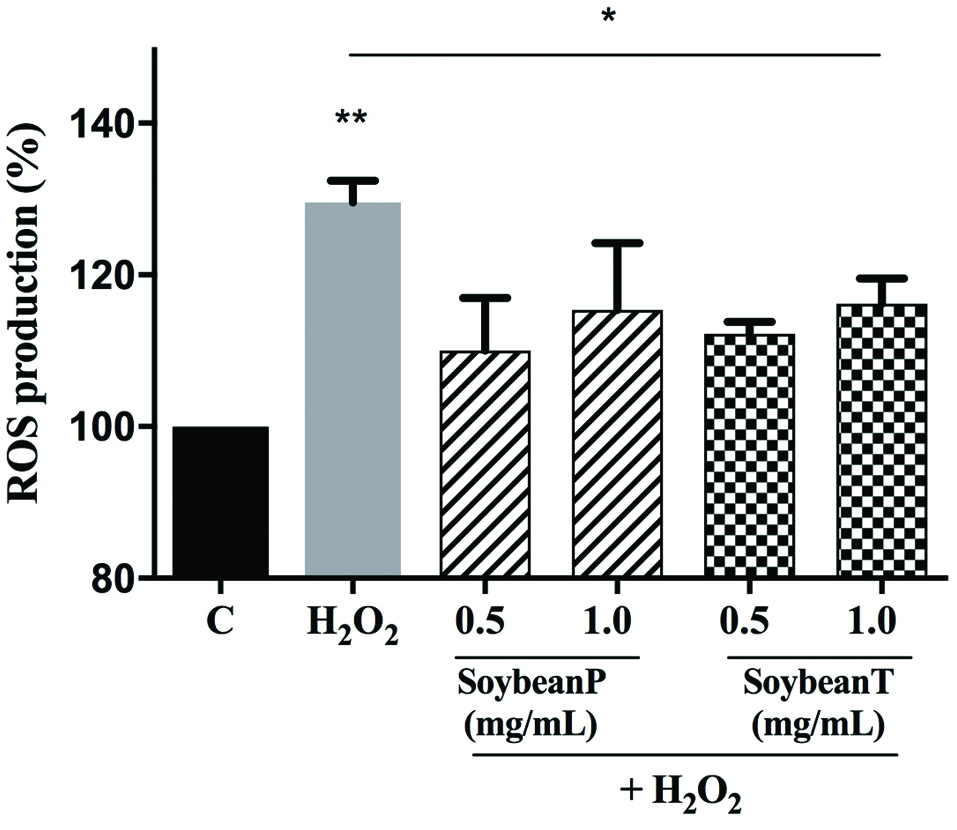 Click for large image | Figure 2. Evaluation of the effects of Soybean P and T hydrolysates on the H2O2-induced ROS production levels at human hepatic HepG2 cells. The data points represent the averages ± sd of six independent experiments in duplicate. (*) p < 0.05, (**) p < 0.01. C: control cells. |
Lipid of cellular membranes are susceptible to an oxidative attack, typically by ROS, resulting in a well-defined chain reaction with the production of end products such as malondialdehyde (MDA). Based on these considerations, the capacity of Soybean P and T hydrolysates to modulate the H2O2-induced lipid peroxidation in human hepatic HepG2 cells was assessed measuring the reaction of MDA with thiobarbituric acid (TBA) to form fluorometric (λex = 532/λem = 553 nm) product, proportional to the MDA present. Figure 2 clearly suggests that, in agreement with the observed increase of ROS after the H2O2 treatment, a significant increase of the lipid peroxidation at cellular level up to 112.4 ± 0.5% was observed (p < 0.001). In addition, the pre-treatment of HepG2 cells with both Soybean P and T hydrolysates determine a significant reduction of lipid peroxidation even under basal conditions (p < 0.01). As illustrated in the Figure 3, Soybean P decreases the lipid peroxidation up to 108.1 ± 2.2% and 95.8 ± 0.2% at 0.5 and 1.0 mg/mL, respectively, whereas Soybean T up to 93.1 ± 5.3% and 93.3 ± 1.0% at 0.5 and 1.0 mg/mL, respectively. Since the lipid peroxidation is a validated marker of oxidative stress, these findings confirm the good antioxidant property of soybean hydrolysates and that the tryptic hydrolysate is more active than the peptic one.
