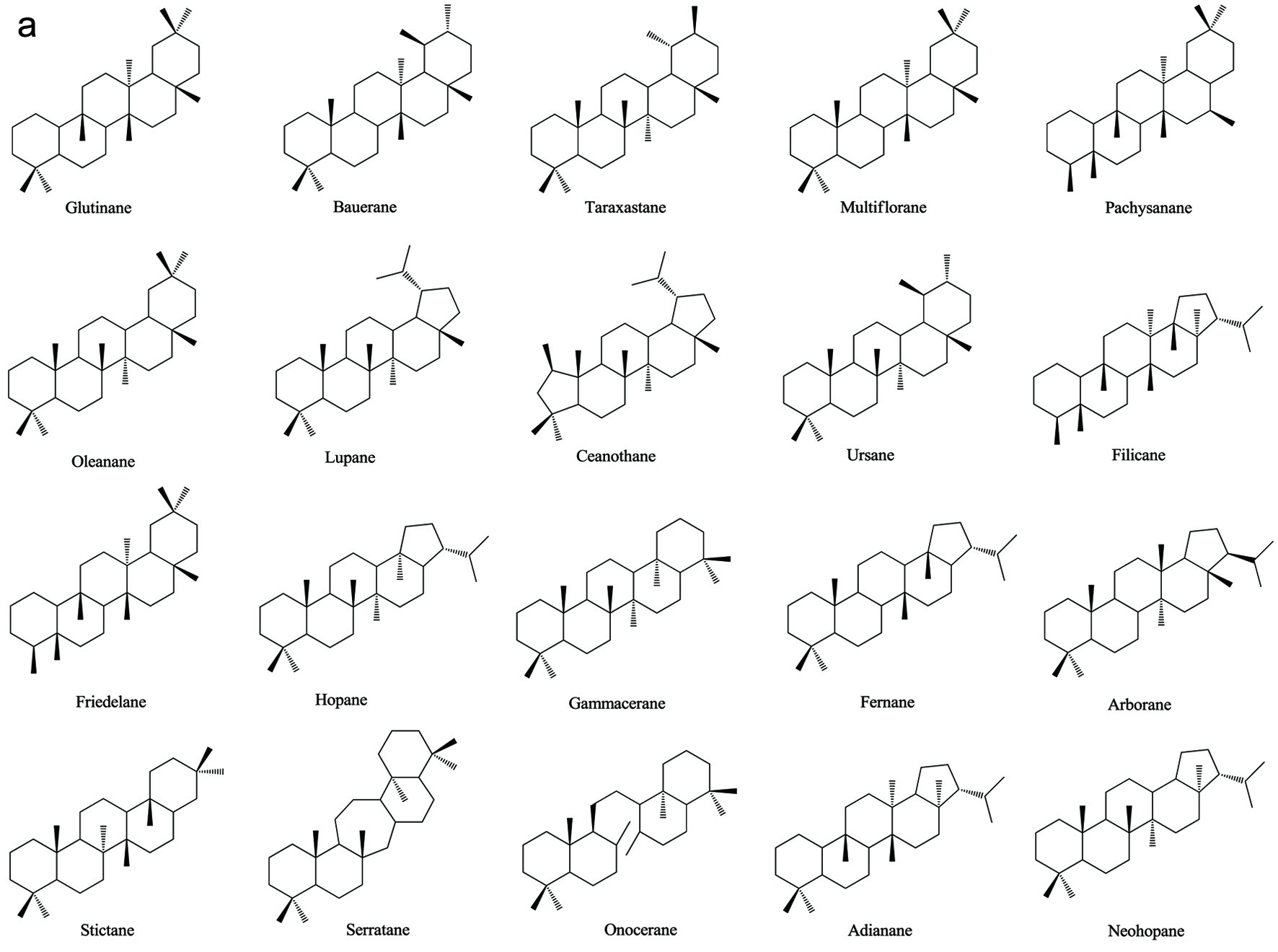
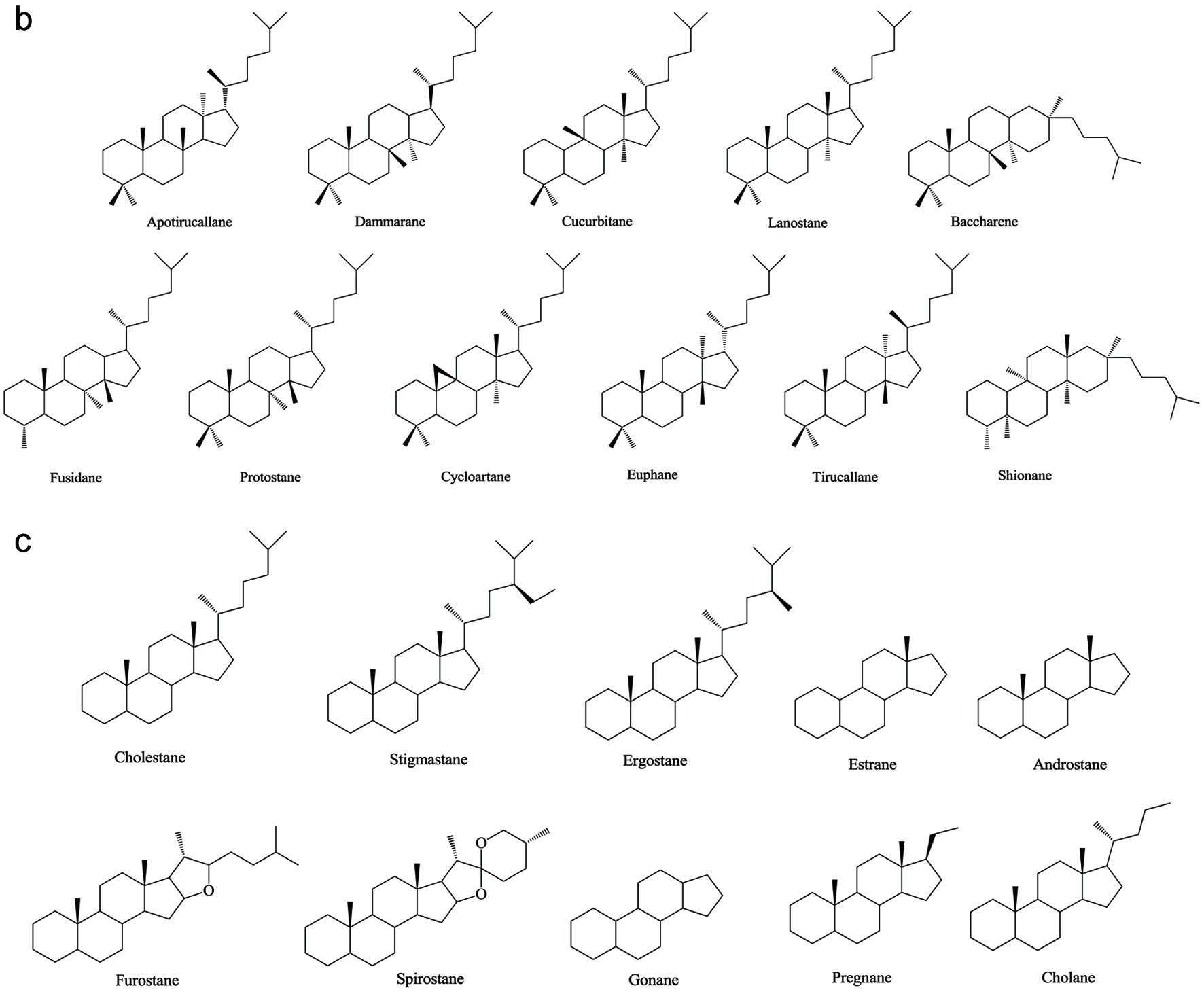
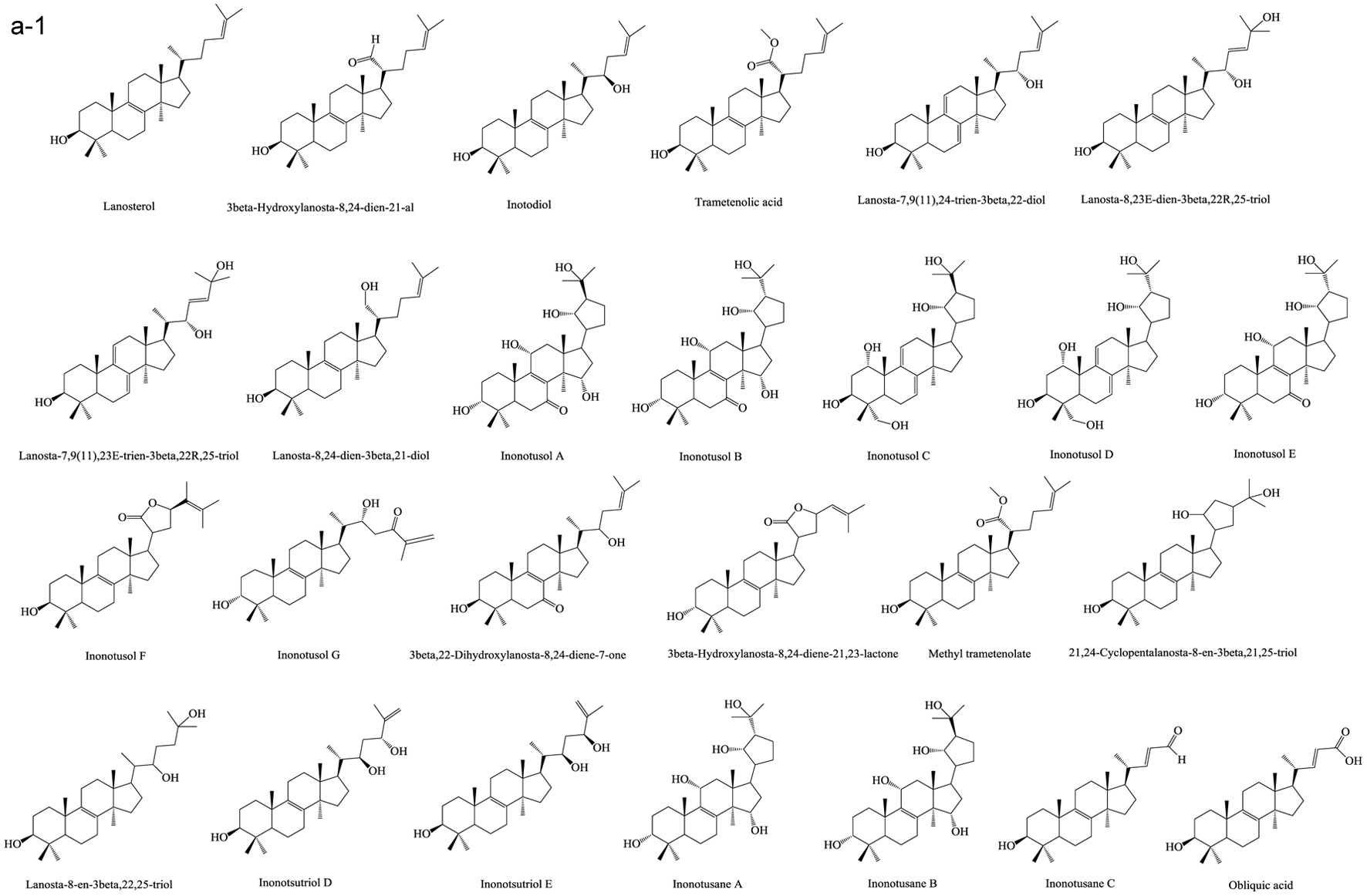
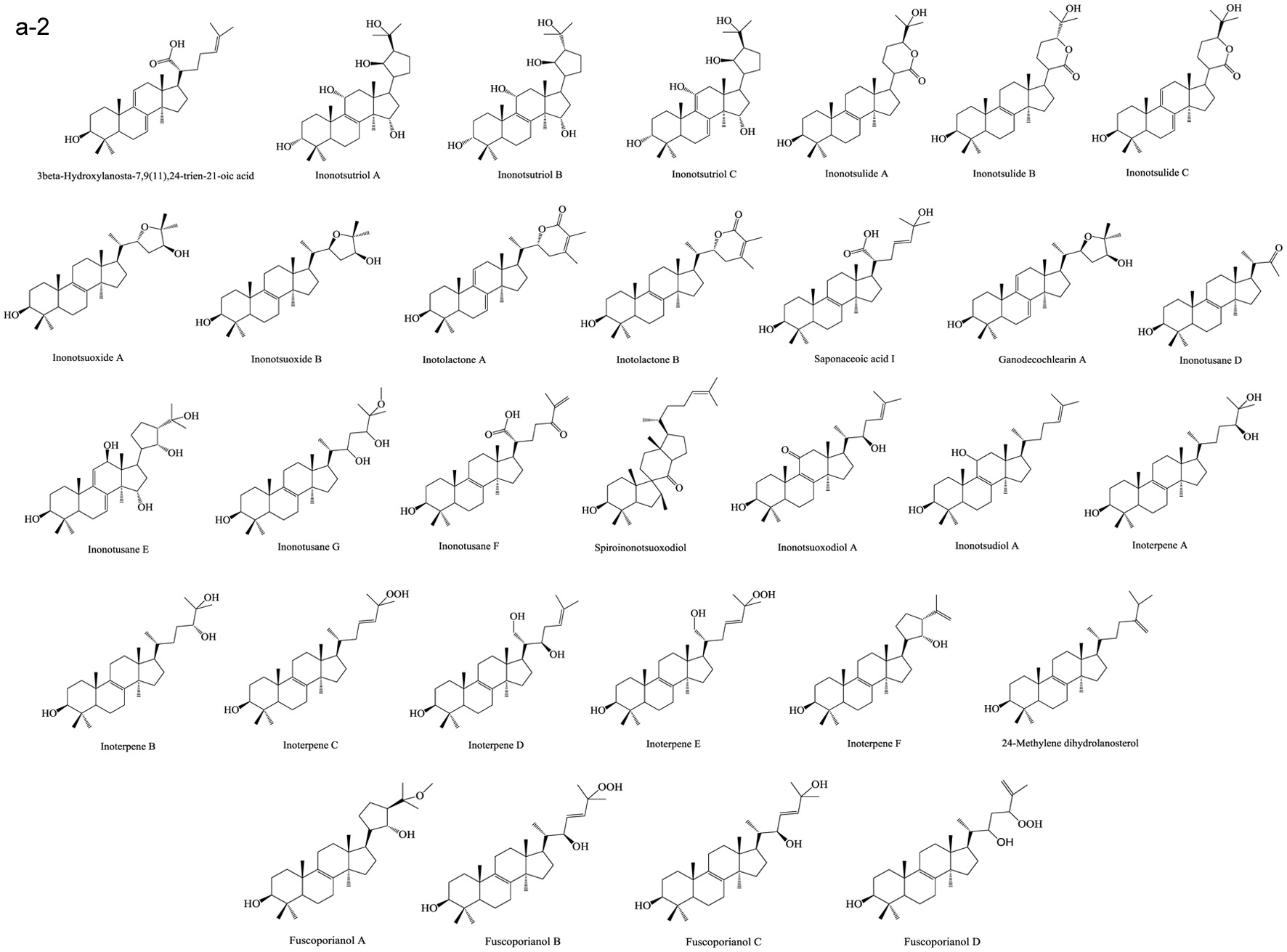
Figure 1. Various skeleton cores of pentacyclic, tetracyclic triterpenoids, and steroids. (a) Types of pentacyclic triterpenoid; (b) Types of tetracyclic triterpenoid; (c) Types of steroid.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 12, December 2020, pages 9-75
Bioactive compounds and bioactive properties of chaga (Inonotus obliquus) mushroom: a review
Figures


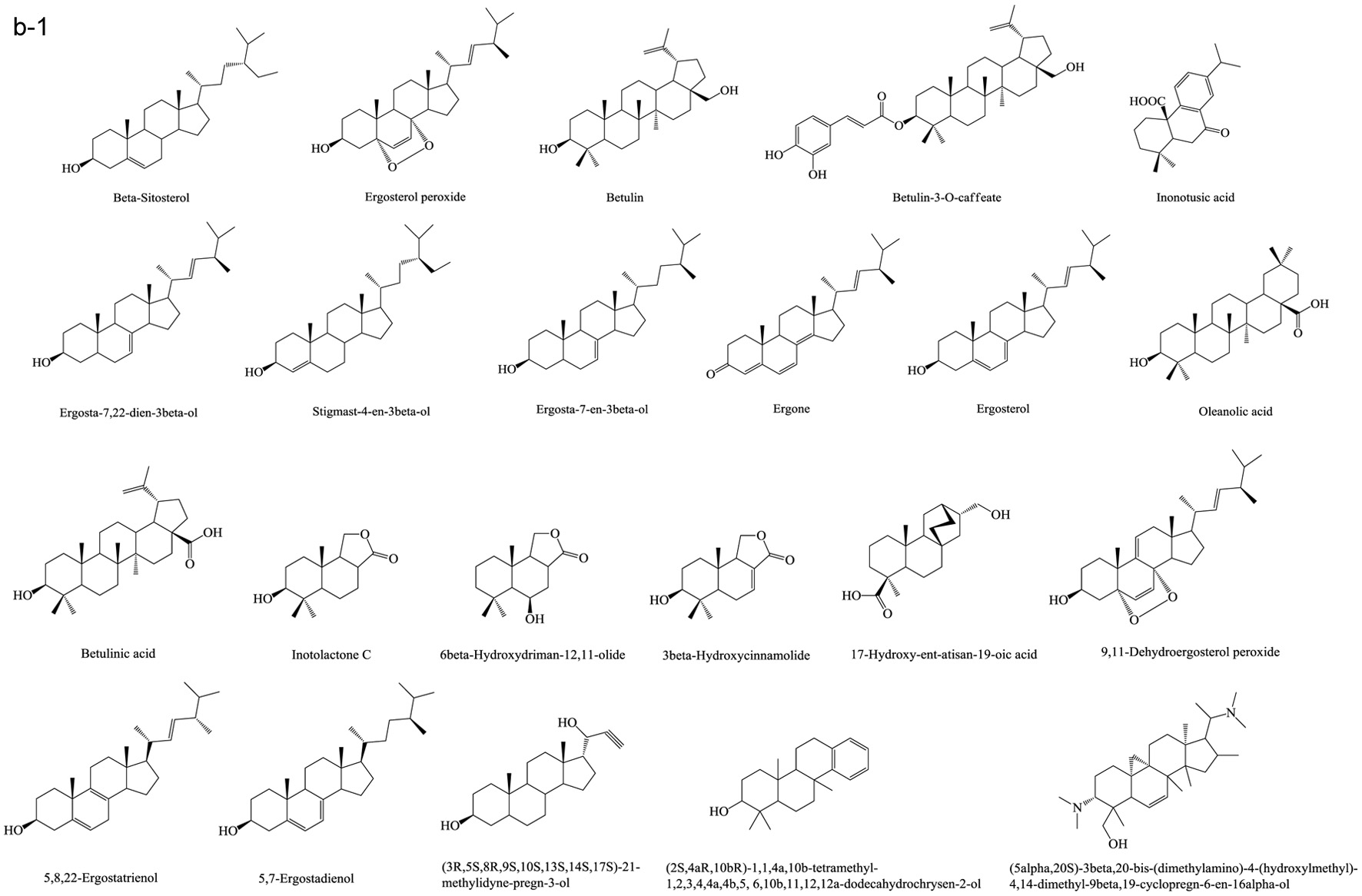
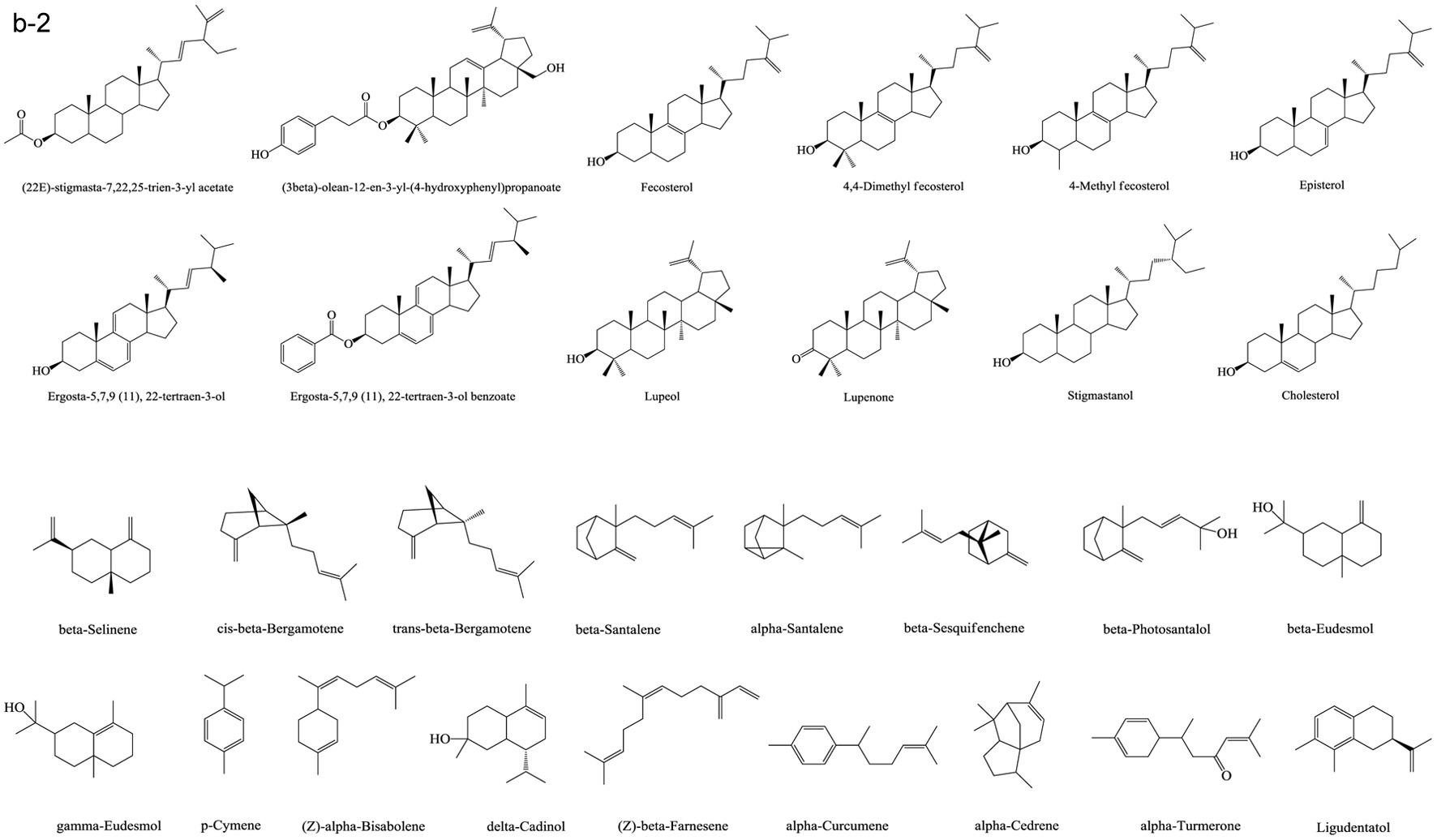
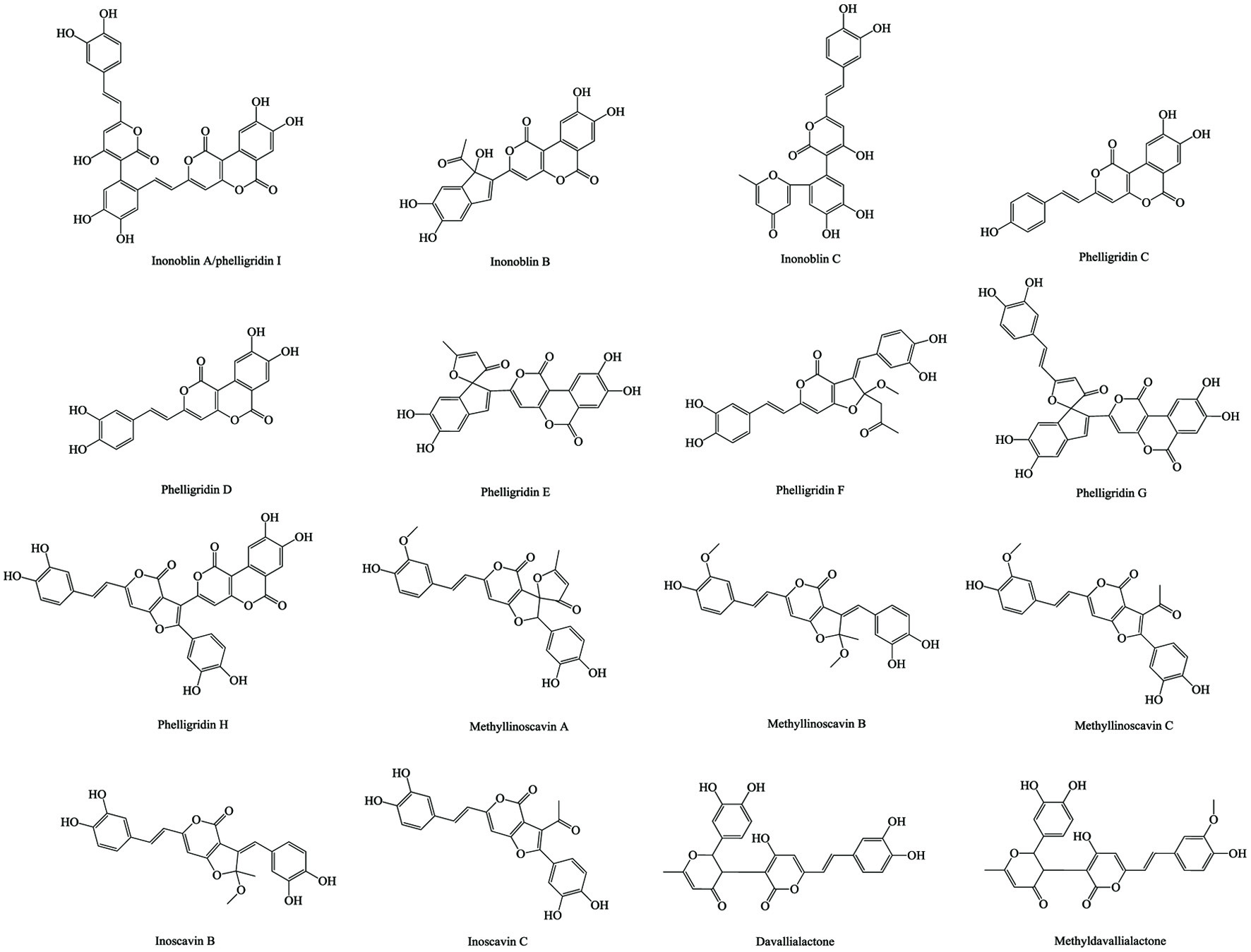
Tables
| Crude extract | Bioactivity | Model | IC50/EC50/LC50 values or experimental dosage (ED) | Specific mechanism or manifestation | Reference |
|---|---|---|---|---|---|
| DSS: dextran sulfate sodium; PPARγ: peroxisome proliferator-activated receptors γ; AP2: adipocyte protein 2; LPL: lipoprotein lipase; CD36: fatty acid translocase; MDCK cell: Madin-Darby Canine Kidney cell; CRFK cell: Crandell-Reese feline kidney cell; FPV: feline panleukopenia virus; FIPV: feline infectious peritonitis virus; FHV-1: feline herpesvirus 1; FCV: feline calicivirus; MMP: matrix metalloproteinase; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; BW: body weight; HFD: high-fat diet; STZ: streptozotocin; MMP: matrix metalloproteinase; MSPKs: mitogen-activated protein kinases; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B; ERK: extracellular signalregulated protein kinase; JNK: c-Jun N-terminal kinase; P38: Cytokinin Specific Binding Protein (CSBP); MAPKs: mitogen-activated protein kinases; NF-κB: nuclear factor κB; COX: cyclooxygenase, STZ: streptozocin; MDA: maleic dialdehyde; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; CAT: catalase; SOD: superoxide dismutase; GPx: glutathione peroxidase; TBARS: thiobarbituric acid-reactive species; PPGE: postprandial plasma glucose excursion; AUC: area under the curve; HBDH: hydroxybutyrate dehydrogenase; LDH: lactate dehydrogenase; MDH: malate dehydrogenase; GGT: gamma-glutamyl transferase; MNNG: N-methyl-N′-nitro-N-nitrosoguanidine; C/EBPα: CCAAT/enhancer-binding protein α); PPARγ: peroxisome proliferator-activated receptors γ; GLUT4: glucose transporter 4; aP2: adipocyte protein 2; LPL: lipoprotein lipase; CD36: fatty acid translocase; STAT: signal transducers and activators of transcription; IFN: interferon: COX: cyclooxygenase; IL: interleukin; Ig: immunoglobulin; ALT: alanine aminotransferase; ACC: acetyl-CoA carboxylase; FAS: fatty acid synthase; AOX: acyl CoA oxidase; CPT1: carnitine palmitoyltransferase 1; PGC1-α: peroxisome proliferator-activated receptor gamma coactivator 1-α; LCAD: long-chain acyl-CoA dehydrogenase; PI 3-K: phosphoinositide 3-kinase; SREBP1-c: sterol-regulatory-element-binding protein 1c. | |||||
| Hexane and dichloromethane fractions of methanol extract | Anti-proliferation activity | Calu-6 lung cancer cell | IC50∼2.30 mg/ml | Baek et al. (2018) | |
| A549 lung cancer cell | IC50∼2.03 mg/ml | ||||
| H1264 lung cancer cell | IC50∼2.03 mg/ml | ||||
| H1299 lung cancer cell | IC50∼2.40 mg/ml | ||||
| Chloroform extract | Anti-proliferation activity | P388 mouse leukemia cell | IC50∼13.9 μM | Nomura et al. (2008) | |
| HeLa human cervical cancer cells | – | ||||
| Ethanol extract | Anti-proliferation activity | NCI-H460 human lung cancer cell line | ED∼50 μg/ml | Sun et al. (2011) | |
| Ethanol extract | Anti-proliferation activity | HT-29 human colon cancer cells | ED∼2.5–10 µg/ml | Arrested cell cycle in G1 phase; decreased expression of CDK2, CDK4, and cyclin D1; increased expression of p21, p27, and p53; inhibited phosphorylation of Rb and E2F1 expression. | Lee et al. (2015a) |
| Ethanol extract | Anti-proliferation activity | DLD-1 human colon cancer cell | ED∼400 μg/ml | Induced nuclear fragmentation | Hu et al. (2009) |
| Methanol extract | Anti-proliferation activity | HL-60 | IC50∼32.2 μg/ml | – | Nguyen et al. (2018) |
| LU-1 | IC50∼38.0 μg/ml | – | |||
| SW480 | IC50∼41.3 μg/ml | – | |||
| HepG2 | IC50∼51.3 μg/ml | – | |||
| KB | IC50∼57.0 μg/ml | – | |||
| LNCaP | IC50∼57.7 μg/ml | – | |||
| 80% Methanol extract | Anti-proliferation activity | A549, PA-1, U937, HL-60 | IC50∼23.2–105.2 μg/ml | – | Nakajima et al. (2009) |
| Methanol extract | Anti-proliferation activity | HT1080 cells | ED∼10–100 μg/ml | – | Ryu et al. (2017) |
| Anti-tumor effect | B16F10 melanoma cell implanted C57BL/6 mice | ED∼30 μM/mouse/day (oral administration) | – | ||
| Ethyl acetate and petroleum ether fractions of 100% ethanol extracts | Anti-proliferation activity | human 29 prostatic cancer cell PC3 and human breast cancer cell MDA-MB-231 | IC50∼19.22 and 46.49 μg/ml | – | Ma et al. (2013) |
| Ethyl ether and water extracts | Anti-proliferation activity | Human cervical cancer HeLa cells | – | Impaired the chromesome in metaphase and lysis; impared the cell membrane; no effects on CAT | Jarosz et al. (1990) |
| 70% ethanol and water extracts | Anti-proliferation activity | MCF-7 human breast cancer cell | IC50∼92.65-239.43 μg/ml | – | Glamočlija et al. (2015) |
| NCI-H460 human non-small cell lung cancer cell | IC50∼80.93–267.27 μg/ml | – | |||
| HeLa human cervical uteri tumor cell | IC50∼217.36–318.19 μg/ml | – | |||
| HepG2 human liver cancer cell | IC50∼94.24–281.12 μg/ml | – | |||
| Cultivation broth | Anti-proliferation activity | Hela cells | – | Inhibited the cell mitosis and increased the catalase activity; induced impairment of chromosome/cellular membrane and cell lysis | Jarosz et al. (1990) |
| unknown-solvent extract | Anti-proliferation activity | SCC-13 human malignant keratinocytes | ED∼10–200 μg/mL | Down-regulated the expression of NF-κB | Song et al. (2004) |
| Water extract | Anti-proliferation activity | A549 lung cancer cell | – | Higher toxicity on cancer-derived cells A549 than on normal transformed cells BEAS-2B | Géry et al. (2018a) |
| Water extract | Anti-proliferation activity | HepG2 human liver cancer cells | ED∼750 μg/ml | Arrested cells in G0/G1 phase; up-regulated the expression of capase-3; down-regulated the expression of cell cycle modulators (p53, pRb, and p27) and G0/G1 regulatory proteins (Cdk2, Cdk4, Cdk6, and Cyclin D1, D2, and E) | Youn et al. (2008) |
| Water extract | Anti-proliferation activity | Hela human cervical uteri tumor cells | – | Decreased the cell protein amount and mitotic index value; decreased the activity of LDH, HBDH, MDH, GGT and increasing the activity of CAT | Rzymowska (1998) |
| Water extract | Anti-proliferation activity | HCT-116 human colorectal cancer cell | ED∼20 mg/ml | Up-regulated Bax, bad, and caspase-3 genes and mRNA expression p53, p21WAF1/CIP1; increased Bax/bcl-2 ratio; increased caspase-3 activity and p53 protein expression and decreased the expression of NF-κB, p65 protein and COX-2 gene; arrested cell at G0/G1 phase ; downregulated CyclinD1 | Tsai et al. (2017) |
| Water extract | Anti-proliferation activity | HT-29 human colon cancer cells | ED∼0–1.0 mg/ml | Arrested the cell cycle; upregulated the level of Bax and caspase-3 proteins and down-regulated Bcl-2 protein | Lee et al. (2009) |
| Silver nanoparticles of water extract | Anti-proliferation activity | A549 human lung cancer cell | ED∼1 mM | – | Nagajyothi et al. (2014) |
| MCF-7 human breast cancer cell | ED∼1 mM | – | |||
| Fermented meterials | Anti-proliferation activity | HepG2 human liver cancer cells | ED∼200 μg/ml | Arrested cell cycle at G0/G1phase | Hou et al. (2018) |
| Water extract | Anti-proliferation activity | Sarcoma 180 cells | ED∼20–100 μg/ml | Arrested the cell cycle at G0-G1 phase | Chen (2007) |
| Water extract | Anti-proliferation and immunomodulatory effect | Sarcoma 180 cell implanted male ICR mouse tumor model | ED∼20–100 mg/kg BW/day (oral administration) | Restored splenic lymphocyte number and proliferation potential; increased the production of TNF-α; inhibited the expression of bcl-2 and bax gene in tumors; reduced the tumor weight | |
| Water extract | Anti-proliferation effects | B16-F10 mouse melanoma cell | ED∼750 μg/ml | Formation of dendrite-like structures; arrested cell cycle in (sub-)G0/G1 phase and activated caspase-3 activity; down-regulated expression of p53, p27, and pRb proteins; decreased the expression of Cdk2 Cdk4, Cyclin D1 and Cyclin E | Youn et al. (2009) |
| Water extract | Anti-tumor effect | B16-F10 cell implanted Balb/c mice | ED∼20 mg/kg/day (intraperitoneal administration) | – | |
| Water extract | Anti-tumor effect | Lewis lung cancer cell-implanted mouse tumor model | ED∼6 mg/kg BW/day (oral administration) | Promoted a decrease of body weight in middle-aged and old mice; slowed tumor progression; decreased tumor vascularization; suppressed lung metastasis; prevented body temperature decrease after tumor implantation | Arata et al. (2016) |
| Water extract | Anti-proliferation ability | HT1080, Hep G2, CT-26 cancer cells and fibroblast CRL-7250 normall cell | ED∼0.2–200 μg/ml | Inhibited the viability of both cancer and normal cells | Song et al. (2007) |
| Anti-tumor effects | CT-26 cell-inoculated BALB/c mice pulmonary metastasis model | ED∼20 or 10 μg/ml (oral and intravenous administration) | Decreased pulmonary metastasis | ||
| Pro-tumor effects | CT-26 cell-inoculated BALB/c mice pulmonary metastasis model | ED∼100 μg/ml (intravenous administration) | Increased pulmonary metastasis | ||
| Immunomodulatory activity | RAW 264.7 cells | ED∼0.2–20 μg/ml | Increased NO production and mRNA expression of iNOS, IL-1β, IL-10; | ||
| Freshly isolated splenocytes | ED∼0.2–20 μg/ml | Stimulated proliferation; up-regulated mRNA expression of IL-2, IL-4, IL-10, IL-12, IFN-γ, TGF-β; increased expression of IL-2, IL-10, TNF-α, IFN-γ; | |||
| NK cells | ED∼0.2–20 μg/ml | Stimulated NK cytotoxic activity | |||
| Cultivation broth | Immunomodulatory activity | Vaccinated chickens | ED∼0.8% of daily diet (oral administration) | Inhibited hemagglutination in negative group; enhanced the neutralizing antibody titers, proliferation of PBMCs, proportions of CD3+, CD3+CD8+, and CD3+CD4+ T lymphocytes, as well as the ratio of Th1/Th2 | Zhang et al. (2018) |
| Water extract | Anti-proliferation/inflammation activities | HCT116 and DLD1 Human colorectal cancer cell | ED∼0.2 and 0.5 mg/ml | Arrested cell cycle in S phase; activated caspase-8, caspase-3, caspase-9, pARP; inhibited the level of NF-κB, c-Myc, β-catenin, and Cox-2 | Mishra et al. (2013) |
| Anti-tumor effect/anti-inflammation effect | APCMin/+ mouse colorectal adenoma model | ED∼100 and 300 mg/kg BW/0.5 day (oral administration) | Reduced the count of large polyps in small/large intestine; surpassed the overexpression of cyclin D1 and c-Myc in intestinal epithelial cells; inhibited the expression of β-catenin and CDK-8, pro-caspase-3 and cleaved PARP; Suppressed iNOS and Cox-2 level | ||
| Anti-cancer effect/anti-inflammation effect | AOM/DSS-induced mouse colon cancer model | ED∼100 and 300 mg/kg BW/0.5 day (oral administration) | Maintained colonic epithelial cell structures and improves histological damage in response to AOMJDSS; suppressed mRNA overexpression of IL-6, IL-1β, TNF-α, IFN-γ | ||
