| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 7, September 2019, pages 27-35
UF-LC-DAD-MSn for discovering enzyme inhibitors for nutraceuticals and functional foods
Li Lia, Rong Tsaob, *
aThe College of Chemistry, Changchun Normal University, Changchun 130032, China
bGuelph Research and Development Center, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario, N1G 5C9 Canada
*Corresponding author: Rong Tsao, Guelph Research and Development Center, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario, N1G 5C9 Canada. Tel: +1 226-217-8108; Fax: +1 226-217-8183; E-mail: rong.cao@canada.ca
DOI: 10.31665/JFB.2019.7195
Received: September 29, 2019
Revised received & accepted: September 30, 2019
| Abstract | ▴Top |
The technique of ultrafiltration liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UF-LC-MSn) in screening enzyme inhibitors has emerged as an efficient tool for high-throughput screening of bioactives. The mini-review discusses about the principles of the UF-LC-MSn method and its applications in the discovery of bioactives in herbs and foods that are specific inhibitors of important proteins/enzymes related to human health. The advantageous and disadvantages of the method is critically assessed, and its potential use in functional food and nutraceutical research is addressed.
Keywords: Ultrafiltration; LC-MS; High-throughput screening; Enzyme inhibitors; Herb extracts; Food bioactives
| 1. Introduction | ▴Top |
The pharmacological activity of many drugs or food bioactives comes from the interactions between small bioactive molecules and biological targets e.g. macromolecules like proteins in vivo. Existing studies have provided convincing evidence that enzymes are major biomarkers in many pathological processes, which are attractive targets for new drug discovery and for understanding the relationship between diet and health. Recent surveys found that nearly half of all the marketed small-molecule drugs are enzyme inhibitors (Jin et al., 2016; Newman and Cragg, 2007; Wang et al., 2011). Many enzymes play critical roles in non-communicable chronic diseases. For example, matrix metalloprotease-2 (MMP-2) is key enzyme involved in cancer progression, tissue remodeling, bone remodeling, wound healing and several aspects of immunity (Szaboa et al., 2004). 5-Lipoxygenase (5-LOX) is an important enzyme in leukotriene biosynthesis and catalysis, and is involved in several pathological processes related to pancreatic cancer, rheumatoid arthritis and inflammatory reactions (Ding et al., 2011; Schneider and Bucar, 2005; Steinhilber, 1994). Cyclooxygenase-2 (COX-2) is essential for the maintenance of the gastrointestinal tract, renal function, arthritis and fever (Chandrasekharan and Simmons, 2004; Yokoyama and Tanabe, 1989). It is also a key enzyme responsible for the production of inflammatory mediators prostaglandins (PG) and their metabolites, and has been associated with pro-inflammatory activities that are related to several acute and chronic diseases. α-Glucosidase is closely related to type 2 diabetes (van de Laar et al., 2005), which has become a worldwide health problem. α-Glucosidase inhibitors are widely used in the treatment of type 2 diabetes, and many dietary components including polyphenols have been found to exhibit excellent α-glucosidase inhibitory activities, thus play significant role in dietary intervention for metabolic syndromes like type 2 diabetes(Wang et al., 2014; Zhang et al., 2015; Tang et al., 2016). Xanthine oxidase (XOD) can generate reactive oxygen species and catalyze the metabolism of hypoxanthine and xanthine to produce uric acid, the over production of which could cause hyperuricemia, a risk factor for gout (Lin et al., 2000; Liu et al., 2012a; Wang et al., 2014). The angiotensin-converting enzyme (ACE) converts the hormone angiotensin I to angiotensin II which is the active vasoconstrictor controlling the blood pressure via regulating the volume of fluids in the body. As a result, ACE indirectly increases blood pressure. ACE inhibitors are widely used as pharmaceutical drugs for treatment of cardiovascular diseases, and many food-originated inhibitors of ACE have been found to exhibit therapeutic value in the prevention of hypertension (Inoue et al., 2011). Acetylcholinesterase (AChE) is an acetylhydrolase that catalyzes