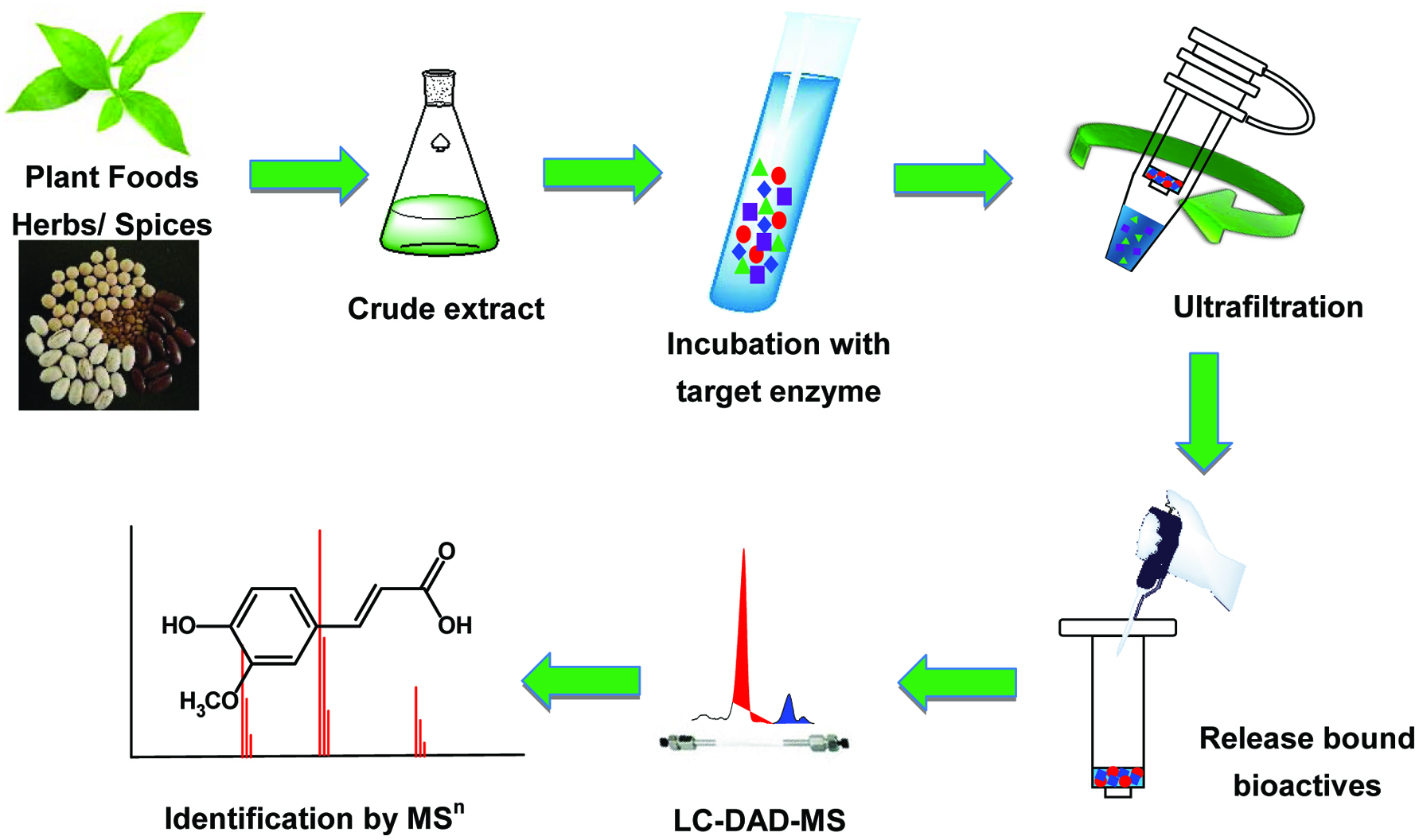
Figure 1. Typical procedures of the ultrafiltration liquid chromatography-mass spectrometry (UF-LC-MS) method.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 7, September 2019, pages 27-35
UF-LC-DAD-MSn for discovering enzyme inhibitors for nutraceuticals and functional foods
Figures

Tables
| Compounds | MWa | Relative binding to enzymeb | IC50d (µM) | Source | Referencese | ||
|---|---|---|---|---|---|---|---|
| MMP-2c | 5-LOXc | MMP-2 | 5-LOX | ||||
| aMW: molecular weight; bRelative binding to enzyme = (amount of compound specifically bound)/(total amount of compound in incubation) × 100%; cConcentration of MMP-2 and 5-LOX were 20 and 0.1ng/μL, respectively. dIC50: Concentration required for 50% inhibition of the enzyme activity under the assay conditions. eRefer to main text. UF-LC-MS: Ultrafiltration liquid chromatography-mass spectrometry. | |||||||
| Hydroxyl safflower yellow A | 612 | 2.01 | 5.76 | 137.3 | 3.72 | C. tinctorius | Li et al. 2014; Zhao et al. 2016 |
| Anhydrosafflor yellow B | 1,062 | 16.29 | 22.93 | 2.82 | 3.45 | ||
| Astilbin | 450 | 14.44 | 40.78 | 107.8 | 8.45 | S. glabra | Li et al. 2015; Zhao et al. 2016 |
| Isoastilbin | 450 | - | 4.30 | - | 46.6 | ||
| Engelitin | 434 | 21.14 | 5.07 | 106.9 | 21.33 | ||
| Isoengelitin | 434 | - | 1.54 | - | 191.31 | ||
| Resveratrol | 228 | 41.59 | 44.26 | 53.1 | 4.38 | ||
| prim-O-Glucosylcimifugin | 468 | 4.40 | 9.22 | 108.87 | 9.76 | S. divaricata | |
| 4′-O-β-D-Glucosyl-5-O- methylvisamminol | 452 | 12.42 | 32.55 | 15.6 | 15.53 | ||
| Cimifugin | 307 | 0.80 | 1.35 | 313.25 | 93.66 | ||
| sec-O-Glucosylhamaudol | 438 | 0.63 | 35.96 | 344.4 | 7.45 | ||
| Puerarin | 416 | - | 25.8 | P. lobata | Zhao et al. 2016 | ||
| Daidzin | 416 | 40.41 | 4.32 | ||||
| 3′-Methoxy-puerarin | 432 | - | - | ||||
| 3′-Hydroxy-puerarin | 446 | 18.28 | 7.36 | ||||
| Daidzein | 254 | 19.68 | 6.65 | ||||
| Compounds | MWa | Relative binding to COX-2b | IC50 (μM)c | Source | Referencesd |