| Ethyl acetate fraction of residue water extract | Pro-inflammatory activity | LPS-induced RAW 264.7 murine macrophage cells | ED∼50–500 μg/ml | Increased (adverse effect) TNF-α and IL-6 production | Van et al. (2009) |
| 80% ethanol extract | Anti-inflammatory activity | LPS-induced RAW 264.7 murine macrophage cells | ED∼50–500 μg/ml | Inhibited NO production; down-regulated IL-6 and TNF-α levels; no effect on IL-1β | |
| 70% Ethanol extract | Anti-inflammatory activity | LPS-induced RAW 264.7 murine macrophage cells | ED∼100 μg/ml | Inhibited NO production and iNOS and COX-2 expression; inhibited the phosphorylation of IκB-α, Akt, and MAPKs (JNK, p38, ERK) | Kim et al. (2007) |
| 70% Ethanol extract | Anti-inflammatory effect | DSS-induced BALB/c mice colitis model | ED∼50 mg/kg BW/day (oral administration) | Decreased TNF-α, COX-2, IL-4, IFN-γ, STAT1, and STAT6; lowered the levels of IgE and IgA in the spleen and mesenteric lymph node; suppressed the DSS-induced colonic tissue destruction | Debnath et al. (2012) |
| 50% Ethanol and water extract | Anti-inflammatory activity | LPS-induced RAW 264.7 murine macrophage cells | ED∼250 μg/ml | Inhibited TNF-α production | Javed et al. (2019) |
| Histamine-induced RAW 264.7 murine macrophage cells | ED∼250 μg/ml | Inhibited TNF-α production | |||
| Histamine-induced microvascular inflammation in male C57BL6 mice | ED∼12.5 μg/ml | Reversed the histamine-induced reduction of conducted vasodilation | |||
| Ethyl acetate and petroleum ether fraction of ethanol extracts | Anti-inflammatory activity | LPS-induced RAW 264.7 macrophage cells | ED∼40 μg/ml | Inhibited NO production | Ma et al. (2013) |
| NF-κB reporter gene-stably transfected RAW264.7 cells, | ED∼40 μg/ml | Inhibited activation of NF-κB luciferase | |||
| Methanol extract | Anti-inflammatory activity | LPS-induced RAW 264.7 murine macrophage cell | ED∼45–135 μg/ml | Suppressed NO and PEG2 production; inhibited protein and mRNA expression of LPS-induced TNF-α, iNOS, COX-2, NF-κB (p65/p50); inhibited the degradation of cytosol IκB-α; reducing the level of nuclear p65 | Park et al. (2005b) |
| Anti-inflammatory effect | Carrageenin-induced paw edema in male Sprague-Dawley rats | ED∼100/200 mg/kg (oral administration) | – | ||
| Water extract | Anti-inflammatory activity | LPS-induced RAW 264.7 macrophage cells | – | Inhibited the production of TNF-α, STAT1, pSTAT1, STAT6, and pSTAT6 | Choi et al. (2010) |
| Anti-inflammatory effect | DSS-induced male BALB/c mouse acute colitis model | ED∼100/200 mg/kg (oral administration) | Maintained the liver weight; decreased the serum IgE level; decreased the expressions of TNF-α, IFN-γ, IL-4, STAT6, and STAT1 proteins in the spleen; | ||
| Water extract | Anti-inflammatory | DSS-induced female C57BL/6 mouse acute colitis model | ED∼50 and 100 mg/kg BW/12 h | Suppressed edema, mucosal damage, and the loss ofcrypts induced by DSS; inhibited iNOS levels and myeloperoxidase accumulation in colon tissues; suppressed mRNA overexpression of TNF-α, IFN-γ, IL-1β, and IL-6 | Mishra et al. (2012) |
| Ethyl acetate, butanol, water fractions of 60% ethanol extract | Antioxidant activity | DPPH, superoxide and hydroxyl radical scavenging assays | EC50∼31.42–336.42 μg/ml | – | Liang et al. (2009) |
| Water and 70% ethanol extracts | Antioxidant activity | DPPH, FRAP, TBARS and β-carotene bleaching assays | EC50∼0.07–9.22 mg/ml | – | Glamočlija et al. (2015) |
| Water and 80% ethanol extract | Antioxidant activity | DPPH, APPH and superoxide scavenging assays | ED∼5 μg/ml | – | Cui et al. (2005) |
| Ethyl acetate fraction of water extract | Antioxidative stress activity | H2O2-treated human HaCaT keratinocytes | ED∼50 μg/ml | – | |
| Water extract | Antioxidative stress activity | Female SKH-1 hairless mice UV irradiation model | ED∼1.0% (external use) | Suppressed UV-induced morphologic skin changes (thickening and wrinkle) | Yun et al. (2011) |
| H2O2-treated human dermal fibroblasts | ED∼1–50 µg/ml | Scavenged intracellular ROS and prevented lipid peroxidation; increased collagen synthesis through inhibition of MMP-1 and MMP-9 activities | |||
| 95% Ethanol extracts | Antioxidative stress activity | BJ normal human skin fibroblast | ED∼1 mg/mL | Increased SOD1, CAT and KI67 mRNA expression and decreased ROS production | Szychowski et al. (2018) |
| Anti-proliferation effect/prooxidative stress activity | Caco-2 human colon cancer cell | ED∼1 mg/mL | Decreased SOD1, CAT and KI67 mRNA expression and increased ROS production | ||
| Water extract | Antioxidant activity | H2O2-treated lymphocyte from gastroenterology patients and healthy volunteers | ED∼50–500 μg/ml | Alleviated oxidative DNA damage | Najafzadeh et al. (2007) |
| Ethanol extract | Antioxidant activity | H2O2-treated lymphocytes from healthy volunteers | ED∼6.25–100 μg/ml | Alleviated oxidative DNA damage | Park et al. (2005a) |
| Water extract | Antioxidant activity | H2O2-treated human lymphocytes | ED∼10–500 μg/ml | Alleviated oxidative DNA damage | Park et al. (2004) |
| Subfractions of Methanol extract | Antimutagenic activity | MNNG and 4NQO induced Salmonella typhimurium strains TA98 and TA100; Trp-P-1 and B(α)P induced Salmonella typhimurium strains TA98 and TA100 in presence with the S-9 rat enzyme system | ED∼50 g/plate | – | Ham et al. (2009) |
| Ethyl acetate extract | Antimutagenic effect | N-methyl-N′-nitro-N-nitrosoguanidine induced mice | ED∼0–1.6 mg/mice/day | – | Ham et al. (2003) |
| Methanol extract | Analgesic activity | Hot plate test in mice | ED∼100 and 200 mg/kg BW (oral administration) | – | Park et al. (2005b) |
| Acetic acid-induced abdominal constriction test in mice | ED∼100 and 200 mg/kg BW (oral administration) | – | |||
| Water and aqueous water extract | Anti-virus | HIV-infected MT-4 lymphoblastoid cells | ED∼5.0 μg/ml | – | Shibnev et al. (2015) |
| Water extract | Anti-virus | Hepatitis C virus-infected porcine embryo kidney cells | – | Inhibited infective properties of virus more than 100-fold and the production of infective virus | Shibnev et al. (2011) |
| Water extract | Anti-virus | HIV-infected MT-4 amd CD4 cell, | ED∼0.01–1,000 µg/ml | Inhibited HIV infection and HIV-induced cell damage | Sakuma (2004) |
| HIV-infected and PHA-stimulated peripheral blood mononuclear cells | ED∼0.01–1,000 µg/ml | ||||
| 70% Ethanol and water extracts | Antibacterial activity | Staphylococcus aureus (ATCC 6538), Bacillus cereus (clinical isolate), Micrococcus flavus (ATCC 10240), Listeria monocytogenes (NCTC 7973), Pseudomonas aeruginosa (ATCC 27853), Salmonella typhimurium (ATCC 13311), Escherichia coli (ATCC 35210), Enterobacter cloacae (human isolate) | – | – | Glamočlija et al. (2015) |
| Antifungal activity | Aspergillus fumigatus (human isolate), Aspergillus versicolor (ATCC 11730), Aspergillus ochraceus (ATCC 12066), Aspergillus niger (ATCC 6275), Trichoderma viride (IAM 5061), Penicillium funiculosum (ATCC 36839), Penicillium ochrochloron (ATCC 9112) Penicillium verrucosum var. cyclopium (food isolate) | – | – | ||
| Silver nanoparticles of water extract | Antibacterial activity | Escherichia coli, Proteus mirabilis, Staphylococcus epidermidis | Nagajyothi et al. (2014) | ||
| Water extract | Pro-adipocyte differentiation | 3T3-L1 preadipocytes | ED∼10, 25, 50, 100 μg/ml | Activated adipogenesis of 3T3-L1 preadipocytes; increased TG accumulation; stimulated gene expression of CCAAT/enhancer-binding protein α and PPARγ during adipocyte differentiation; induced the expression of AP2, LPL, and CD 36; increased the expression of PPARγ and GLUT4 | Joo et al. (2010) |
| Water extract | Antihyperglycemic activity | 3T3-L1 adipocytes | ED∼100–2,000 μg/ml | Increased both non-insulin-stimulated and insulin-stimulated glucose uptake; activated PI 3-K and increased the Akt phosphorylation; increased mRNA expression of lipogenic genes FAS; increased the mRNA expression of fatty acid oxidation genes including CPT-1, AOX, and LCAD | Lee and Hyun (2014a) |
| HepG2 and C2C12 cells incubated with the conditioned media from 3T3-L1 adipocyte cultures | – | Increased the phosphorylation of AMPK | |||
| Subcellular membrane | – | Increased translocation of GLUT4 from cytoplasmic vesicles to plasma membrane | |||
| Antihyperglycemic effect | High fat-fed obese mice | ED∼50 mg/kg BW/day (oral administration) | Improved insulin sensitivity and reduced adiposity; increased mRNA expression of adiponectin in epididymal adipose tissue; increased the mRNA expression of fatty acid oxidative genes (CPT-1, AOX, and PGC1α) | ||
| Chloroform extract of cultivation broth | Anti-hyperglycemic activity | Dipeptidyl peptidase-4 assay | ED∼200 μg/ml | – | Geng et al. (2013) |
| Dry material of cultivation broth | Anti-hyperglycemic and antioxidative stress effects | Alloxan-induced type-1 diabetic mice | ED∼500 and 1,000 mg/kg BW/day (oral administration) | Decreased serum contents of FFA, TC, TG and LDL-C; increased HDL-C, insulin level and hepatic glycogen contents in liver; increased CAT, SOD and GPx activities, and decreased MDA content in liver; restored the damage of pancreatic β-cells | Sun et al. (2008) |
| 80% Ethanol extract of dry material of culture broth | Anti-hyperglycemic and antioxidative stress effects | Alloxan-induced type-1 diabetic mice | ED∼30 and 60 mg/kg BW/day (oral administration) | Decreased serum contents of FFA, TC, TG and LDL-C; increased HDL-C, insulin level and hepatic glycogen contents; increased CAT, SOD and GPx activities, and decreased MDA content in liver; restored the damage of pancreatic β-cells | Xu et al. (2010a) |
| Ethyl acetate extract | Anti-hyperglycemic and antioxidative stress effects | Alloxan-induced type-1 diabetic mice | ED∼500 mg/kg BW/day (oral administration) | Decreased serum contents of TC and TG; increased serum HDL-C and hepatic glycogen contents; increased GPx activities, and decreased MDA content in liver; | Lu et al. (2010) |
| Water extract | Anti-hyperglycemic effect | KK-Ay mice (Genetically type-2 diabetic mice) | ED∼100 and 300 mg/kg (single dose, oral administration) | Reduced the blood glucose and plasma insulin | Miura (2007) |
| Raw power | Anti-hyperglycemic and hepatoprotective effect | Otsuka long-evans tokushima fatty rat (genetically diabetic rat oral administration | ED∼50 g/kg BW/day | Decreased serum contents of TC and TG; reduced the serum ALT level and liver fatty accumulation | Cha et al. (2006) |
| Ethanol extract | Platelet aggregation inhibitory activity | Human blood samples | ED∼2.5 mg/ml | – | Hyun et al. (2006) |
| Water extract | Anti-hypertension effect | Stroke-prone spontaneously hypertensive rats, | ED∼extracts of 0.03 g dry material/day | Decreased mean arterial pressure and the rate of rise of mean arterial pressure; decreased blood pressure in the cross-sectional area of the subendocardial cardiomyocytes; increased the blood pressure in the capillaries; decreased the alkaline phosphatase and IL-6 expression in the capillaries; lowered the HbA1c level | Koyama et al. (2006) |