the breakdown of acetylcholine, one of the most neurotransmitters. AChE inhibitors prevent the hydrolysis of acetylcholine into choline and acetate, thus increase both the level and duration of action of the neurotransmitter acetylcholine in the central nervous system, which is considered beneficial to alleviating Alzheimer’s disease. Phenolics such as cinnamic acids and flavonoids, and other phytochemicals such as ginsenosides have been found to inhibit AChE activity and (Zhang et al., 2018; Yang et al., 2019). Other enzymes have been known for their role in tumour formation and cancer. Quinone reductase-2 (QR-2) is a cytosolic enzyme involved in several possible mechanisms of action including antimalarial and antitumor activities (Fu et al., 2005; Kwiek et al., 2004), and aromatase represents an important target for the treatment of hormone dependent breast cancer (Kendall and Dowsett, 2006). Another target of antiviral agents is neuraminidase (NA) which is a glycoside hydrolase enzyme that breaks the glycosidic linkages of neuraminic acids. Inhibitors of neuraminidase are particularly important drugs against influenza infection (Meanwell and Krystal, 1996). Discovering new inhibitors of these important enzymes from medicinal and functional foods is a good practice although there are challenges. Synthetic enzyme inhibitors are mostly pharmaceutical drugs that can ameliorate symptoms of various diseases, however, they can also cause severe side effects as have been found in clinical applications in recent years (Borges et al., 2002; Gnant, 2006; Kitching et al., 2009; Nelson et al., 2000; van de Laar et al., 2005). For this reason, increasing attention has been given to researches targeting on naturally occurring enzyme inhibitors such as those derived from herb or food extracts. However, screening and identifying active constituent(s) by conventional methods are time consuming and inefficient (Wen et al., 2007; Shi et al., 2013). Rapid and effective screening strategies must therefore be developed for identifying inhibitors from complex mixtures. Membrane ultrafiltration with different molecular cut-offs has been used for isolation and purification of macromolecules (He et al, 2013). However in the last few years, this technology has been coupled with liquid chromatography-diode array detector-mass spectrometry (LC-DAD-MS) for high-throughput screening and discovery of enzyme inhibitors from herb extracts and plant foods (Cao et al., 2010; Choi et al., 2011; Li et al., 2014; Li et al., 2015; Li et al., 2009; Liu et al., 2013; Liu et al., 2013; Liu et al., 2012a, b; Liu et al., 2011; Shi et al., 2013; van Breemen et al., 2011; Wang et al., 2014; Wang et al., 2014; Yang et al., 2012; Zhao et al., 2016; Zhang et al., 2012; Shi et al., 2010; Zhou et al., 2012 ). In ultrafiltration LC-DAD-MS (UF-LC-MS) experiments, when a mixture of compounds (ligands) is in contact with the target enzyme (a macromolecular receptor), the ultrafiltration membrane retains the macromolecules and the ligand-receptor complexes, but allows unbound, low molecular weight compounds to pass through. Bound ligands are then dissociated by disrupting the ligand-receptor complex with an organic solvent or changing pH. The released ligands in the organic solvent are then analyzed and identified on-line using LC-DAD-MSn (Johnson et al., 2002) (Figure 1). In UF-LC-MSn, the tandem mass spectrometric fragmentation pattern of compounds has been studied and used extensively in the characterization of unknown compounds. This is especially useful when standard references are not available (Cuyckens and Claeys, 2004; 2005). UF-LC-MSn is a high throughput screening and identification technique for active compounds, and is advantageous in low sample requirement and reuse of enzymes, and it does not involve any enzyme immobilization process (Zhou et al., 2012) (Figure 1). More importantly, UF-LC-MSn can effectively solve one of the mostchallenging problems in functional foods and nutraceuticals research, i.e. thecompound with the highest concentration in a particular food or its extract is often assumed as the most active component, yet in many cases, minor components of a mixture may be more bioactive when compounds are purified. There lacks information on the bioactivity of individual compounds of mixtures.