|---|---|---|---|---|---|
| aMW: molecular weight; bRelative binding to COX-2 = (amount of compound specifically bound)/(total amount of compound in incubation) × 100%; cIC50: Concentration required for 50% inhibition of the enzyme activity under the assay conditions; dRefer to main text. UF-LC-MS: Ultrafiltration liquid chromatography-mass spectrometry. | |||||
| 10-Gingerol | 350 | 0.17 | 32.0 | Z. officinale | Van et al. 2011 |
| 8-Shogaol | 304 | 0.27 | 17.5 | ||
| 10-Shogaol | 332 | 0.35 | 7.5 | ||
| Senkyunolide O | 380 | 52 | 5 | L. chuanxiong | Cao et al. 2010 |
| Phenethyl-trans-ferulate | 297 | 25 | 31 | N. incisum | |
| Cryptoanshinone | 296 | 31 | 22 | S. miltiorrhiza | |
| Roburic acid | 440 | 45 | 9 | G. macrophylla | |
| Compounds | MWa | Source | Referencesb |
|---|---|---|---|
| aMW: molecular weight; bRefer to main text. UF-LC-MS: Ultrafiltration liquid chromatography-mass spectrometry. | |||
| Quercetin | 448 | G. biloba; A. senticosus | Liu et al. 2013; Zhou et al. 2012 |
| Apigenin | 270 | G. biloba | Liu et al. 2013 |
| Kaempferol | 286 | ||
| Isorhamnetin | 316 | ||
| Rutin | 610 | A. senticosus | Zhou et al. 2012 |
| Hyperin | 464 | ||
| Isoquercitrin | 464 | ||
| 1,5-Dicaffeoylquinic acid | 516 | ||
| 1,4-Dicaffeoylquinic acid | 516 | ||
| 4,5-Dicaffeoylquinic acid | 516 | ||
| 5,7,3,2′,6′-Pentahydroxy flavanone | 304 | S. baicalensis | Wang et al. 2014 |
| Chrysin-6-C-arabinosyl-8-C-glucoside | 548 | ||
| Chrysin-6-C-glucosyl-8-C-arabinoside | 548 | ||
| 5,7,2′,5′-Tetrahydroxy-8,6′-dimethoxy flavonne | 346 | ||
| Baicalin | 446 | ||
| Oroxylin A-7-O- glucuronide | 460 | ||
| Wogonoside | 460 | ||
| Baicalein | 270 | ||
| Skulllcapflavon II | 374 | ||
| Wogonin | 284 | ||
| Oroxylin A | 284 | ||
| Quercetin-3-O-rha- (1–4)-glcosyk-rhamnoside | 756 | C. oxyacantha | Li et al. 2009 |
| Vitexin-2″-O-glucoside | 594 | ||
| Vitexin-2″-O-rhamnoside | 578 | ||
| Vitexin | 432 | ||