| 100% Ethanol | Anti-hyperuricemia effect | Potassium oxonate/hypoxanthine-induced hyperuricemic mice | ED∼30, 60, 120 mg/kg BW (single oral administration) | Suppressed xanthine oxidase activity in serum and liver; down-regulated renal uric acid transporter 1 | Yong et al. (2018) |
| 50% Methanol fraction of 100% ethanol extract | Anti-hyperuricemia activity | Xanthine oxidase Inhibition assay | IC50∼20.5 µg/mL | – | Wold et al. (2020) |
| 80% Methanol extract | Anti-hyperuricemia activity | Xanthine oxidase inhibitory assay | IC50∼34.37 µg/mL | – | Szychowski et al. (2018) |
| 80% Ethanol extract | Anti-obesity and probiotic effects | High-fat diet fed C57BL6/J mice | ED∼500 mg/kg BW per day | Improved the obesity of mice, including the adjustment of body weight gain, energy intake, energy efficiency, liver glucose metabolism and triglyceride metabolism, tricarboxylic acid (TCA) cycle, and degradation of three major nutrients (carbohydrate, lipid, and protein); Increased cecal propionate based on Bacteroides and Akkermansia, thereby inhibiting energy intake and fat accumulation in mice | Yu et al. (2020) |
| Cases related to patients/healthy volunteers | |||||
| Raw power | Anti-hyperglycemic effect | Type-2 diabetic patients | ED∼100 mg (single dose, oral administration) | Decreased the postprandial peak glucose, PPGE, AUC glucose; improved the postprandial endothelial dysfunction | Maenaka et al. (2008) |
| Food product containing chaga extract | Anti-hypertension effect | Healthy adults | ED∼5 ml for two times or single dose of 15 ml/person/day | Lowered systolic blood pressure and diastolic blood pressure | Yonei et al. (2007) |
| Anti-oxidative stress effect | Suppressed lipid peroxide | ||||
| Adverse effect | Frequent micturition and increased sweating | ||||
| Water extract | Anti-virus effect | HIV-infected patients | – | One succeeded, one failed | Sakuma (2004) |
| Ethanol extract | Anti-psoriasis effect | psoriasis patients | – | – | Frost (2016); Dosychev and Bystrova (1973) |
| Medicinal product | Anti-peptic ulcers effect | peptic ulcer patients | – | – | Frost (2016); Fedotov and Rodsolaĭnen (1981) |
| Terpenoid | Molecular formula | Extraction Method | Qualification Method | Purification mMethod | Reference |
|---|---|---|---|---|---|
| alanostane-type triterpenoids and steroids; bergostane-type steroids; coleanane-type triterpenoids; dlupane-type triterpenoids; eabietane-type diterpenoids; fdrimane-type sesquiterpenoids; gatisane-type diterpenoids; hcholestane-type steroids; istigmastane-type steroids; jcycloartane-type triterpenoids and steroids; kpregnane-type steroids; leudesmane-type sesquiterpenoids; msantalane-type sesquiterpenoids; nbergamotane-type sesquiterpenoids; ocholane-type triterpenoids; pcadinane-type sesquiterpenoids; qcedrane-type sesquiterpenoids; rfarnesane-type sesquiterpenoids; sbisabolane-type sesquiterpenoids; tmenthane-type monoterpenoid; ucurcumane-type sesquiterpenoids; vnoreudesmane-type sesquiterpenoids. | |||||
| Lanosterol/lanosta-8,24-dien-3β-ola | C30H50O | Methanol, six times | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column/RP-HPLC/Sephadex LH-20 column | Kim et al. (2011) |
| β-Sitosterol/24R-ethylcholesta-5-en-3β-ol I | C29H50O | ||||
| 3β-Hydroxylanosta-8,24-dien-21-ala | C30H48O2 | ||||
| Ergosterol peroxide/5,8-epidioxyergosta-6,22-dien-3β-olb | C28H44O3 | ||||
| Inotodiol/Lanost-8,24-dien-3β,22R-diola | C30H50O2 | ||||
| Trametenolic acid/3β-hydroxylanosta-8,24-dien-21-oic acida | C30H48O3 | ||||
| Betulind | C30H50O2 | ||||
| Betulin-3-O-caffeated | C39H56O5 | Dichloromethane, 48 h, reflux | MS and 1H-NMR/13C-NMR | Silica gel column, RP-HPLC (C18 column) | Wold et al. (2020) |
| Lanosta-7,9(11),24-trien-3β,22-diola | C30H50O3 | n-Hexane | IR spectra, MS, and 1H-NMR/13C-NMR | Alumina column | Kahlos and Hiltunen (1986) |
| Lanosta-8,23E-dien-3β,22R,25-triol/3β,22R,25-trihydroxylanosta-8,23E-dienea | C30H50O3 | Chloroform, 20 days, 60 °C | IR spectra, MS, and 1H-NMR/13C-NMR | Silica gel column and RP-MPLC/HPLC | Taji et al. (2008b) |
| Lanosta-7,9(11),23E-trien-3β,22R,25-triol/3β,22,25-trihydroxylanosta-7,9(11),23E-trienea | C30H48O3 | ||||
| Lanosta-8,24-dien-3β,21-diol/3β,21-dihydroxylanosta-8,24-diene/uvariol/21-hydroxylanosterola | C30H50O2 | ||||
| Inonotusol A/(−)-(3R,5S,10S,11R,15S,17R,20R,21S,24S)-21,24-cyclopenta-3,11,15,21,25-pentahydroxylanosta-8-en-7-onea | C30H48O6 | 95% Ethanol, 2 h, three times | IR spectra, MS, and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column, RP-HPLC (C18 column) | Liu et al. (2014) |
| Inonotusol B/(−)-(3R,5S,10S,11R,15S,17S,20R,21S,24R)-21,24-cyclopenta-3,11,15,21,25-pentahydroxylanosta-8-en-7-onea | C30H48O6 | ||||
| Inonotusol C/(17α,20β,24α)-21,24-cyclopenta1α,3β,21α,25,28-pentahydroxy-5α-lanosta-7,9(11)-dienea | C30H48O5 | ||||
| Inonotusol D/(17β,20β,24β)-21,24-cyclopenta-1α,3β,21α,25,28-pentahydroxy-5α-lanosta-7,9(11)-dienea | C30H48O5 | ||||
| Inonotusol E/(−)-(3R,5S,10S,11R,17S,20R,21S,24R)-21,24-cyclopenta-3,11,21,25-tetrahydroxylanosta-8-en-7-onea | C30H48O5 | ||||
| Inonotusol F/(17α,21α,23α)-24-methyl-3β-hydroxy-5α-lanosta-8,24-dien-21,23-lactonea | C31H48O3 | ||||
| Inonotusol G/3β,22-dihydroxy-5α-lanosta-8,25-dien-24-onea | C30H48O3 | ||||
| Inonotusic acid/(−)-(5S,10S)-13-isopropyl-7-oxo-abieta-8,11,13-trien-20-oic acide | C21H28O2 | ||||
| 3β,22-Dihydroxylanosta-8,24-dien-7-onea | C30H48O3 | ||||
| Ergosta-7,22-dien-3β-olb | C28H46O | ||||
| Lawsaritol/stigmast-4-en-3β-oli | C29H50O | ||||
| Fungisterol/ergosta-7-en-3β-olb | C28H48O | ||||
| Ergone/ergosta-4,6,8(14),22-tetraen-3-oneb | C28H40O | ||||
| Ergosterolb | C28H44O | ||||
| 3β-Hydroxylanosta-8,24-dien-21,23-lactonea | C30H46O3 | 95% Ethanol, 24 h, room temperature, 5 times | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column | Shin et al. (2000) |
| Methyl trametenolatea | C31H50O3 | – | – | – | |
| 21,24-Cyclopentalanosta-8-en-3β,21,25-triola | C30H50O3 | 95% Ethanol, 24 h, room temperature, 5 times | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column | Shin et al. (2001b) |
| Lanosta-8-en-3β,22,25-triola | C30H52O3 | 95% Ethanol, 24 h, room temperature, 5 times | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column | Shin et al. (2002) |
| Inonotsutriol D/lanosta-8-en-3β,22R,24R-triola | C30H50O3 | Chloroform, 7 days, 50 °C | IR spectra, MS, and 1H-NMR/13C-NMR | Silica gel column and RP-MPLC (silica gel column)/HPLC (C18 column) | Tanaka et al. (2011) |
| Inonotsutriol E/lanosta-8-en-3β,22R,24S-triola | C30H50O3 | ||||
| Oleanolic acidc | C30H48O3 | 95% Ethanol, 1 h, reflux, 5 times | IR spectra, MS, and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column, Sephadex LH-20 and RP-HPLC (C18 column) | Zhao et al. (2015a) |
| Betulinic acidd | C30H48O3 | ||||
| Inonotusane A/(21S, 24R)-24-cyclolanost-8-en-3β,21,25-triola | C30H50O3 | ||||
| Inonotusane B/(21S, 24S)-24-cyclolanost-8-en-3β,21,25-triola | C30H50O3 | ||||
| Inonotusane C/3β-hydroxy-4,4,14-trimethylchola-8,22E-dien-24-alo | C27H42O4 | ||||
| Obliquic acid/3β-hydroxy-25,26,27-trinorlanosta-8,22E-dien-24-oic acida | C27H42O3 | ||||
| 3β-Hydroxylanosta-7,9(11),24-trien-21-oic acida | C30H46O3 | ||||
| (+)-Fuscoporianol C/3β,22α,25-trihydroxylanosta-8,23E-dienea | C30H50O3 | ||||
| Inonotsutriol A/(20R,21R,24S)-21,24-cyclopentalanosta-8-en-3β,21,25-triola | C30H50O3 | Chloroform, 20 days, 60 °C | IR spectra, 1H-NMR/13C-NMR, and MS | Silica gel column and RP-MPLC (silica gel column)/HPLC (C18 column) | Taji et al. (2008a) |
| Inonotsutriol B/(20R,21R,24R)-21,24-cyclopentalanosta-8-en-3β,21,25-triola | C30H50O3 | ||||
| Inonotsutriol C/(20R,21R,24S)-21,24-cyclopentalanosta-7,9(11)-dien-3β,21R,25-triola | C30H48O3 | ||||
| Inonotsulide A/(20R,24S)-3β,25-dihydroxylanost-8-en-20,24-olidea | C30H48O4 | Chloroform, 20 days, 60 °C | IR spectra, 1H-NMR/13C-NMR, and MS | Silica gel column and RP-MPLC (silica gel column)/HPLC (C18 column) | Taji et al. (2007) |
| Inonotsulide B/(20R,24R)-3β,25-dihydroxylanost-8-en-20,24-olidea | C30H46O4 | ||||
| Inonotsulide C/(20R,24S)-3β,25-dihydroxylanosta-7,9(11)-dien-20,24-olidea | C30H46O3 | ||||
| Inonotsuoxide A/22R,25-epoxylanost-8-en-3β,24S-diola | C30H50O3 | Chloroform, 7 days, 50 °C | IR spectra, 1H-NMR/13C-NMR, and MS | Silica gel column and RP-MPLC (silica gel column)/HPLC | Nakata et al. (2007) |
| Inonotsuoxide B/22S,25-epoxylanost-8-en-3β,24S-diola | C30H50O3 | ||||
| Inotolactone B/3β-hydroxy-24-methyl-lanosta-8,24(25)-dien-26,22R-olidea | C31H48O3 | 95% Ethanol, 3 days, room temperature | IR spectra, 1H-NMR/13C-NMR, and MS | Silica gel column and RP-HPLC (C8 column) | Ying et al. (2014) |
| Inotolactone A/3β-hydroxy-24-methyl-lanosta-7,9,24(25)-trien-26,22R-olidea | C31H46O3 | ||||
| Inotolactone C/3β-hydroxydriman-12,11-olidef | C15H24O3 | ||||
| 6β-Hydroxydriman-12,11-olidef | C15H24O3 | ||||
| 3β-Hydroxycinnamolidef | C15H22O3 | ||||
| 17-Hydroxy-ent-atisan-19-oic acidg | C20H32O3 | ||||
| Saponaceoic acid I/3β,25-dihydroxy-4,4,14-trimethyl-5α-cholesta-8,23-dien-21-oic acida | C30H48O4 | 95% Ethanol, 1 h, reflux, 5 times | IR spectra, MS, and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column, Sephadex LH-20 and RP-HPLC (C18 column) | Zhao et al. (2016a) |
| Ganodecochlearin A/22R,25-epoxylanost-7,9-dien-3β,24S-diola | C30H48O3 | ||||
| 9,11-Dehydroergosterol peroxideb | C28H42O3 | ||||
| Inonotusane D/3β-hydroxy-24,25,26,27-tetranorlanosta-8-en-22-onea | C26H42O2 | ||||
| Inonotusane E/3β,12β,15α,21R,25-pentahydroxy-21,24S-cyclopentalanosta-7,9(11)-dienea | C30H48O5 | ||||
| Inonotusane G/lanosta-8-en-3β,22,24,25-tetraol-25-methyl oxidea | C31H54O4 | ||||
| Inonotusane F/Chagabusone A/3β-hydroxylanosta-8,25-dien-24-on-21-oic acida | C30H46O4 | 80% Methanol, 2 days, twice, room temperature | IR spectra, MS, and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column/RP-HPLC (C18 column) | Baek et al. (2018) |
| Spiroinonotsuoxodiol/3S,7S-dihydroxy-7(8→9R) abeo-lanost-24-en-8-onea | C32H52O4 | Chloroform | IR spectra, MS, and 1H-NMR/13C-NMR | Silica gel column, MPLC (silica gel column) and RP-HPLC (C18 column) | Handa et al. (2010) |
| Inonotsuoxodiol A/3β,22-dihydroxylanosta-8,24-dien-11-onea | C30H48O3 | ||||
| Inonotsudiol A/lanosta-8,24-dien-3 β,11β-diola | C38H48O2 | ||||
| 5,8,22-Ergostatrienolb | C28H44O | Petroleum, 14 h, room temperature | GC-MS | – | Sun et al. (2011) |
| 5,7-Ergostadienolb | C28H46O | ||||
| Inoterpene Aa | C30H52O3 | Methanol, 3 h, reflux, 3 times | IR spectra, MS, and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column, and HPLC (C18 column) | Nakamura et al. (2009) |
| Inoterpene Ba | C30H52O3 | ||||
| Inoterpene Ca | C30H52O3 | ||||
| Inoterpene Da | C30H50O3 | ||||
| Inoterpene Ea | C30H50O4 | ||||
| Inoterpene Fa | C30H48O2 | ||||
| (3R,5S,8R,9S,10S,13S,14S,17S)-21-Methylidyne-pregn-3-ol/(3R,5S,8R,9S,10S,13S,14S,17S)-17-(1-hydroxyprop-2-ynyl)-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopent-a[a]phenanthren-3-olk | C22H33O2 | Chloroform, 12 h, room temperature, three times | UPLC-Q-TOF-MSn | Silica gel column/RP-HPLC (C18 column) | Geng et al. (2013) |