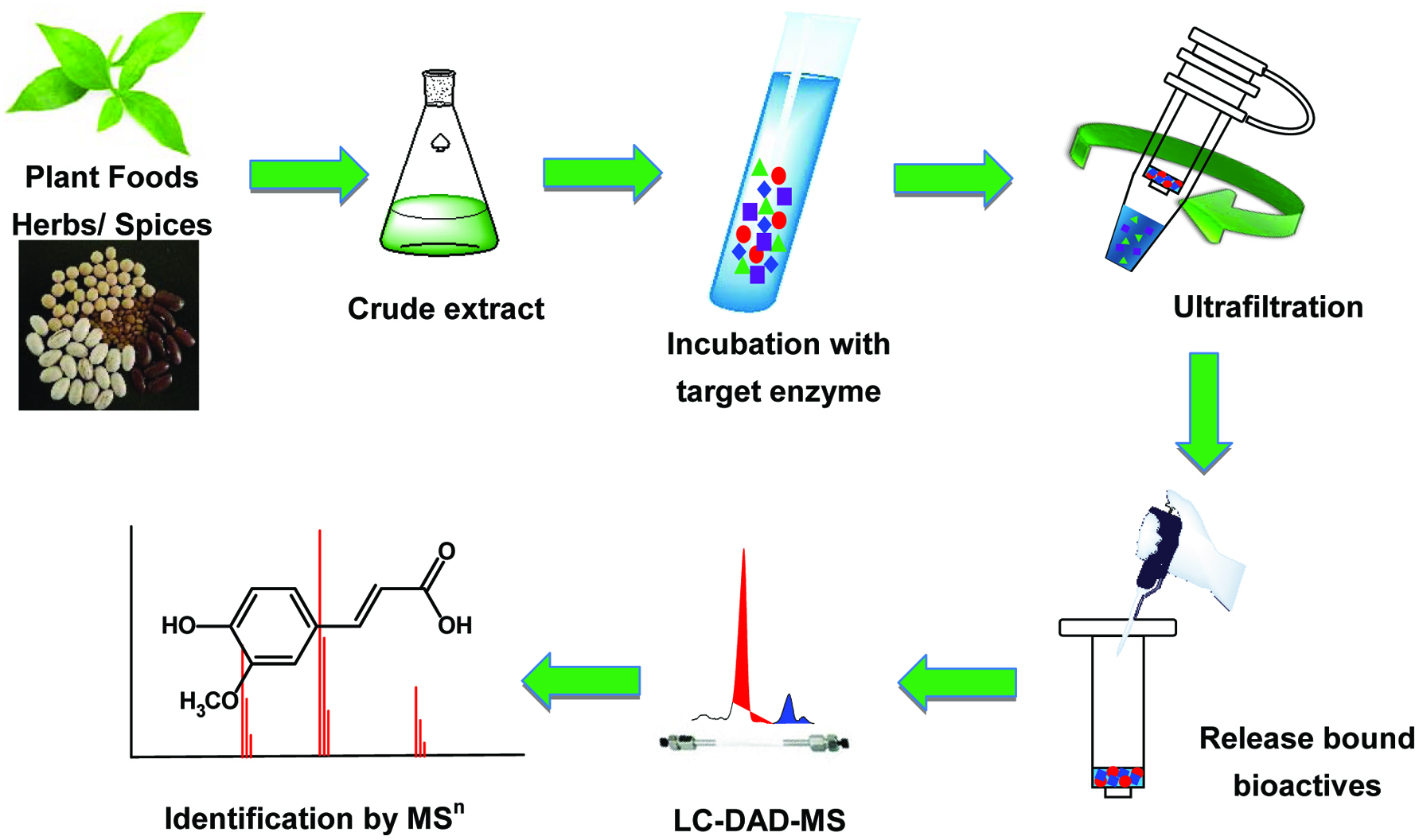 Click for large image | Figure 1. Typical procedures of the ultrafiltration liquid chromatography-mass spectrometry (UF-LC-MS) method. |
While the UF-LC-MSn technique has been used in discovering bioactives from medicinal herbs, its application in food bioactives has not been widely adopted. This paper is therefore intended to review the most recent studies reported for the utilization of UF-LC-MSn for high-throughput screening and identification of food and herb originated enzyme inhibitors, and to discuss its possibility in functional foods and nutraceuticals, and health research.