| Compounds | MWa | Source | Referencesb |
|---|---|---|---|
| aMW: molecular weight; bRefer to main text. UF-LC-MS: Ultrafiltration liquid chromatography-mass spectrometry. | |||
| Amentoflavone | 538 | S. tamariscina | Wang et al. 2014 |
| Robustaflavone | 538 | ||
| Tanshinone II B | 310 | S. miltiorrhizae | Liu et al. 2013 |
| Tanshindiol B | 312 | ||
| Tanshindiol A | 312 | ||
| 15,16-Dihydrotanshinone I | 278 | ||
| 1,2-Dihydrotanshinone I | 278 | ||
| Danshenxinkun B | 280 | ||
| Cryptotanshinone | 296 | ||
| Tanshinone I | 276 | ||
| 3-Hydroxy methylenetanshinquinone | 294 | ||
| Mmethylene tanshinquinone | 278 | ||
| Tanshinone II A | 294 | ||
| Compounds | MWa | Relative binding to NAb | IC50 (μM)c | Source | Referencesd |
|---|---|---|---|---|---|
| aMW: molecular weight; bRelative binding to Neuraminidase = (amount of compound specifically bound)/(total amount of compound in incubation) × 100%; cIC50: Concentration required for 50% inhibition of the enzyme activity under the assay conditions; dRefer to main text. UF-LC-MS: Ultrafiltration liquid chromatography-mass spectrometry. | |||||
| Baicalin | 446 | 20.32 | 49.34 | R. scutellaria | Liu et al. 2011 |
| oroxylin A-7-O-gluacid | 460 | 18.05 | |||
| wogonin | 460 | 20.01 | 29.83 | ||
| Baicalein | 270 | 25.16 | 30.52 | ||
| wogonoside | 284 | 26.08 | |||
| oroxylin A | 284 | 28.23 | |||
| Compounds | MWa | Relative binding To QR-2b | IC50 (μM)c | source | Referencesd |
|---|---|---|---|---|---|
| aMW: molecular weight; bRelative binding to Quinone reductase-2 = (amount of compound specifically bound)/(total amount of compound in incubation) × 100%; cIC50: Concentration required for 50% inhibition of the enzyme activity under the assay conditions; dRefer to main text. UF-LC-MS: Ultrafiltration liquid chromatography-mass spectrometry. | |||||
| xanthohumol | 354 | 196 | H.lupulus | Choi et al. 2011 | |
| xanthohumol D | 370 | 110 | |||
| tetrangulol methyl ether | 318 | 0.16 | Actinomycessp | ||