| (2S,4aR,10bR)-1,1,4a,10b-Tetramethyl-1,2,3,4,4a,4b,5, 6,10b,11,12,12a-dodecahydrochrysen-2-ol | C22H31O | ||||
| (5α,20S)-3β,20-Bis-(dimethylamino)-4-(hydroxylmethyl)-4,14-dimethyl-9β,19-cyclopregn-6-en-16α-olk | C28H47N2O2 | ||||
| (22E)-Stigmasta-7,22,25-trien-3-yl acetatei | C31H47O2 | ||||
| (3β)-Olean-12-en-3-yl-(4-hydroxyphenyl)propanoatec | C39H57O3 | ||||
| Ligudentatolv | C14H17O | ||||
| 24-Methylene dihydrolanosterola | C31H52O | 80% Ethanol, 24 h, room temperature | GC-MS | – | Zheng et al. (2007a) |
| 4,4-Dimethyl fecosterolb | C32H50O | ||||
| 4-Methyl fecosterolb | C31H48O | ||||
| Fecosterol/δ-8(24),28-Ergostadienolb | C30H46O | ||||
| Episterol/ergosta-7,24(28)-dien-3-olb | C28H46O | ||||
| Ergosta-5,7,9(11),22-tertraen-3-olb | C28H42O | ||||
| Ergosta-5,7,9(11),22-tertraen-3-ol benzoateb | C35H46O2 | ||||
| Fuscoporianol D/3β,22α-dihydroxy-lanosta-8,25(27)-dien-24-peroxidea | C30H50O4 | 80% Ethanol, 24 h, room temperature | GC-MS, 1H-NMR/13C-NMR, X-ray, and IR spectra, | Silica gel column and macroporous resin | |
| Fuscoporianol A/25-methoxy-21, 22-cyclolanosta-8-en-3β,21α-diola | C31H52O3 | Petroleum ether, reflux | IR spectra, MS and 1H-NMR/13C-NMR | Silica gel column | He et al. (2001) |
| Fuscoporianol B/3β,22α-dihydroxy-lanosta-8,23E-dien-25-peroxidea | C30H50O4 | ||||
| Fuscoporianol C/3β,22α,25-trihydroxy-lanosta-8,23E-dienea | C30H50O3 | ||||
| Lupeold | C30H50O | – | GC and GC-MS | – | Kahlos and iltunen (1987); Kahlos (1994) |
| Lupenoned | C30H48O | ||||
| Stigmastanol/sitostanoli | C29H52O | ||||
| Cholesterolh | C27H46O | ||||
| β-Selinenel | C15H24 | – | GC and GC-MS | – | Ayoub et al. (2009) |
| cis-Bergamotenen | C15H24 | ||||
| trans-Bergamotenen | C15H24 | ||||
| α-Santalenem | C15H24 | ||||
| β-Sesquifenchene | C14H22 | ||||
| epi-β-Santalenem | C15H24 | ||||
| Photosantalolm | C15H24O | ||||
| β-Eudesmoll | C15H26O | ||||
| γ-Eudesmoll | C15H26O | ||||
| p-Cymenet | C10H14 | Hydrodistillation | GC and GC-MS | – | Kahlos et al. (1992) |
| α-Bisabolenes | C15H24 | ||||
| δ-Cadinolp | C15H26O | ||||
| (Z)-β-Farnesener | C15H24 | ||||
| α-Curcumeneu | C15H22 | ||||
| α-Cedreneq | C15H24 | ||||
| α-Turmeroneu | C15H22O | ||||
| Terpenes | Bioactivity | Model | IC50 value or experimental dosage (ED) | Mechanism or manifestation | Reference |
|---|---|---|---|---|---|
| PTKs: protein tyrosine kinases; EBV-EA: Epstein–Barr virus early antigen activation; AOM: Azoxymethane; DSS: Dextran sulfate sodium; PNPG: p-nitrophenyl-α-D-glucopyranoside. | |||||
| Osmundacetone | Anti-proliferation activity | Bel-7402 cell line | IC50∼4.7 μM | – | Liu et al. (2014) |
| PTKs inhibitory activity | ELISA assay | IC50∼7.7 μM | – | ||
| Ergosterol | Anti-proliferation activity | PC3 | IC50∼9.82 μM | – | Ma et al. (2013) |
| Anti-inflammatory activity | LPS-induced RAW 264.7 macrophages | ED∼40 μg/ml, inhibition rate∼6% and 23.46% | Inhibited the NO production and NF-κB luciferase activity | ||
| Ergosterol peroxide | Anti-inflammatory activity | LPS-induced RAW 264.7 macrophages | ED∼40 μg/ml, inhibition rate∼36.88% and 53.63% | Inhibited the NO production and NF-κB luciferase activity | Ma et al. (2013) |
| Anti-proliferation activity | PC3 human prostatic carcinoma cell | IC50∼38.19 μM | – | ||
| MDA-MB-231 breast carcinoma cell | IC50∼30.23 μM | – | |||
| A549 human lung cancer cell | IC50∼17.04 μM | – | Kim et al. (2011) | ||
| L1210 mouse lymphocytic leukemia cell | IC50∼36.40 μM | – | |||
| HepG2 human liver cancer | IC50∼13.19 μM | – | |||
| MCF-7 breast cancer cell | IC50∼9.06 μM | – | |||
| HCT116 human colorectal cancer cell | ED∼10 μg/ml | Induced subG1 arrest; increased cleaved PARP and decreased uncleaved caspase-3; reduced expression of β-catenin, c-Myc, cyclin D1 and CDK-8 | Kang et al. (2015) | ||
| HT-29 human colorectal cancer cell | ED∼5 μg/ml | ||||
| SW620 human colorectal cancer cell | ED∼10 μg/ml | – | |||
| DLD-1 human colorectal cancer cell | ED∼10 μg/ml | – | |||
| Anti-tumor effect | AOM/DSS-induced colorectal cancer in mice | ED∼15 mg/kg/12 h (oral administration) | Suppressed colon tumor growth and total tumor count but not the tumor incidence in mice; suppressed the overexpression of β-catenin, c-Myc and cyclin D1 | ||
| Lanosterol | Hepatoprotective activity | D-galactosamine-induced toxicity in WB-F344 cells | ED∼10 μM | Protection rate∼74.8% | Liu et al. (2014) |
| Anti-cancer activity | TPA-induced Raji cell | ED∼10–1,000 ratio/TPA | Inhibited EBV-EA activation | Nakata et al. (2007) | |
| Anti-proliferation activity | L1210 cell line | IC50∼37.15 μM | – | Zhao et al. (2015a) | |
| HT1080 cells | ED∼10–100 μg/ml | – | Ryu et al. (2017) | ||
| A549 | ED∼62.5–250 μg/ml | – | Chung et al. (2010) | ||
| AGS | ED∼62.5–250 μg/ml | – | |||
| MCF-7 | ED∼62.5–250 μg/ml | – | |||
| Hela | ED∼62.5–250 μg/ml | – | |||
| Anti-tumor effect | Sarcoma-180 cells implanted Balbc/c mice | ED∼0.1/0.2 mg/mice/day | – | ||
| Pro-proliferation activity | human follicle dermal papilla cells | ED∼1–25 μg/ml | – | Sagayama et al. (2019) | |
| Trametenolic acid | Hepatoprotective activity | D-galactosamine-induced toxicity in WB-F344 cells | ED∼10 μM | Protection rate∼75% | Liu et al. (2014) |
| Anti-cancer activity | TPA-induced Raji cell | ED∼10–1,000 ratio/TPA | Inhibited EBV-EA activation | Nakata et al. (2007) | |
| Anti-inflammatory activity | LPS-induced RAW 264.7 macrophages | ED∼40 μg/mL | Inhibited the NO production and NF-κB luciferase activity, inhibition rate∼50.04% and 18.42% | Ma et al. (2013) | |
| Pro-proliferation activity | human follicle dermal papilla cells | ED∼1–25 μg/ml | – | Sagayama et al. (2019) | |
| Inonotusol F | Hepatoprotective activity | D-galactosamine-induced toxicity in WB-F344 cells | ED∼10 μM | Protection rate∼71.9% | Liu et al. (2014) |
| 3β,22-Dihydroxylanosta-8,24-dien-11-one | Hepatoprotective activity | D-galactosamine-induced toxicity in WB-F344 cells | ED∼10 μM | Protection rate∼81.2% | Liu et al. (2014) |
| Inonotusol G | Anti-proliferation activity | KB cell line | IC50∼9.9 μM | – | Liu et al. (2014) |
| Inonotsutriol A | Anti-proliferation activity | A549 cell line | IC50∼2.34 μM | – | Zhao et al. (2015a) |
| Inonotsutriol D | Anti-proliferation activity | Hela cell | IC50∼29.56 μM | – | Zhao et al. (2015a) |
| A549 cell line | IC50∼8.39 μM | – | |||
| P388 cell line | IC50∼10.20 μM | – | Tanaka et al. (2011) | ||
| L1210 cell line | IC50∼10.00 μM | – | |||
| KB cell line | IC50∼11.60 μM | – | |||
| Inonotsutriol E | Anti-proliferation activity | HT29 | IC50∼37.43 μM | – | Zhao et al. (2015a) |
| Hela | IC50∼32.08 μM | – | |||
| L1210 | IC50∼38.23 μM | – | |||
| A549 cell line | IC50∼1.63 μM | – | |||
| 3β,22α-Dihydroxylanosta-8,25-diene-24-one | Anti-proliferation activity | A549 cell line | IC50∼5.39 μM | – | Zhao et al. (2015a) |
| Hela cell line | IC50∼20.20 μM | – | |||
| Betulin | Anti-proliferation activity | NCI-H460 lung cancer cell | IC50∼2.8 μM | – | Wold et al. (2020) |
| HT29-MTX colon cancer cell | IC50∼1.6 μM | – | |||
| A549 | IC50∼28.81 μM | – | Zhao et al. (2015a) | ||
| Betulinic acid | Anti-proliferation activity | NCI-H460 lung cancer cell | IC50∼2.10 μM | – | Wold et al. (2020) |
| HT29-MTX colon cancer cell | IC50∼0.80 μM | – | |||
| Hela | IC50∼30.30 μM | – | Zhao et al. (2015a) | ||
| Inonotsuoxide A | Anti-proliferation activity | Hela cell line | IC50∼12.15 μM | – | Zhao et al. (2015a) |
| L1210 cell line | IC50∼19.40 μM | – | Tanaka et al. (2011) | ||
| Anti-cancer activity | TPA-induced Raji cell | ED∼10–1,000 ratio/TPA | Inhibited EBV-EA activation | Nakata et al. (2007) | |
| Inonotsuoxide B | Anti-proliferation activity | Hela cell line | IC50∼14.22 μM | – | Zhao et al. (2015a) |
| HT29 cell line | IC50∼22.27 μM | – | |||
| L1210 cell line | IC50∼16.30 μM | – | Tanaka et al. (2011) | ||
| Inonotusane C | Anti-proliferation activity | human lung cancer A549 cell line | IC50∼22.50 μM | – | Zhao et al. (2015a) |
| Hela cell line | IC50∼29.18 μM | – | |||
| Inotodiol | Anti-proliferation activity | NCI-H460 lung cancer cell | IC50∼3.8 μM | – | Wold et al. (2020) |
| HT29-MTX colon cancer cell | IC50∼3.8 μM | – | |||
| L1210 cell line | IC50∼12.40 μM | – | Tanaka et al. (2011) | ||
| human lung cancer A549 cell | – | Down-regulated the expression of Ki-67 and Bcl-2 protein; up-regulated the expression of p53 and bax protein; arrested A549 cells in S phase | Zhong et al. (2011) | ||
| mouse leukemia P388 cell | ED∼30 μM | Up-regulated the expression of caspase-3/7 | Nomura et al. (2008) | ||
| HT1080 cells | ED∼10–100 μg/ml | – | Ryu et al. (2017) | ||
| A549 | ED∼62.5–250 μg/ml | – | Chung et al. (2010) | ||
| AGS | ED∼62.5–250 μg/ml | – | |||
| MCF-7 | ED∼62.5–250 μg/ml | – | |||
| Hela | ED∼62.5–250 μg/ml | – | |||
| Anti-tumor effect | mouse leukemia P388-bearing female CDF1 mice | ED∼3 and 10 mg/kg for day 1 and 4, respectively | – | Nomura et al. (2008) | |
| Sarcoma-180 cells implanted Balbc/c mice | ED-0.1/0.2 mg/mice/day | – | Chung et al. (2010) | ||
| DMBA/TPA-induced skin carcinogenesis in pathogen-free female ICR mice | ED∼85 nmol/0.1 ml acetone/day for 20 weeks | – | Nakata et al. (2007) | ||
| Anti-cancer activity | TPA-induced Raji cell | ED∼10–1,000 ratio/TPA | Inhibited EBV-EA activation | ||
| Anti-inflammatory activity | LPS-induced RAW 264.7 macrophages | ED∼40 μg/ml | Inhibited the NO production, inhibition rate∼3.13% | Ma et al. (2013) | |
| Pro-proliferation activity | human follicle dermal papilla cells | ED∼1–25 μg/ml | – | Sagayama et al. (2019) | |
| 3β-Hydroxylanos-8,24-dien-21-al | Anti-proliferation activity | NCI-H460 lung cancer cell | IC50∼33.00 μM | – | Wold et al. (2020) |
| L1210 cell line | IC50∼10.70 μM | – | Tanaka et al. (2011) | ||
| KB cell line | IC50∼14.70 μM | – | |||
| MDA-MB-231 | IC50∼36.5 μM | – | Ma et al. (2013) | ||
| HT1080 cells | ED∼10–100 μg/ml | – | Ryu et al. (2017) | ||
| A549 | ED∼62.5–250 μg/ml | – | Chung et al. (2010) | ||
| AGS | ED∼62.5–250 μg/ml | – | |||
| MCF-7 | ED∼62.5–250 μg/ml | – | |||
| Hela | ED∼62.5–250 μg/ml | – | |||
| Anti-tumor effect | Sarcoma-180 cells implanted Balbc/c mice | ED-0.1/0.2 mg/mice/day | – | ||
| 3β-Hydroxylanos-8,24-dien-21-ol | Anti-proliferation activity | L1210 cell line | IC50∼10.40 μM | – | Tanaka et al. (2011) |
| KB cell line | IC50∼32.1 μM | – | |||
| Pro-proliferation activity | human follicle dermal papilla cells | ED∼1–25 μg/ml | – | Sagayama et al. (2019) | |
| Inonotusane D | Anti-proliferation activity | HT29 cell line | IC50∼24.23 μM | – | Zhao et al. (2016a) |
| L1210 cell line | IC50∼19.93 μM | – | |||
| MCF-7 cell line | IC50∼19.20 μM | – | |||
| 4T1 | IC50∼9.40 μM | – | |||
| Inonotusane E | Anti-proliferation activity | HT29 | IC50∼37.72 μM | – | Zhao et al. (2016a) |
| HepG2 | IC50∼24.29 μM | – | |||
| 4T1 | IC50∼26.67 μM | – | |||
| Inonotusane F | Anti-proliferation activity | HT29 | IC50∼31.31 μM | – | Zhao et al. (2016a) |
| Hela | IC50∼26.99 μM | – | |||
| L1210 cell line | IC50∼27.70 μM | – | |||
| HepG2 | IC50∼35.83 μM | – | |||
| MCF-7 cell line | IC50∼15.20 μM | – | |||
| 4T1 | IC50∼24.10 μM | – | |||
| Inonotusane G | Anti-proliferation activity | Hela | IC50∼31.88 μM | – | Zhao et al. (2016a) |