| 2. Ultrafiltration LC-MS screening of enzyme inhibitors | ▴Top |
2.1. Screening for 5-LOX, MMP-2 and COX-2 inhibitors
5-LOX, MMP-2 and COX-2 are three key enzymes that are closely related to immunity and inflammation, and are involved in several pathological processes, such as cancer, fever, wound healing, rheumatoid arthritis and inflammatory reactions (Brown, 1997; Ding et al., 2011; Ra and Parks, 2007; Schneider and Bucar, 2005; Steinhilber, 1994; Yokoyama and Tanabe, 1989). Finding selective inhibitors of MMP-2, 5-LOX or COX-2 from herb extracts has become increasingly a hot research topic in recent years. UF-LC-MSn has been proven useful for high-throughput screening and identification of MMP-2, 5-LOX or COX-2 inhibitors from some plant extracts (Li et al., 2014; Li et al., 2015; Zhao et al., 2016).
UF-LC-MSn was successfully used to screen and identify a high-affinity inhibitor of 5-LOX from four Chinese medicinal herbs/foods, Carthamus tinctorius L. Smilax glabra Roxb., Saposhnikovia divaricata (Turcz.) Schischk and Pueraria lobata (Zhao et al., 2016). Only compounds with certain binding ability to 5-LOX were detected by LC-DAD-MSn after affinity based binding and ultrafiltration purification, and the selected results are shown in Table 1 and Figure 2. Resveratrol had the highest binding ability to 5-LOX, followed by astilbin, daidzin, sec-O-glucosylhamaudol, 4′-O-β-D-glucosyl-5-O-methylvisamminol, anhydrosafflor yellow B. This corresponded exactly to the results found in vitro enzyme assays (Table 1). Results from the ultrafiltration LC-DAD-MS assay proved that the relative quantity of the compounds in an extract mixture may not automatically guarantee the same in bioactivity, and minor components such as anhydrosafflor yellow B, sec-O-glucosylhamaudol, resveratrol and daidzin in extracts may play more important role than major components in bioactivity, in this case 5-LOX inhibition. This can be exemplified in the study of kudzu, a widely accepted functional food in China. The results also suggest a greater number of binding