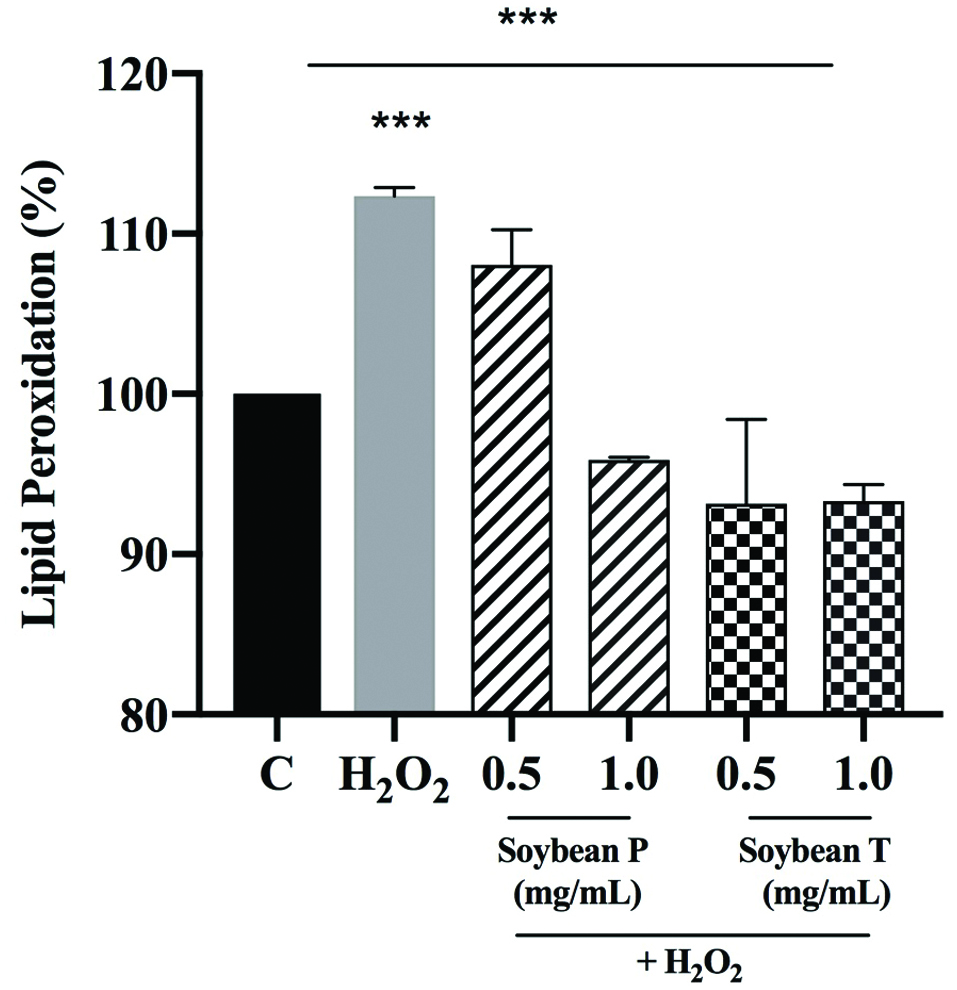 Click for large image | Figure 3. Evaluation of the effects of Soybean P and T hydrolysates on the H2O2-induced lipid peroxidation levels at human hepatic HepG2 cells. The data points represent the averages ± sd of six independent experiments in duplicate. (***) p < 0.001. C: control cells. |
3.3. Soybean P and T hydrolysates modulate the H2O2-induced NO production in human hepatic HepG2 cells
The ROS act either as a signalling molecule or a mediator of inflammation (Mittal, Siddiqui, Tran, Reddy, & Malik, 2014). Superoxide can rapidly combine with NO to form reactive nitrogen species (RNS), such as peroxynitrite, with a reaction rate that is faster than the dismutation of superoxide by superoxide dismutase (Beckman, 1996). In addition, the RNS lead to a nitrosative stress, which parallels the pro-inflammatory activity of ROS (Sunil, Shen, Patel-Vayas, Gow, Laskin, & Laskin, 2012). More and more emerging evidences clearly underline the intricate relation between oxidative stress and inflammation (Mittal et al., 2014).
Based on these considerations, the Soybean P and T hydrolysates effects on the NO level production were evaluated at human hepatic HepG2 cells, after oxidative stress induction. A H2O2 treatment was used to induce the oxidative stress and the NO levels, produced at intracellular levels, were measured. Figure 4 clearly indicates that the H2O2 treatment dramatic increases the NO levels up to 383.6 ± 94.1% (p < 0.05) and that the pre-treatment with soybean peptides reduces the H2O2-induced NO levels leading their values closer to the basal levels (p < 0.01). In particular, Soybean P (0.5 and 1.0 mg/mL) decreases the NO level up to 139.1 ± 34.7% and 125.9 ± 14.7%, whereas Soybean T (0.5 and 1.0 mg/mL) up to 125.8 ± 22.6% and 125.6 ± 35.8%, respectively. Interestingly, these findings confirm the same trend that was observed assessing the effect of soybean peptides on the modulation of H2O2-induced cellular lipid peroxidation. In particular, Soybean T hydrolysate seems to be also in this case slightly more active to restore the basal intracellular NO levels.