| HepG2 | IC50∼36.32 μM | – | |||
| 4T1 | IC50∼20.90 μM | – | |||
| Inotolactone B | Anti-proliferation activity | MCF-7 cell line | IC50∼36.34 μM | – | Zhao et al. (2016a) |
| 4T1 | IC50∼39.39 μM | – | |||
| α-Glucosidase inhibitory activity | PNPG hydrolysis assay | – | – | ||
| Inotolactone A | Anti-proliferation activity | MCF-7 cell line | IC50∼30.72 μM | – | Zhao et al. (2016a) |
| α-Glucosidase inhibitory activity | PNPG hydrolysis assay | – | – | ||
| Ganodecochlearin A | Anti-proliferation activity | A549 cell line | IC50∼35.11 μM | – | Zhao et al. (2016a) |
| HepG2 | IC50∼35.98 μM | – | |||
| 4T1 | IC50∼10.91 μM | – | |||
| Saponaceoic acid I | Anti-proliferation activity | A549 cell line | IC50∼39.39 μM | – | Zhao et al. (2016a) |
| HT29 | IC50∼12.78 μM | – | |||
| Hela | IC50∼24.23 μM | – | |||
| L1210 cell line | IC50∼37.98 μM | – | |||
| MCF-7 cell line | IC50∼8.35 μM | – | |||
| 4T1 | IC50∼7.79 μM | – | |||
| Inonotusol A | Anti-proliferation activity | 4T1 | IC50∼33.80 μM | – | Liu et al. (2014) |
| Inonotusol C | Anti-proliferation activity | HepG2 | IC50∼30.56 μM | – | Liu et al. (2014) |
| 4T1 | IC50∼34.29 μM | – | |||
| Inonotusol B | Anti-proliferation activity | HepG2 | IC50∼31.37 μM | – | Liu et al. (2014) |
| 4T1 | IC50∼30.45 μM | – | |||
| 9,11-Dehydroergosterol peroxide | Anti-proliferation activity | A549 cell line | IC50∼10.77 μM | – | Zhao et al. (2016a) |
| HT29 | IC50∼30.76 μM | – | |||
| Hela | IC50∼35.82 μM | – | |||
| L1210 cell line | IC50∼29.31 μM | – | |||
| HepG2 | IC50∼10.93 μM | – | |||
| MCF-7 cell line | IC50∼8.40 μM | – | |||
| 4T1 | IC50∼9.31 μM | – | |||
| Spiroinonotsuoxodiol/(3S,7S,9R)-3,7-dihydroxy-7(8→9)abeo-lanost-24-en-8-one | Anti-proliferation activity | P388 | IC50∼29.5 μM | – | Handa et al. (2010) |
| L1210 | IC50∼12.5 μM | – | |||
| HL-60 | IC50∼30.1 μM | – | |||
| KB | IC50∼21.2 μM | – | |||
| Inonotsuoxodiol A/lanosta-8,24-dien-3β,11β-diol | Anti-proliferation activity | P388 | IC50∼23.8 μM | – | Handa et al. (2010) |
| L1210 | IC50∼23.8 μM | – | |||
| HL-60 | IC50∼27.2 μM | – | |||
| KB | IC50∼14.5 μM | – | |||
| Inonotsudiol A/(22R)-3β,22-dihydroxylanosta-8,24-dien-11-one | Anti-proliferation activity | P388 | IC50∼15.2 μM | – | Handa et al. (2010) |
| L1210 | IC50∼19.7 μM | – | |||
| HL-60 | IC50∼17.7 μM | – | |||
| Betulin-3-O-caffeate | Anti-inflammatory activity | LPS + IFNγ-activated C57BL/6 primary macrophages | IC50∼17.6 μM | Reduced NO production | Wold et al. (2020) |
| Antioxidant activity | DPPH radical scavenging assay | IC50∼52 μM | – | ||
| Inotolactone A | α-Glucosidase inhibitory activity | PNPG hydrolysis assay | IC50∼0.24 mM | – | Ying et al. (2014) |
| Inotolactone B | IC50∼0.24 mM | – | |||
| 3β-Hydroxycinnamolide | IC50∼3.39 mM | – | |||
| Phenolics | Molecular formula | Extraction Method | Qualification Method | Purification Method | Reference |
|---|---|---|---|---|---|
| SEC: size exclusion chromatography; HPSEC: high performance size exclusion chromatography. | |||||
| Gallic acid | C7H6O5 | Water or 70% ethanol, 70–80 °C, 2–24 h | LC | – | Zheng et al. (2008b); Glamočlija et al. (2015) |
| Protocatechuic acid | C7H6O4 | Water or 70% ethanol, 70–80 °C, 2–24 h/boiling water, 1 h | LC, LC-MS and GC-MS, MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, HP-20 column and RP-HPLC (C18 column) | Ju et al. (2010); Nakajima et al. (2007); Glamočlija et al. (2015) |
| p-Hydroxybenzoic acid | C7H6O3 | Water or 70% ethanol, 70–80 °C, 2–24 h | LC | – | Glamočlija et al. (2015) |
| Vanillic acid | C8H8O4 | High-pressure steam, 35% methanol, 35% acetone, 30% water | LC-MS and GC-MS | Liquid-liquid extraction | Ju et al. (2010) |
| 2,5-Dihydroxyterephthalic acid | C8H6O6 | High-pressure steam, 35% methanol, 35% acetone, 30% water or Water boiling, 1 h | LC-MS and GC-MS, MS and 1H-NMR/13C-NMR | Liquid-liquid extraction or/and HP-20 column and RP-HPLC (C18 column) | Nakajima et al. (2009); Nakajima et al. (2007); Ju et al. (2010) |
| Caffeic acid | C9H8O4 | Water boiling, 1 h | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, HP-20 column and RP-HPLC (C18 column) | Nakajima et al. (2007) |
| 3,4-Dihydroxybenzalacetone | C10H10O3 | Methanol, six times or water boiling, 1 h | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, HP-20 column and RP-HPLC (C18 column) or Sephadex LH-20 column/silica gel column | Kim et al. (2011); Nakajima et al. (2007) |
| 3,4-Dihydroxybenzaldehyde | C7H6O3 | Methanol, two times, room temperature | IR spectra, MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, HP-20 column and RP-HPLC (C18 column) | Nakajima et al. (2007); Liu et al. (2014) |
| 6,7-Dihydroxycoumarin | C9H6O4 | High-pressure steam, 35% methanol, 35% acetone, 30% water | GC-MS | Liquid-liquid extraction | Ju et al. (2010) |
| 4-Hydroxy-3,5-dimethoxy benzoic acid 2-hydroxy-1-(hydroxymethyl) ethyl ester | C12H16O7 | Water boiling, 1 h | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, HP-20 column and RP-HPLC (C18 column) | Nakajima et al. (2007) |
| 2,5-Dihydroxybenzaldehyde | C7H6O3 | Methanol, six times | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column, MPLC, RP-HPLC/Sephadex LH-20 column | Kim et al. (2011) |
| Inonoblin A/phelligridin I | C33H20O13 | Methanol, two times, room temperature | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, Sephadex gel LH-20 column | Lee et al. (2007) |
| Inonoblin B | C23H14O10 | ||||
| Inonoblin C | C25H18O9 | ||||
| Phelligridin D | C20H12O8 | ||||
| Phelligridin E | C25H14O10 | ||||
| Phelligridin G | C32H18O12 | ||||
| Methylinoscavin A | C26H20O9 | Petroleum ether, chloroform, ethyl acetate, acetone, ethanol and water, reflux for three times | 1H-NMR | – | Zheng et al. (2011b) |
| Methylinoscavin B | C25H22O8 | ||||
| Methylinoscavin C | C24H18O8 | ||||
| Phelligridin C | C20H12O7 | ||||
| Phelligridin H | C33H18O13 | ||||
| Phelligridin F | C26H22O9 | ||||
| 2,3-Dihydroxy-1-(4-hydroxy-3-methoxyphenyl)propan-1-one | C10H12O5 | 95% Ethanol, 2 h, reflux, three times | IR spectra, MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column, Sephadex gel LH-20 and RP-HPLC (C18 column) | Liu et al. (2014) |
| 2,3-Dihydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone | C11H14O6 | ||||
| 4-(3,4-Dihydroxyphenyl)-(E)-3-buten-2-one | C11H14O6 | ||||
| Davallialactone | C25H20O9 | – | LC and 1H-NMR/13C-NMR | – | Zhao et al. (2015b) |
| Methyl davallialactone | C26H22O9 | ||||
| Inoscavin C | C23H16O8 | ||||
| p-Coumaric acid | C9H8O3 | 70% Aqueous acetone, 24 h, room temperature, three times | LC | – | Zheng et al. (2009b) |
| Rhoifolin/apigenin-7-O-neohesperidoside | C27H30O14 | ||||
| Isorhoifolin/apigenin-7-O-rutinoside | C27H30O14 | ||||
| Naringin/naringenin 7-O-neohesperidoside | C27H32O14 | ||||
| Isorhamnetin-3-O-rutinoside | C28H32O16 | ||||
| Rutin | C27H30O16 | ||||
| Narirutin | C27H32O14 | ||||
| Kaempferol | C15H10O6 | ||||
| Quercetin | C15H10O7 | ||||
| Isohamnetin | C16H12O7 | ||||
| Luteolin | C15H10O6 | ||||
| Naringenin | C15H12O5 | ||||
| Apigenin | C15H10O5 | ||||
| Fortuneletin/5,7-dihydroxy-3′-methoxyflavone | C16H12O5 | ||||
| EGCG | C22H18O11 | ||||
| ECG | C22H18O10 | ||||
| Inoscavin B | C24H20O8 | ||||
| Homogentisic acid | C8H8O4 | HCl-acetonitrile, 2 h, room temperature | LC | – | Kim et al. (2008b) |
| Ferulic acid | C10H10O4 | ||||
| o-Coumaric acid | C9H8O3 | ||||
| Resveratrol | C14H12O3 | ||||
| 2,6-Dimethoxyphenol | C8H10O3 | HCl-water, 5 h, reflux; then hot ethyl acetate and methanol | IR spectra and GC-MS | – | Mazurkiewicz (2006) |
| Resorcinol | C6H6O2 | ||||
| 3-Hydroxy-4,5-dimethoxybenzoic acid | C9H10O5 | ||||
| 3-Hydroxy-2-oxo-2Hchromene-4,6-dicarboxylic acid | C11H6O7 | 70% Methanol, 12 h, 60 °C | IR spectra, MS, UV and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column, Sephadex gel LH-20/ODS-Sepak cartridge and RP-HPLC (C18 column) | Hwang et al. (2016) |
| 6,6′-Dihydroxy-(1,1′-biphenyl)-3,3′-dicarboxylic acid | C14H10O6 | ||||
| 4-Hydroxy-3,5-dimethoxybenzoic acid/syringic acid | C9H10O5 | ||||
| 4-Hydroxyisophthalic acid | C8H6O5 | ||||
| Eriocitrin | C27H32O15 | 50% Methanol, 24 h, room temperature | LC | – | Zheng et al. (2008b) |
| Isorhamnetin | C16H12O7 | ||||
| EGC | C15H14O7 | ||||
| 2,3-Dihydroxybenzaldehyde | C7H6O5 | ||||
| (2′R)4-[1-(Hydroxymethyl)-2-methoxy-2-oxoethoxy]-3,5-dimethoxy benzoic acid methyl ester | C14H18O8 | – | MS and 1H-NMR/13C-NMR | Chiralpak IG column | Zou et al. (2019) |
| (2′S)4-[1-(Hydroxymethyl)-2-methoxy-2-oxoethoxy]-3,5-dimethoxy benzoic acid methyl ester | C14H18O8 | ||||
| 4-Hydroxy-3,5-dimethoxy-2-butoxy-2-oxoethyl ester | C15H20O7 | ||||
| Lignin-carbohydrate complexes (37.9 and 24.5 kDa, 75–80% lignin) | – | Water, 4 h, 60 °C | HPSEC | Anion-exchange chromatography (DEAE-cellulose column); SEC (Sephadex G-25 column); dialysis | Wang et al. (2015) |
| Lignin-carbohydrate complexes (29, 35, and 61 kDa, 64% lignin) | – | NaOH-water, 12 h, 4 °C | HPSEC | Anion-exchange chromatography (DEAE-cellulose column); SEC (Sephadex G-25 column); dialysis | Niu et al. (2016) |
| Compounds | Bioactivity | Model | IC50 value or experimental dosage (ED) | Mechanism or manifestation | Reference |
|---|---|---|---|---|---|
| Phenolics | |||||
| 3,4-dihydroxybenzaldehyde | Anti-proliferation activity | A549 cell line | IC50∼23.63 μM or 3.1 μM | – | Liu et al. (2014); Zhao et al. (2016a) |
| Anti-proliferation activity | 4T1 | IC50∼16.40 μM | – | Zhao et al. (2016a) | |
| Anti-proliferation activity | Bel-7402 cell line | IC50∼3.7 μM | – | Liu et al. (2014) | |
| PTKs inhibitory activities | ELISA assay | IC50∼24.6 μM | – | ||
| 4-(3,4-dihydroxyphenyl)-(E)-3-buten-2-one | Anti-proliferation activity | A549 cell line | IC50∼24.23 μM | – | Zhao et al. (2016a) |
| MCF-7 cell line | IC50∼30.71 μM | – | |||
| 4T1 | IC50∼26.67 μM | – | |||
| 3,4-dihydroxybenzalacetone | Anti-proliferation activity | PA-1 | IC50∼12.2 μM | – | Nakajima et al. (2009) |
| HL-60 | IC50∼32.9 μM | – | |||
| A549 | IC50∼23.6 μM | – | Kim et al. (2011) | ||
| HL-60 | IC50∼21.8 μM | – | |||
| HCT116 | ED∼10 and 100 μM | – | Kuriyama et al. (2013) | ||
| 3,4-dihydroxybenzaldehyde | Anti-proliferation activity | PA-1 | IC50∼12.1 μM | – | Nakajima et al. (2009) |
| caffeic acid | Anti-proliferation activity | HL-60 | IC50∼27.4 μM | – | Nakajima et al. (2009) |
| HCT116 | ED∼10 and 100 μM | – | Kuriyama et al. (2013) | ||
| (2′S)4-[1-(hydroxymethyl)-2-methoxy-2-oxoethoxy]-3,5-dimethoxy benzoic acid methyl ester | Anti-proliferation activity | Hep3B cells | ED∼25 μM | – | Zou et al. (2019) |
| 4-hydroxy-3,5-dimethoxy-2-butoxy-2-oxoethyl ester | – | ||||
| Caffeic acid | Anti-proliferation activity | DNA topoisomerase II inhibitory assays | IC50∼15.0 μM | – | Kuriyama et al. (2013) |
| 3,4-Dihydroxybenzalacetone | IC50∼10 μM | – | |||
| Gallic acid | IC50∼50 μM | – | |||
| Syringic acid | IC50∼175 μM | – | |||
| Protocatechuic acid | IC50∼80 μM | – | |||
| 3,4-Dihydroxybenzaldehyde | IC50∼150 μM | – | |||
| 2,5-Dihydroxy-terephthalic acid | IC50∼170 μM | – | |||
| Inonoblin A/Phelligridin I | Antioxidant activity | ABTS and DPPH scavenging assays | IC50∼0.43 and 1.45 μM | – | Lee et al. (2007) |