can be achieved by increasing receptor concentrations, and revealed that enzyme inhibition can also be a competitive process among compounds coexisted in herb extracts (Figure 2). HPLC-UV chromatogram of the extract shows that not all detected peaks bound to the enzyme. The largest peak (peak 1, puerarin) detected by DAD did not have any affinity. Binding capacity or affinity also depended on the concentration of enzyme (Figure 2).
 Click to view | Table 1. Major MMP-2/5-LOX inhibitors identified in plant extracts by UF-LC-MS |
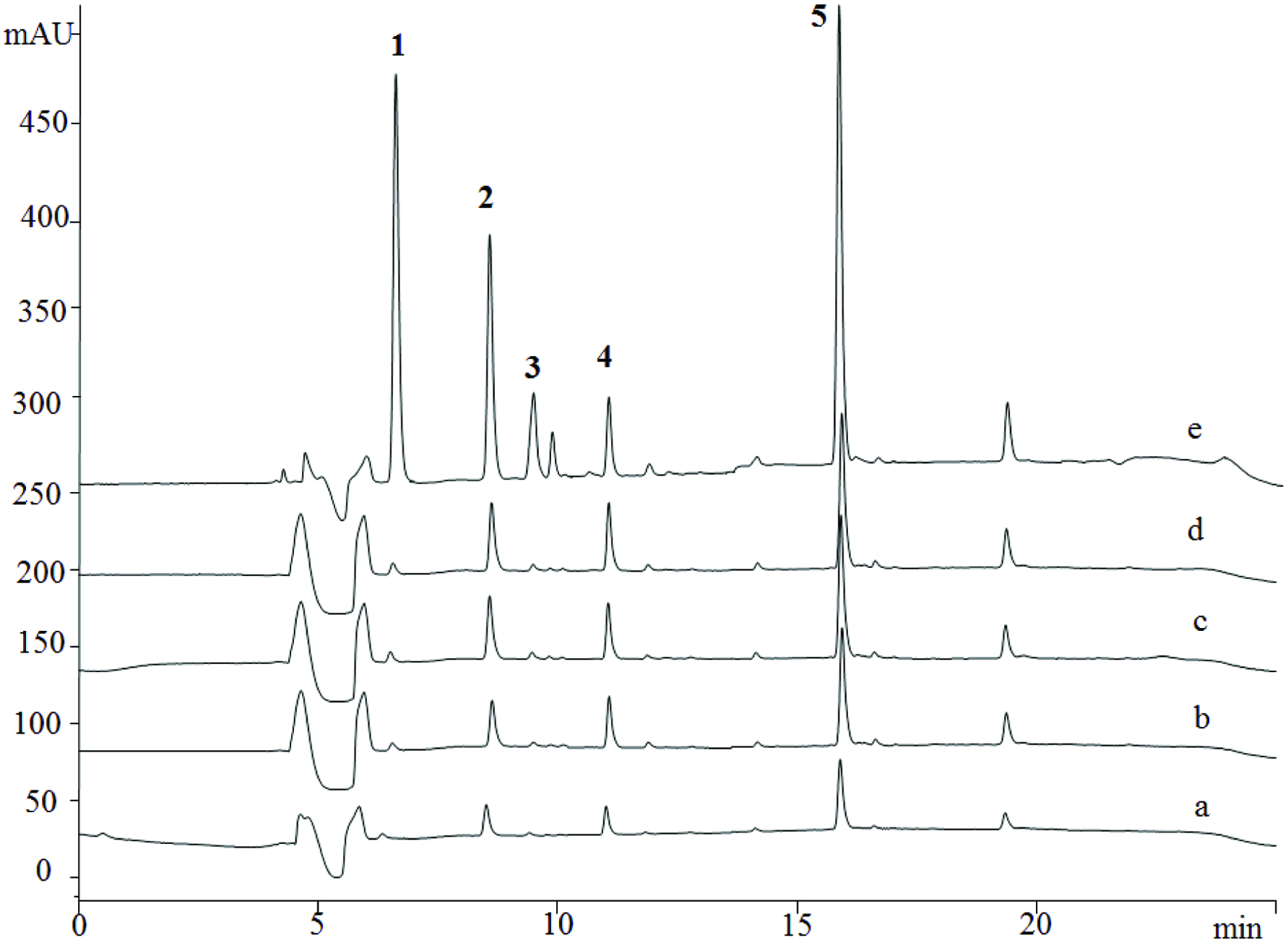 Click for large image | Figure 2. Ultrafiltration LC-DAD chromatograms of potential 5-LOX inhibitors in kudzu (Pueraria lobate) as compared to that of the crude extract without affinity purification. 5-LOX concentration: a, 0 ng/µL; b, 0.1 ng/µL; c, 0.2 ng/µL; d, 0.3 ng/µL; e, crude extract of P. lobata. Peaks 1, Puerarin; 2, Daidzin; 3, 3′-Methoxy-puerarin; 4, 3′-Hydroxy-puerarin; 5, Daidzein. Figure adopted from Zhao et al., 2016 with permission. |
Similar ultrafiltration LC-MS screening has been conducted for MMP-2 inhibitors from C. tinctorius, S. glabra, Smilax china L. and S. divaricate (Li et al., 2014; Li et al., 2015). S. glabra and S. china are widely consumed by Chinese as functional/medicinal foods for improving immunity and general health. Among the compounds identified, resveratrol showed the strongest binding to MMP-2, followed by engelitin, anhydrosafflor yellow B, astilbin and 4′-O-O-D-glucosyl-5-O-methylvisamminol (Table 1). These studies proved that the relative quantity of the compounds in an extract may not follow the relative strength in bioactivity. It was also found that the same compound may have binding ability to different enzymes. Compounds such as resveratrol and daidzin showed strong inhibition against both 5-LOX and MMP-2, suggesting a wider significance as these are phytochemicals found in many commonly consumed foods, such as grapes, mulberries, peanuts, grapes, red wine, soybean and soy-based foods (Tsao, 2010; Lastra and Villegas, 2005).