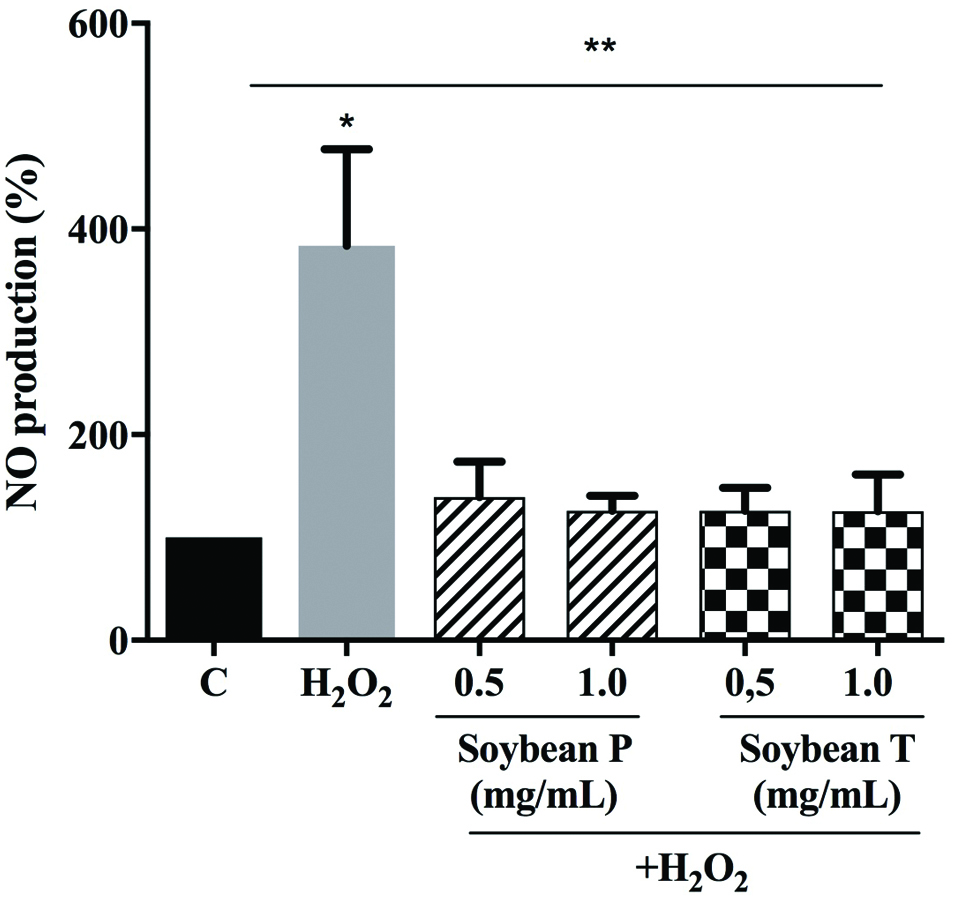 Click for large image | Figure 4. Investigation of the ability of Soybean P and T hydrolysates to modulate the H2O2-induced NO level production at human hepatic HepG2 cells. The data points represent the averages ± sd of six independent experiments in duplicate. (*) p < 0.05, (**) p < 0.01. C: control cells. |
| 4. Conclusion | ▴Top |
Soybean is an interesting source of bioactive peptides useful for the development of functional foods and nutraceuticals. Many evidences clearly suggest that soybean peptides mediate hypocholesterolemic, hypotensive and hypoglycaemic activities which are strictly related to oxidative stress. Our results indicate that soybean peptides could also contribute to an antioxidant activity which is linked to the modulation of intracellular ROS and NO levels leading to a reduction of lipid degradation.
Acknowledgments
We acknowledge Carlo Sirtori Foundation (Milan, Italy) for having provided part of equipment used in this experimentation and the Fondazione Cariplo, project “SUPER-HEMP: Sustainable Process for Enhanced Recovery of Hempseed Oil” number: 2017-1005. Moreover, we thank the project “DISCOVERY—Disaggregation of conventional vegetable press cakes by novel techniques to receive new products and to increase the yield”, bando ERA-NET SUSFOOD2
Experiment ideation and design, CL.; Biological experiments, C.L. C.B. Data analysis, C.L. and C.B.; Discussion of the results, C.L., Manuscript writing, C.L. and A.A.
Conflict of interest
The authors declare they have no conflicts of interest.
| References | ▴Top |