| Inonoblin B | IC50∼0.58 and 1.42 μM | – | |||
| Inonoblin C | IC50∼0.65 and 0.82 μM | – | |||
| Phelligridin D | IC50∼0.33 and 1.51 μM | – | |||
| Phelligridin E | IC50∼0.40 and 1.57 μM | – | |||
| Phelligridin G | IC50∼0.43 and 1.48 μM | – | |||
| Caffeic acid | IC50∼0.66 and 0.41 μM | – | |||
| Lignin fraction | Anti-virus activity | HIV-protease | IC50∼1.4 μg/ml | – | Ichimura et al. (1998) |
| Lignin–carbohydrate complex | Immunomodulatory activity | RAW 264.7 macrophages | ED∼50 or 100 μg/ml | Stimulated NO production and phagocytic activity | Niu et al. (2016) |
| Antioxidant activity | DPPH, hydroxyl radical scavenging and FRAP assays | ED∼0.25–1.00 mg/ml | – | ||
| Lignin–carbohydrate complex | Anti-proliferation activity | A549, LO2, Bel-7402 or HEK 293 | IC50∼150 and 200 µg/mL (for A549) | Arrested cells at S phase of A549; | Wang et al. (2015) |
| Anti-inflammatory activity | LPS-induced HEK 293/NF-B-Luc cells | ED∼1 mg/ml | Inhibited the activation of NF-κB | ||
| Purified phenolic extract | Immunomodulatory ability | CYP-induced ICR mice | ED∼50 mg/kg BW/day (oral administration) | Inhibited the CYP-induced reduction of body weight, spleen index and the viability of peripheral lymphocytes | Zheng et al. (2008b) |
| Compound | Molecular formula | Extraction Method | Qualification Method | Purification Method | Reference |
|---|---|---|---|---|---|
| UPLC-Q-TOF-MS/MS: ultra-high-performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry; RP-HPLC: reverse-phase high performance liquid chromatography; MALLS: multi-angle laser light scattering; SEC: size exclusion chromatography; HPSEC: high performance size exclusion chromatography; AEC: anion-exchange chromatography; HPAEC: high-performance anion-exchange chromatography. | |||||
| Polysaccharides | |||||
| Glycoprotein (230 kDa) | – | Water, 3 h, 80 °C | SEC | Alcohol precipitation, AEC (DEAE-Sepharose fast flow column), SEC (SepharoseCL-6B column), dialysis | Huang et al. (2012) |
| Proteoglycan (40 kDa) | – | Water, 2 h, 100 °C, two times | HPSEC (refractive index, UV, and MALLS detectors), AEC, and FT-IR | Liquid-liquid extraction | Liu et al. (2019) |
| α-Linked fucoglucomannan (1,000 kDa) | – | Water, 6 h, 121 °C | SEC | Alcohol precipitation, AEC (DEAE-cellulose column), SEC (Toyopearl HW65F column), dialysis | Kim et al. (2006) |
| Purified fractions of polysaccharide (93 kDa) | – | Water, 3 h, 80 °C | GC and HPSEC | Alcohol precipitation, AEC (DEAE-Sepharose CL-6B column), SEC (sepharose CL-6B column), dialysis | Fan et al. (2012) |
| Purified fractions of polysaccharide (122 kDa) | – | Water, 80 min, 75 °C, ultrasonication | SEC | Deproteination (Sevag reagent), alcohol precipitation, DEAE-52 cellulose column, dialysis | Ma et al. (2012); Zhang et al. (2013b) |
| Purified fractions of polysaccharide (32.5 kDa) | – | Water, 2.5 h, 60 °C | SEC | Anion-exchange DEAE cellulose column and SEC (Sephadex G-200) | Hu et al. (2016) |
| Purified fractions of polysaccharide (111.9 kDa) | – | Water, 2 h, 90 °C | UV, IR spectra, HPSEC | Alcohol precipitation, DEAE-52 column, SEC (Sephadex G-100) | Han et al. (2019) |
| Purified homogeneous polysaccharide fraction (37.354 kDa) | – | Water, 2.5 h, 60 °C | FT-IR, HPSEC, 1H-NMR/13C-NMR | Deproteination (Sevag reagent), alcohol precipitation, AEC (DEAE cellulose column), Sephadex G-200 gel | Hu et al. (2017a) |
| Neutral polysaccharides (60–73 kDa) | – | Water, 2 h, 100 °C, two times | SEC-MALLS, IR spectra, 1H-NMR/13C-NMR, and GC-MS | AEC (ANX Sepharose™ 4 Fast Flow), SEC (Superose® 6 column), dialysis | Wold et al. (2018) |
| Acidic polysaccharides (melanin-polysaccharide complex) (10–31 kDa) | – | AEC (ANX Sepharose™ 4 Fast Flow), SEC (Hiload™ 16/60 Superdex™ 200 column), dialysis | |||
| Alkaline polysaccharides (>450 kDa) | – | AEC (ANX Sepharose™ 4 Fast Flow), SEC (Sephacryl S-500 HR column), dialysis | |||
| Alkaloids | |||||
| 3,3-Dimethyl-9-(propylamino)-3,4-dihydro-1(2H)-acridinone | C18H21N2O | Chloroform, 12 h, room temperature, three times | UPLC-Q-TOF-MSn | Silica gel column/RP-HPLC (C18 column) | Geng et al. (2013) |
| 2-Butyl-3-(3-methylphenyl)-4(3H)-quinazolinone | C19H19N2O | ||||
| 1-(4-Methyl-1-piperazinyl)-2-{[3-(2-methyl-1-piperidinyl)propyl]amino}ethanone | C16H31N4O | ||||
| 1-{[2-(Diethylamino)ethyl]amino}-3-(4-methyl-1-piperazinyl)-2-propanol | C14H31N4O | ||||
| N-{(1S,2S)-1-benzyl-3-[1-(cyclohexylmethyl)hydrazino]-2-hydroxypropyl}-N2-[(2-methoxyethoxy)carbonyl]-L-valinamide | C26H43N4O5 | ||||
| 1,1-Dimethyl-3,3-bis(2,2,6,6-tetramethyl-1-prop-2-en-1-ylpiperidin-4-yl)urea | C27H49N4O | ||||
| 1-(3,6-Dihydropyridin-1(2H)-yl)-3-[3-(dimethylamino)propyl]urea | C11H21N4O | ||||
| (2R,4S,5S,7S)-5-Amino-N-butyl-7-{4-[4-(dimethylamino)-butoxy]-3-(3-methoxypropoxy)benzyl}-4-hydroxy-2,8-dimethylnonanamide | C32H57N3O5 | ||||
| 2,2-Bis[2,2,6,6-tetramethyl-1-(octyloxy)piperidin-4-yl]-hexanedioate | C40H73N2O6 | ||||
| 3-(4-Cyclohexylbutyl)-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocine | C24H36N | ||||
| Other organic compounds | |||||
| 2-(1,4,4-Trimethylcyclohex-2-en-1-yl)ethyl acetate | C13H22O2 | HCl-water, 5 h, reflux; then hot ethyl acetate and methanol | IR spectra and GC-MS | – | Mazurkiewicz (2006) |
| 4-Oxopentanoic acid | C5H8O3 | ||||
| Docosane | C22H46 | ||||
| Hexatriacontane | C36H74 | ||||
| O-Acetyl-all-trans-Retinol | C22H32O2 | ||||
| Hexadecanoic acid | C16H32O2 | ||||
| Heneicosane | C21H44 | ||||
| Benzyl alcohol | C7H8O | ||||
| Oxalic acid | C2H2O4 | Water or 70% ethanol, 2–24 h, 70-80 °C | LC | None | Glamočlija et al. (2015) |
| Cinnamic acid | C9H8O2 | ||||
| Isocitric acid | C6H8O7 | High-pressure steam, 35% methanol, 35% acetone, 30% water | LC-MS and GC-MS | Liquid-liquid extraction | Ju et al. (2010) |
| 1-Dodecanol | C12H26O | Petroleum, 14 h, room temperature, | GC-MS | – | Sun et al. (2011) |
| 2,10-Dimethyl-9-undecenol | C13H26O | ||||
| Ethyl octadecanoate | C20H40O2 | ||||
| Isopropyl linoleate | C20H38O2 | ||||
| Ethyl oleate | C20H38O2 | ||||
| Ethyl hexadecanoate | C18H36O2 | ||||
| Ethyl dodecanoate | C14H28O2 | ||||
| Ethyl tetradecanoate | C16H32O2 | ||||
| Di-isobutyl phthalate | C16H22O4 | ||||
| Di-iso-octyl phthalate | C24H38O4 | ||||
| Ethyl pentadecanoate | C17H34O2 | ||||
| Ethyl Heptadecanoate | C19H38O2 | ||||
| 2,6,10,14-Tetramethyl heptadecane | C21H44 | ||||
| 2,6,10,14-Tetramethyl pentadecane | C19H40 | ||||
| Hexadecane | C16H34 | ||||
| Octadecane | C18H38 | ||||
| Heptadecane | C17H36 | ||||
| Nonadecane | C19H40 | ||||
| Dibutyl phthalate | C16H22O4 | ||||
| Methyl-8,11-octadecadienoate | C19H34O2 | ||||
| Ethyl linoleate | C20H36O2 | ||||
| Pentadecanal | C15H30O | ||||
| Linoleic acid | C18H32O2 | ||||
| Benzaldehyde | C7H6O | HCl-water, 5 h, reflux; then hot ethyl acetate and methanol | IR spectra and GC-MS | – | |
| (2S)-2-[(1S)-1-Phenylethyl]-3,6-dihydro-2H-pyran | C13H15O | Chloroform, 12 h, room temperature, three times | LC-Q-TOF-MSn | Silica gel column/RP-HPLC | Geng et al. (2013) |
| 1-Octen-3-ol | C8H16O | Hydrodistillation | GC and GC-MS | – | Kahlos (1994) |
| Linolenic acid | C18H30O2 | Hexane | TLC, GLC, and GC-MS | – | Kahlos et al. (1989) |
| 1,6-Dideoxy-3,4-O-(1,5,9-trimethyl-decylidene)-Dmannitol | C19H37O4 | Chloroform, 12 h, room temperature, three times | LC-Q-TOF-MSn | Silica gel column/RP-HPLC | Geng et al. (2013) |
| (1S,4aR,5R,8aS)-5-[(1R)-5-Hydroxy-1,5-dimethylhexyl]-4a-methyldecahydronaphthalen-1-ol | C19H35O2 | ||||
| Glucitol | C6H14O6 | 95% Ethanol, 24 h, room temperature, 5 times | MS and 1H-NMR/13C-NMR | Liquid-liquid extraction, silica gel column | Shin et al. (2001a) |
| Trp-Gly-Cys | C16H20N4O4S | Hyun et al. (2006) | |||
| Phenylalanine | C9H11NO2 | 50% Ethanol, 24 h, room temperature | HPLC | Sephadex LH-20 column | Zheng et al. (2008b) |
| Tyrosin | C9H11NO3 | ||||
| Purified melanin fractions (56–60 kDa or 100–120 kDa or more) | – | NaOH-water, 2 h, boiling | SEC (Toyopearl HW-65 resin column) | SEC (Sephadex G-75 column) | Babitskaya et al. (2000) |
| Purified melanin fractions (2–20 kDa or 90–100 kDa or more) | – | 50–95% ethanol, 2 h, 100 °C; then water, 1 h, 100 °C; then KOH-water, 1–3 h, 20 °C | IR spectra, 13C NMR | Ethanol precipitation, acid precipitation, Sephadex G-100 column | Olennikov et al. (2012) |
| Purified melanin-polysaccharide (<10 kDa, ∼5% polysaccharide) | – | Water, 2 h, boiling, three times | HPSEC (diol-300 column) | Ethanol precipitation, dialysis, acid precipitation | Wold et al. (2020) |
| Purified polysaccharide-melanin complex (10–31 kDa, ∼4.2–9.7% melanin) | – | Water, 2 h, boiling, 2 times | SEC-MALLS, IR spectra, 1H-NMR/13C-NMR, and GC-MS | AEC (ANX Sepharose™ 4 Fast Flow); SEC (Hiload™ 16/60 Superdex™ 200 column); dialysis | Wold et al. (2018) |
| Crude melanin | – | Water, 10 h, 70 °C or microwave-assisted extraction | – | Acid precipitation | Burmasova et al. (2019); Parfenov et al. (2019) |
| Compounds | Bioactivity | Model | IC50 value or experimental dosage (ED) | Mechanism or manifestation | Reference |
|---|---|---|---|---|---|
| HFD: high-fat diet; STZ: streptozotocin; MDA: maleic dialdehyde; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; CAT: catalase; SOD: superoxide dismutase; GPx: glutathione peroxidase; GSH: glutathione; TBARS: thiobarbituric acid-reactive substances; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BW: body weight; CYP: cyclophosphamide; DDC: diethyldithiocarbamate; TAOC: total antioxidant capacity; AMS: amylase; MMP: matrix metalloproteinase; MSPKs: mitogen-activated protein kinases; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B; ERK: extracellular signalregulated protein kinase; JNK: c-Jun N-terminal kinase; P38: Cytokinin Specific Binding Protein (CSBP); MAPKs: mitogen-activated protein kinases; NF-κB: nuclear factor κB p65; COX: cyclooxygenase, IL-2R: interleukin-2 receptor; Bax: Bcl-2 associated X protein; Keap1: Kelch-like ECH-associated protein 1; Bcl-2: B-cell lymphoma-2; Nrf2: NF-E2p45-related factor 2; HO-1: heme oxygenase-1; APP/PS1: amyloid precursor protein/presenilin 1 ; NO: nitric oxide; IL-6: interleukin-6; IL-1β: interleukin-1β; INF-γ: interferon-γ; IL-4: interluekin-4; TLR2: toll-like receptor 2; TLR4: toll-like receptor 4; IκBα: inhibitor kappaBα of NF-κB, or nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; TGF-β: transforming growth factor; Fox-p3: forkhead box; ROR-γt retinoic acid-related orphan receptor; STAT-3: signal transducer and activator of transcription; STAR: steroidogenic acute regulatory protein; NQO-1: NADPH quinoneoxidoreductase-1; p-AKT: phospho-protein kinase B; p-mTOR: phospho-mammalian target of rapamycin; Nrf2: erythroid 2-related factor 2; GM-CSF: granulocyte macrophage-colony stimulating factor. | |||||
| Polysaccharides | |||||
| Crude polysaccharides | Antioxidant activity | DPPH test, hydroxyl radical/superoxide anion radical scavenging test | IC50∼0.27–7.0 mg/ml | – | Mu et al. (2012) |
| Antioxidative stress activity | H2O2-induced cell death of PC12 cell | ED∼5, 10, 20 µg/ml | |||