Ginger root (Zingiber officinale) has been used for both culinary and medicinal purposes, and UF-LC-MSn studies have shown that gingerol-related compounds from the roots of Z. officinale were inhibitors of COX-2 (van Breemen et al., 2011). Among them, 10-gingerol, 8-shogaol and 10-shogaol showed specific binding to the active site of COX-2 with IC50’s of 32, 17.5 and 7.5 µM, respectively (Table 2). UF-LC-MSn has also been used to identify COX-2 inhibitors from traditional Chinese herbs; some of the COX-2 inhibitors identified such as phenyl ferulate are also known for common foods (Cao et al., 2010).
 Click to view | Table 2. Major COX-2 inhibitors identified in plant extracts by UF-LC- MS |
2.2. Screening for α-glucosidase inhibitors
α-Glucosidase is an enzyme present in the brush border of the small intestine, whose inhibitors act by a reversible inhibition, could delay the absorption of carbohydrates from the small intestine, and suppress the postprandial blood glucose and insulin levels (Laar et al., 2005). Recent studies in the discovery of food and other natural sources of α-glucosidase inhibitors have attracted considerable interest as α-glucosidase is an important biomarker for treating type 2 diabetes mellitus (Li et al., 2009; Liu et al., 2013; Ramdath et al., 2014; Tang et al., 2016; Wang et al., 2014; Zhou et al., 2012; Zhang et al., 2015).
The fruit of hawthorn (Crataegus oxyacantha L.) has been part of foods or confectionaries in different cultures, however hawthorn tree leaves also contained flavonoids that were α-glucosidase inhibitors in a UF-LC-MSn screening study (Li et al., 2009). Similar results were found in other studies. Polyphenols including flavonoids and phenolic acids of Siberian ginseng Acanthopanax senticosus Harms, Ginkgo biloba leaves and other medicinal foods, especially rutin, hyperin, isoquercitrin, quercitrin, 1,5-dicaffeoylquinic acid, 1,4-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid were found to be strong inhibitors of α-glucosidase due to their higher affinity (Zhou et al., 2012) (Table 3). ( Liu et al., 2013; Wang et al., 2014) (Table 3). These polyphenols are commonly found in fruits, vegetables, cereals and pulse grains, thus consumption of these foods are beneficial to blood glucose management Ramdath et al., 2014; Tang et al., 2016; Zhou et al., 2012; Zhang et al., 2015). UF-LC–MSn was used to rapidly and selectively screen and identify major α-glucosidase ligands or inhibitors in Radix Astragali and polyphenols particularly calycosin, formononetin and other isoflavones were found to be strong α-glucosidase inhibitors. The activity of eight isoflavone aglycones was evaluated and confirmed using in vitro enzymatic assay (Zhao et al., 2015).
 Click to view | Table 3. α-Glucosidase inhibitors identified by UF-LC-MS |
2.3. Screening for xanthine oxidase inhibitors
Xanthine oxidase (XOD) is an important enzyme involved in chronic gout, cardiovascular disease and many other diseases related to hyperuricemia (Dawson et al., 2007; Lin et al., 2000). In purine metabolism, xanthine and hypoxanthine are oxidized to uric acid by XOD, which is highly expressed in the liver and intestine (Borges et al., 2002). UF-LC-MS has been used for the discovery of XOD inhibitors (Table 4) (Liu et al., 2012a; Liu et al., 2013; Wang et al., 2014).
 Click to view | Table 4. XOD inhibitors identified by UF-LC-MS |
An ultra high performance liquid chromatography and triple quadrupole mass spectrometry (UHPLC-TQ-MS) method was established to simultaneously screen and identify inhibitors of both superoxide anion scavengers and XOD in a single analysis by adding WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt) to the enzymatic reaction (Liu et al., 2012a). This method not only distinguishes the specific inhibitory effects of some of the commonly found polyphenols in foods, but more interestingly, because of the introduction of LC-MS which can directly measure substrates and/or products of the enzyme reaction, it can also effectively eliminate the false positive and negative results. Some food flavonoids such as quercetin and apigenin were found to be strong inhibitors of both superoxide anion radical and XOD, and others such as catechin was a strong radical scavenger but lacked XOD inhibitory activity, or in the case of the isoflavone genistein, a strong XOD inhibitor but not a radical scavenger. Although ultrafiltration was not part of this unique method, it proved the usefulness of LC-MS in kinetic studies of enzyme inhibition by food bioactives. UF-LC-MSn has been used to screen and identify XOD inhibitors including the unique flavonoids from medicinal foods Selaginella tamariscina (Beauv) Spring and Radix Salviae Miltiorrhizae extracts (Liu et al., 2013, Wang et al., 2014). Structural features of these bioactives were important for the affinity, and inhibited XOD activity significantly (Liu et al., 2013). Application of UF-LC-MSn in screening food bioactives needs to be further explored considering the importance of the two marker enzymes in inflammation and chronic diseases.
2.4. Screening for neuraminidase inhibitors
Neuraminidase (NA) is an enzyme present in the surface of the influenza virus. This enzyme is recognized as an attractive target for developing agents against influenza infection and its inhibitors have been widely used in the treatment (Meanwell and Krystal, 1996; von Itzstein et al., 1993; Palese et al., 1974). Although synthetic NA inhibitors exemplified by oseltamivir and zanamivir have been designed to halt the spread of the virus in the body, adverse side effects such as nausea, vomiting, diarrhea, abdominal pain have been observed (Kitching et al., 2009). Hence, naturally occurring NA inhibitors have been intensely studied as safer alternatives. Liu et al. (2011) reported an UF-LC-MSn method for screening NA inhibitors from Radix scutellaria extract. As a result, some unique flavonoids have been identified NA inhibitors (Table 5). However, not all flavonoids are NA inhibitors. Using the same screening method, Liu et al. (2012b) found no compounds in the n-butanol extract of Indigowoad Root Granule had NA inhibitory activity.
 Click to view | Table 5. Screening the NA inhibitors from R. scutellaria extract by ultrafiltration LC-MS |
2.5. Screening for certain QR-2 inhibitors
Quinone reductase-2 (QR-2) is a cytosolic enzyme that is a target for antimalarial activity and antitumor activities due to several possible mechanisms of action. QR-2 inhibitors act to antimalarial activity and antitumor activities or can function as chemoprevention agents by prevent the metabolic activation of toxic quinones (Table 6). A well known polyphenol resveratrol has been shown to be a good QR-2 inhibitor and recognized as a cancer chemopreventive agent (Knox et al., 2000). Discovery of natural QR-2 inhibitors like resveratrol has instigated further studies in this direction using effective high-throughput screening methods. Choi et al. (2011) developed an UF-LC-MS method to screen ligands of QR-2 from a hop (Humulus lupulus L.), and identified two prenylated chalcones (open chain flavonoids) xanthohumol and xanthohumol D as strong QR-2 inhibitors. Hops are widely used in the brewery of beers, and xanthohumols are known as the bittering agents. Other research has also led to the discovery of novel prenylated bichalcone and chalcone compounds from hops as -2 inhibitors (Yu et al., 2014).
 Click to view | Table 6. Screening the QR-2 inhibitors from extracts of marine sediment bacterial cultures by ultrafiltration LC-MS |
2.6. Screening for ACE and AChE inhibitors
Some natural food colourants and food and beverage formulations with different food matrices, including infant formula, soy paste, ketchup, mayonnaise, wheat flour, orange juice, supplement drink, tea and coffee were found to contain strong angiotensin-converting enzyme (ACE) inhibitory activities, using high-throughput LC/SID-MS/MS. ACE is a target enzyme that regulates blood pressure, and inhibitors of ACE therefore have therapeutic value in the prevention of hypertension (Inoue et al., 2011).
UF-LC-MS screening has led to the discovery of several cinnamic acids and flavonoids as acetylcholinesterase (AChE) inhibitors that might be potentially used for the treatment and prevention of Alzheimer’s disease (Zhang et al., 2018). Most recently, the same researchers also used this approach to screen AChE inhibitors from stem-leaf and found saponins, particularly the ginsenosides F1 , Rd, Rk3 , 20(S)-Rg3 , F2 and Rb2 that are also main bioactives of ginseng root, were strong AChE inhibitor (Yang et al., 2019). Similarly were some alkaloids (Zhao et al., 2016).