| Polysaccharides-chromium (III) complex (115 kDa) | Antioxidative stress activity | H2O2-induced oxidative damage in hepatic L02 cells | ED∼500 μg/mL | Improved the cell viability; inhibited the morphology alteration and maintained the integrity of mitochondria | Wang et al. (2018a) |
| Purified polysaccharide (97.12 kDa) | Antioxidative stress activity | H2O2-induced oxidative damage in hepatic L02 cells | ED∼50–500 μg/mL | Improved the cell viability; restored the morphology alterations of cells and maintained the integrity of mitochondria | Wang et al. (2018c) |
| Antioxidant activity | DPPH radical scavenging test | IC50∼498.35 μg/mL | – | ||
| Crude protein-polysaccharide complex | Antioxidant activity | DPPH assay | IC50∼1.33–4.35 mg/ml | – | Xiang et al. (2012) |
| Crude exo/endo-polysaccharide from submerged cultures | Antioxidant activities | DPPH, TBARS assays | ED∼0.5–3 mg/ml | – | Xu et al. (2011a) |
| Crude exo-polysaccharide from submerged cultures | Antioxidant activities | Hydroxyl and superoxide radicals scavenging abilities | IC50∼1.08 mg/ml and 174.1 µg/ml | – | Chen et al. (2011) |
| Crude polysaccharide | Antioxidative stress activity | Fe2+-Cys-induced lipid peroxidation in fresh mouse liver homogenate | ED∼100, 200, 300, and 400 μg/ml | – | Song et al. (2008) |
| Fe2+-VC-induced mitochondria swelling | ED∼100, 200, 400, and 800 μg/ml | – | |||
| Purified polysaccharide (40 kDa) | Antioxidant activities | DPPH radicals scavenging, TEAC, and FRAP assays | ED∼50–1,000 μg/ml | – | Liu et al. (2019) |
| Purified polysaccharide (32.5 kDa) | Antioxidant activities | DPPH and hydroxyl radicals scavenging assays | IC50∼1.3–3.2 mg/ml | – | Hu et al. (2016) |
| Purified polysaccharide (122 kDa) | Antioxidant activities | FRAP and anti-liver-lipid peroxidation model | ED∼0.5–5 mg/ml | – | Ma et al. (2012) |
| Purified polysaccharide (122 kDa) | Antioxidant activities | FRAP and anti-liver-lipid peroxidation model | ED∼0.5–5 mg/ml | – | Zhang et al. (2013b) |
| Purified homogeneous polysaccharide (37.354 kDa) | Antioxidant activities | DPPH and hydroxyl radical Scavenging | ED∼1.0–5.0 mg/mL | – | Hu et al. (2017a) |
| Purified homogeneous selenized polysaccharide (28.071 kDa) | – | ||||
| Purified homogeneous polysaccharide (37.354 kDa) | Antioxidative stress activity | D-gal-induced oxidant damage in mice | ED∼100 mg/kg DW | Increased SOD and GPx levels coupled with reduction in MDA level | |
| Purified homogeneous selenized polysaccharide (28.071 kDa) | |||||
| Unknown polysaccharides | Antioxidant protective activity | Tacrine induced apoptosis of HepG2 cells | – | Reduced tacrine-induced apoptosis; Inhibited tacrine-induced ROS generation, 8-OHdG formation in mitochondrial DNA, and loss of the mitochondrial transmembrane potential; decreased tacrine-induced the cytochrome c release and activation of caspase-3 | Li et al. (2019) |
| Purified polysaccharide | Antioxidative stress and anti-proliferation activity | Zebrafish embryos | ED∼1–5 mg/mL | Reduced levels of intracellular ROS and apoptosis in the developing embryos; arrested the cells at G1 stage | Eid and Das (2020a) |
| Purified polysaccharide | Anti-genotoxic effects | UVB-exposed zebrafish embryos | ED∼2.5 mg/mL | Reduced DNA damage and ameliorated the deformed structures; upregulated mRNA expressions of XRCC-5, XRCC-6, RAD51, P53, and GADD45 | Eid et al. (2020b) |
| Purified polysaccharide | Antioxidative stress activity | H2O2-treated RINm5F pancreatic β-cells | ED∼1–100 µg/ml | Decreased DNA fragmentation and the rate of apoptosis; upregulated phosphorylation of MAPK (JNK, ERK, and p38); Suppressed cleaved caspase-3 | Sim et al. (2016) |
| Purified polysaccharide (42 kDa) | Anti-inflammatory and anti-oxidative stress effects, and protective effect of reproductive function | Toxoplasma gondii-induced male mouse | ED∼100, 200, and 400 mg/kg BW/day (oral administration) | Improved the spermatogenic capacity and ameliorated pathological damage of testis; increased serum testosterone, luteinizing hormone and follicular-stimulating hormone levels; Decreased the levels of MDA and NO, but increased the activities of SOD and GSH; Up-regulated testicular StAR, P450scc and 17β-HSD expressions; up-regulated the expressions of Nrf2, HO-1 and NQO-1, and suppressed the apoptosis of testicular cells by decreasing Bax and cleaved caspase-3 expresisions; enhanced testicular PI3K, p-AKT and p-mTOR expression | Ding et al. (2020) |
| Purified polysaccharide (42 kDa) | Anti-inflammatory and anti-oxidative stress effects, and protective effect of pregnancy | Toxoplasma gondii-induced adverse pregnancy in female mouse | ED∼100, 200, and 400 mg/kg BW/day (oral administration) | Reduced the abortion rate; inhibited the decreases of serum progesterone and estriol levels and the increase of MDA level; increased the activities of SOD and GSH in blood and/or placenta; Inhibited the production of TNF-α, IL-6, IFN-γ, IL-1β and IL-17A; and promoted the production of anti-inflammatory cytokine IL-10 and TGF-β in placenta; Up-regulated the expression of Fox-p3, whereas down-regulated the expressions of ROR-γt, STAT-3 and TLR-4, and inhibited the phosphorylations of NF-κB and IκBα in placental tissues | Xu et al. (2020) |
| Purified polysaccharide (42 kDa) | Anti-inflammatory, anti-oxidative stress, and hepatoprotective effect | Toxoplasma gondii-induced mouse liver injury | ED∼100, 200, and 400 mg/kg BW/day (oral administration) | Decreased the liver coefficient, the levels of ALT, AST, MDA, and NO; increased the contents of SOD and GSH in liver/serum; Decreased the expression of serum TNF-α, IL-6, IL-1β, IFN-γ and IL-4; down-regulated TLR2, TLR4, phosphorylation of NF-κB and IκBα; up-regulated the expressions of Nrf2 and HO-1 | Xu et al. (2019a) |
| Low-molecular-weight polysaccharide (10–100 kDa) | Renal protective effect | HFD/STZ-Induced diabetic nephropathy in C57BL/6 male mice | ED∼300 and 1,000 mg/kg BW/day (oral administration) | Restored the integrity of the glomerular capsules and increased the numbers of glomerular mesangial cells; alleviated the glucotoxicity in renal tubular cells; | Chou et al. (2016) |
| Anti-hyperglycemic effect | Decreased insulin tolerance, triglyceride levels, urinary albumin/creatinine ratio and LDL/HDL ratio | ||||
| Anti-inflammatory effect | Decreased NF-κB and TGF-β expression; decreased expression of TGF-β on renal cortex | ||||
| Purified polysaccharide (32.5 kDa) | Anti-inflammatory and anti-oxidative stress effects | DDC-induced chronic pancreatitis mice | ED∼100, 200 and 400 mg/kg BW/day (oral administration) | Alleviated pancreatic acinar atrophy and weight loss; increased SOD and MDA level in pancreatic tissue; decreased LDH, hydroxyproline, AMS, IFN-γ, and IL-1 levels in serum | Hu et al. (2016) |
| Unknown polysaccharide | Anti-inflammatory effect | DSS-induced colitis mice | ED∼100–300 mg/kg BW/day (oral administration) | Reduced the losses of tight junction proteins Occludin and ZO-1 in colon tissues; regulated imbalanced Th1/Th2 and Th17/Treg in colon tissues, mesenteric lymph nodes and spleen; upregulated p-STAT1 and p-STAT3; down-regulated expression of p-STAT6 | Chen et al. (2019b) |
| Crude polysaccharide | Anti-inflammatory activity | LPS-induced RAW 264.7 murine macrophage cells | ED∼50–500 μg/ml | Down-regulated IL-6 and TNF-α levels; no effect on IL-1β; reduced NO production | Van et al. (2009) |
| Crude endo-polysaccharide from submerged cultures | Anti-inflammatory activity | LPS-induced RAW 264.7 murine macrophage cell | ED∼1–10 μg/ml | Up-regulated the mRNA expression of the INOS and inflammatory effector cytokines (IL-1β, IL-6 and TNF-α); Increased total nitrite-producing activity of macrophages | Kim et al. (2005) |
| Crude endo-polysaccharide from submerged cultures | Immunomodulatory activity | Fractionated fresh B and T cells | ED∼1–100 μg/ml | Stimulated proliferation and differentiation of B cells into antibody-producing plasma cells; stimulated IgM antibody yield | Kim et al. (2005) |
| Crude polysaccharide | Immunomodulatory activity | Macrophage and splenocytes | ED∼20 and 100 μg/ml | Promoted cell proliferation and production of IL-2 and GM-CSF | Lee et al. (2017b) |
| Purified polysaccharide (40 kDa) | Immunomodulatory activity | RAW 264.7 murine macrophage cell | ED∼50–500 μg/ml | Stimulated NO production | Liu et al. (2019) |
| Purified α-linked fucoglucomannan (∼1,000 kDa) | Immunomodulatory activity | RAW 264.7 murine macrophage cell | ED∼1–100 μg/ml | Stimulated proliferation and NO production | Kim et al. (2006) |
| Purified proteoglycan (40 kDa) | Immunomodulatory activity | LPS-induced RAW 264.7 murine macrophage cell | ED∼50–500 µg/ml | Increased the release of NO | Liu et al. (2019) |
| Purified polysaccharides (32–119 kDa) | Immunomodulatory activity | Human peripheral blood mononuclear cells | ED∼15–150 µg/ml | Stimulated cell proliferation and secretion of TNF-α, IFN-γ, IL-1β, and IL-2 | Xu et al. (2014b) |
| Alkaline (>450 kDa) and acidic polysaccharides (10–31 kDa) | Immunomodulatory activity | J774.A1 murine macrophage cell and D2SC/1 murine dendritic cell | ED∼100 µg/ml | Increased NO production | Wold et al. (2018) |
| Neutral polysaccharides (60–73 kDa) | ED∼10 µg/ml | ||||
| Crude protein-polysaccharide complex | Anti-proliferation activity | Unclear cellular model | – | Inhibited the activity of cdc25 and cdc2/cyclin B; | Mizuno et al. (1999) |
| Purified α-linked fucoglucomannan (∼1,000 k Da) | Anti-proliferation activity | MCF-7, Hur7 cells | ED∼10 and 50 μg/ml | – | Kim et al. (2006) |
| Anti-tumor effect | B16F10 melanoma cells-implanted (SPF) BDF1 mice | ED∼30 mg/kg BW/day (intraperitoneal administration) or 300 mg/kg BW/day (oral administration) | Enhanced survival rate; decreased tumor incidence | ||
| Purified polysaccharides (48.82 kDa) | Anti-tumor effect | Jurkat cells implanted Kunming mice | ED∼20–80 mg/kg BW/day (oral administration) | Increase Bax expression and inhibit Bcl-2 expression | Chen et al. (2015) |
| Immunomodulatory effect | Stimulated proliferation splenocyte and lymphocyte; promoted cytokine secretion (IL-2, IL-6, IL-12 and TNF-) and macrophage phagocytosis in mice; | ||||
| Crude polysaccharide | Anti-tumor effect | B16-F10 melanoma cells implanted female C57BL/6 mice | ED∼200 mg/kg BW/day (oral administration) | Inhibited the growth of the peritoneal tumor mass | Won et al. (2011) |
| Immunomodulatory effect | Female C57BL/6 mice | ED∼300 and 500 μg/mice (intraperitoneal administration) | Promoted phagocytosis, NO/ROS production, and TNF-α secretion of peritoneal macrophages | ||
| Fractionated fresh mouse splenocyte | ED∼10–1,000 μg/ml | Promoted cell proliferation, comitogenic effect and IFN-γ/IL-4 secretion | |||
| RAW 264.7 murine macrophage cell | ED∼100, 300 and 500 μg/ml | Induced NO/ROS production and TNF-α secretion; induced the phosphorylation of three MAPKs (ERK, JNK and p38) and nuclear translocation of NF-κB; secretion of TNF-α were inhibited by anti-TLR2 mAb | |||
| Purified polysaccharide (93k Da) | Anti-tumor effect | SGC7901 cells implanted nude mice | ED∼50, 75 and 100 mg/kg BW/day | – | Fan et al. (2012) |