2.7. Screening for other inhibitors
Inhibitors of other enzymes such as aromatase have also been studied using UF-LC-MSn. Aromatase is the enzyme that catalyzes a key aromatization step in the synthesis of estrogen from androgens which stimulates growth of breast and ovarian cancers, thus naturally occurring aromatase inhibitors of food origin can be used for chemoprevention or even the treatment of breast cancer in women of high risk (Bulun et al., 2005; Santen, 2003; Brueggemeier, 2006; Brueggemeier et al., 2005; Evans et al., 1986). Studies on use of UF-LC-MS-MS toward aromatase screening are relatively scarce, but it has been used for screening aromatase inhibitors from a well known medicinal herb Corydalis yanhusuo (Shi et al., 2010), which successfully identified several quaternary protoberberine alkaloids including berberine, which is a common bioactive found in barberry and Oregon grape and other edible wild fruits. Considering the prevalence of the breast cancer, further studies using UF-LC-MSn for identifying aromatase and other important enzyme inhibitors in food plants should be carried out. UF-LC-MSn has also been used to screen for synthetic antimicrobial agents that inhibit shikimate kinase (Mulabagal and Calderón, 2010). Yang et al. found that cinnamic acids such as chlorogenic acid and other caffeic acid derivatives, and flavonol glycosides such as rutin, isoquercitrin and other quercetin and kaempferol glycosides, were inhibitors of tyrosinase, a key enzyme involved in browning of fruits and vegetables, and in melanin synthesis. Inhibitors of tyrosinase are widely used in the agri-food industry and in the cosmeceutical industry for treating hyperpigmentation in human skin (Yang et al., 2012).
| 3. Pros and cons of ultrafiltration LC-MSn as a method of screening enzyme inhibitors | ▴Top |
Ultrafiltration LC-MSn as a screening method has been proven to be efficient, and can be used to find specific enzyme inhibitors from a mixture of natural compounds. While enzyme inhibition is a complexed process, and many factors, including pH, temperature, substrate concentration and the amount of enzyme, can all affect the outcome. There are different types of enzyme inhibition, but most food bioactives are reversible inhibitors. Natural inhibitors such as flavonoids and phenolic acids bind with target enzymes by non-covalent bonding, i.e. via hydrogen bonds, hydrophobic interactions, and ionic bonds. For this reason, binding of food bioactives in a mixture to a specific enzyme can be complicated. Different compounds in a extract or a particular food are likely to be competitive inhibitors of an enzyme. These potential inhibitors compete with each other to bind to the enzyme, and may have similar affinity for the active site. Increasing concentration of these compounds or enzyme can normally separate the strong inhibitors from weaker ones.
Binding of a compound to a target enzyme may not necessarily suggest inhibition of the enzyme activity, as study on the binding to a specific site of the enzyme, i.e. it may be caused by simple adsorption of a compound to the enzyme. Such false positive identification can be easily avoided by using denatured enzyme following the same incubation protocol. Merchanism of binding of a bioactive compound to a specific target protein/enzyme can also be assessed using molecular docking approach (Zhang et al., 2017).
| 4. Conclusion | ▴Top |
This mini-review confirms the increasing role of UF-LC-MS in the searching for enzyme inhibitor from different herb extracts. In summary, we found that only certain components of the herb extracts actively and competitively bound to the enzyme using UF-LC-MS, suggesting these phytochemicals can play a potential role in preventing and maintaining good health. Moreover, several 5-LOX or MMP-2 inhibitors such as resveratrol, daidzin, and α-glucosidase inhibitors, i.e. rutin, hyperin, isoquercitrin, quercitrin are also major components of foods. Z. officinale and C. oxyacantha screened for COX-2 and α-glucosidase inhibitors are consumed as foods all around the world. The usefulness of the UF-LC-MSn assay therefore can be a valid and effective screening and identification method for many phytochemicals rich fruits, vegetables, grains and soybean, and for a wider range of key enzymatic biomarkers of different chronic diseases.
Acknowledgments
This project was supported by A-base funding of Agriculture & Agro-Food Canada (Project # J-001322.001.04; J-002252.001.04), and by the National Natural Science Foundation of China (No. 81373899), the Natural Science Foundation of Jilin Province of China (No. 20180101153JC).
| References | ▴Top |