| Immunomodulatory effect | Spleen lymphocyte and Macrophage | ED∼25–400 g/mL | – | ||
| Purified polysaccharide (111.9 kDa) | Anti-oxidative stress activity, | L-glutamic acid-damaged HT22 hippocampal neuronal cells | ED∼5 or 10 μg/mL | Reduced the release of lactate dehydrogenase; restored the dissipated mitochondrial membrane potential; enhanced levels of Bcl-2, Nrf2, HO-1, SOD-1, and cysteine ligase catalytic subunit and suppressed the excess accumulation of intracellular ROS | Han et al. (2019) |
| Anti-apoptotic activity | Inhibited cellular apoptosis and caspase-3 activity; reduced levels of Bax and Keap1 | ||||
| Anti-Alzheimer’s disease effect | APP/PS1 transgenic mice | ED∼25 or 50 mg/kg BW/day (oral) | Improved the pathological behaviors related to memory and cognition; reduced the deposition of β-amyloid peptides and neuronal fiber tangles induced by enhanced phosphor-Tau in the brain; modulated the levels of anti- and prooxidative stress enzymes; Enhanced the expression levels of Nrf2 and its downstream proteins, including HO-1 and SOD-1, in the brains of APP/PS1 mice | ||
| Purified polysaccharide (45 kDa) | Anti-proliferation activity | LLC1 Lewis lung cancer cell | ED∼0.1 or 1 mg/mL | Activated AMPK via phosphorylation of threonine 172 by LKB1; downregulates Bcl-2, upregulates Bax; enhances cleavage of Caspase-3 and PARP | Jiang et al. (2019) |
| Anti-tumor effect | LLC1 cells implanted C57BL/6J mice | ED∼50 mg/kg BW/day (intraperitoneal injection) | Inhibited allograft tumor growth | ||
| Crude polysaccharide | Anti-proliferation activity | A549 human non-small cell lung cancer cell | ED∼50 and 100 µg/ml | Suppressed the migration and invasive ability of A549 cells throughout reducing MMP expression and inhibiting NF-κB nuclear translocation and phosphorylation of JNK/AKT | Lee et al. (2017a) |
| Crude polysaccharide | Anti-proliferation activity | B16-F10 mouse melanoma cell | ED∼50 and 100 µg/ml | No effects on migration of B16-F10 cells; inhibited the invasion of B16-F10 cells and suppressed the expression of MMPs (2/7/9); inhibited NF-κB nuclear translocation; inhibited the phosphorylation of c-Jun N-terminal kinases and AKT | Lee et al. (2016) |
| Crude polysaccharide | Anti-proliferation activity | B16-F10 mouse melanoma cell | ED∼25, 50 and 100 µg/ml | Suppressed the migration and invasive ability of B16-F10 cells and decreased the expression levels and activities of MMP-2 and MMP-9; decreased the phosphorylation levels of MAPKs (ERK, JNK and p38); decreased the expression level of COX-2, and inhibited the nuclear translocation of NF-κB; | Lee et al. (2014b) |
| Crude polysaccharide | Anti-proliferation activity | A549 human non-small cell lung cancer cell | ED∼25, 50 and 100 µg/ml | Suppressed the migration and invasive ability of A549 cells; decreased the expression levels and activity of MMP-2 and MMP-9; decreased the phosphorylation levels of MAPKs and PI3K/(AKT) as well as the expression level of COX-2, and inhibited the nuclear translocation of NF-κB | Lee et al. (2014c) |
| Crude polysaccharide | Anti-proliferation activity | U251 human Neurogliocytoma Cells | ED∼25–500 µg/ml | Decreased the expression of Bcl-2 and increased the expression of caspase-3 | Ning et al. (2014) |
| Crude polysaccharide | Anti-proliferation activity | Human T lymphadenoma jurkat cell and human B lymphadenoma daudi cell | ED∼0.7–200 µg/ml | – | Chen et al. (2010) |
| Anti-tumor effect | Jurkat tumor cells-implanted Balb/c-nu/nu nude mice | ED∼50 and 100 mg/kg BW/day (oral administration) | – | ||
| Crude protein-polysaccharide complex | Anti-proliferation activity | SMMC7721 hepatoma cell | ED∼150 μg/ml | – | Mizuno et al. (1999) |
| Crude polysaccharide | Antihyperglycemic activity | α-Glucosidase Inhibitory assay | IC50∼24.34–82.97 μg/ml | – | Wang et al. (2019) |
| Crude protein-polysaccharide complex | Anti-hyperglycemic effect | Type-1 diabetic mice | – | Maintained hypoglycemic effect for 3−48 h after injection | Mizuno et al. (1999) |
| Crude polysaccharide | Antihyperglycemic and antihyperlipidemic effects | STZ and high-fat-diet-induced type-2 diabetic mice | ED∼900 mg/kg BW/day (oral administration) | Restored the body and fat mass weight, reduced fasting blood glucose levels, improved glucose tolerance ability, increased hepatic glycogen level and ameliorate insulin resistance; Enhanced the cholesterol transportation in the liver; increased HDL-C levels and decreased TC, TG and LDL-C levels; improved the antioxidant activities of liver and alleviate the STZ-lesioned organ tissues (liver, kidney, and pancreas); Up-regulated expressions of PI3K-p85, p-Akt (ser473), GLUT4 | Wang et al. (2017c) |
| Crude polysaccharide (46–41,508 kDa) | Antihyperglycemic, anti-inflammatory and anti-oxidative stress effects | STZ-induced diabetic mice | ED∼50 mg/kg BW/day (oral administration) | Increased the insulin and pyruvate kinase levels in serum; improved the synthesis of glycogen; restored the serum levels of SOD, CAT, GPx, and MDA; down-regulated IL-2R and MMP-9, and enhanced IL-2 level; decreased the expression of phosphor-NF-κB in the kidneys; repaired the damage on kidney tissues, inhibited inflammatory infiltrate and extracellular matrix deposit injuries | Wang et al. (2017b) |
| Crude polysaccharide of submerged cultures | Antihyperglycemic, antihyperlipidemic, and antioxidant effects | Alloxan-induced type-1 diabetic mice | ED∼150 and 300 mg/kg BW/day (oral administration) | Reduced blood glucose level; decreased serum contents of free fatty acid, TC, TG, and LDL-C; increased HDL-C, insulin levels, and hepatic glycogen contents in the liver; increased CAT, SOD, and GPx activities and decreased MDA level; restored the damage of pancreatic tissues | Xu et al. (2010b) |
| Crude polysaccharide | Antihyperglycemic effects | STZ-induced diabetic mice | ED∼10–30 mg/kg BW/day (oral administration) | Restored the altered in vivo glycoprotein components; diminished the focal necrosis, congestion in central vein; protect β-cells from selective destruction | Diao et al. (2014) |
| Polysaccharides-Cr(III) complex | Antihyperglycemic and antihyperlipidemic effects | STZ and high-fat-diet-induced type-2 diabetic mice | ED∼300, 600, and 900 mg/kg BW/day (oral administration) | Improved the glucose tolerance capacity; promoted the metabolism of glucose and synthesis of glycogen; reduced TG, TC, LDL-C levels; promoted the activities of SOD, CAT, GPx and reduced the MDA levels in liver; ameliorated severe pathological kidney damages including mesangial expansion, glomeruli partly sclerosis and glomerular hypertrophy | Wang et al. (2017a) |
| β-pyran-type purified polysaccharide fractions (200 kDa) | Antihyperglycemic activity | HepG2 Cell and insulin resistant HepG2 Cell | ED∼10–40 μg/ml | Increased the glucose consumption in both HepG2 Cell and insulin resistant HepG2 Cell | Xue et al. (2018) |
| α-pyran-type purified polysaccharide (20 kDa) | |||||
| α/β-type purified polysaccharide (13.6 kDa) | – | Liu et al. (2018) | |||
| β-type purified polysaccharide (15.2 kDa) | – | ||||
| α/β-type purified polysaccharide (13.6 kDa) | Antihyperglycemic effects | STZ-induced diabetic mice | ED∼4.5 mg/kg BW/day (oral administration) | – | Liu et al. (2018) |
| α-Glucosidase inhibitory activities | α-Amylase inhibitory assay | IC50∼7.875 μg/ml | – | ||
| β-type purified polysaccharide (15.2 kDa) | Antihyperglycemic effects | STZ-induced diabetic mice | ED∼4.5 mg/kg BW/day (oral administration) | – | |
| α-Glucosidase inhibitory activities | α-Amylase inhibitory assay | IC50∼3.841 μg/ml | – | ||
| Purified polysaccharide (105.02 kDa) | α-Amylase and α-glucosidase inhibitory ability | α-Amylase and α-glucosidase inhibitory assays | ED∼40–200 μg/ml | – | Wang et al. (2018b) |
| Polysaccharides-chromium (III) complex (115 kDa) | α-Amylase and α-glucosidase inhibitory ability | α-Amylase and α-glucosidase inhibitory assays | ED∼3.0 mg/mL | – | Wang et al. (2018a) |
| Purified polysaccharide (97.12 kDa) | α-Amylase inhibitory ability | α-Amylase inhibitory assay | IC50∼482.49 μg/ml | – | Wang et al. (2018c) |
| α-Glucosidase inhibitory ability | α-Glucosidase inhibitory assay | IC50∼51.47 μg/ml | – | ||
| H2O2-induced hemolysis inhibitory assay | Hemolysis inhibitory assay | IC50∼47.63 μg/ml | – | ||
| Purified polysaccharide (114.30 kDa) | α-Amylase inhibitory ability | α-Amylase inhibitory assay | IC50∼2.83 mg/ml | – | |
| α-Glucosidase inhibitory ability | α-Glucosidase inhibitory assay | IC50∼159.73 μg/ml | – | ||
| H2O2-induced hemolysis inhibitory assay | Hemolysis inhibitory assay | IC50∼58.53 μg/ml | – | ||
| Purified polysaccharide (75.94 kDa) | α-Glucosidase inhibitory ability | α-Glucosidase inhibitory assay | IC50∼55.20 μg/ml | – | |
| H2O2-induced hemolysis inhibitory assay | Hemolysis inhibitory assay | IC50∼51.53 μg/ml | – | ||
| Crude polysaccharide | Anti-obesity and probiotic effects | High-fat diet fed C57BL6/J mice | ED∼1,000 mg/kg BW per day | Improved the obesity of mice, including the adjustment of body weight gain, energy intake, energy efficiency, liver glucose metabolism and triglyceride metabolism, tricarboxylic acid (TCA) cycle, and degradation of three major nutrients (carbohydrate, lipid, and protein); Improved the level of cecal butyrate by Lactobacillus and the Bacteroidales S24-7 group, resulting in increased energy consumption, and fat degradation by regulating the TCA cycle of the host | Yu et al. (2020) |
| Purified polysaccharide (32.5 kDa) | Anti-inflammation, antioxidative stress and probiotic effects | DDC-induced chronic pancreatitis in mice | ED∼100, 200, 400 mg/kg BW/day (oral administration) | Increased GPx and TAOC levels in pancreas, and decreased TNF-α, TGF-β, lipase and trypsin levels in serum; Increased the proportion of Bacteroidetes and decreased that of Firmicutes at phylum level; maintained the microbiota structure and richness to normal level | Hu et al. (2017b) |
| Purified polysaccharide | Anti-fatigue effect | Forced sports test of male Kunming mice | ED∼50 mg/kg BW/day (oral administration) | Increase the climbing duration and swimming time as well as reduced the immobility time; Decreased the level of blood lactic acid, urea nitrogen and lactic dehydrogenase; Decreased the 5-HT concentrations in the mice brain | Zhang et al. (2020) |
| Crude polysaccharide | Anti-fatigue effect | Forced sports test of male Kunming mice | ED∼100, 200, 300 mg/kg BW/day (oral administration) | Extended the swimming time; Enhanced liver and muscle glycogen content; Decreased the level of blood lactic acid and urea nitrogen | Zhong et al. (2015) |
| Purified polysaccharide (32.5 kDa) | Anti-virus | FHV-infected CRFK cells | IC50∼18.15 μg/ml | Shoed a low cytotoxicity to CRFK and MDCK cells and broad-spectrum antiviral activity against feline calicivirus | Tian et al. (2017) |
| FPV-infected CRFK cells | IC50∼45.33 μg/ml | ||||
| FIPV-infected CRFK cells | IC50∼22.87 μg/ml | ||||
| H5N6-infected MDCK cells | IC50∼68.47 μg/ml | ||||
| H3N2-infected MDCK cells | IC50∼48.51 μg/ml | ||||
| Other compounds | |||||
| Peptide | |||||
| Trp-Gly-Cys | Platelet aggregation inhibitory activity | 83.3% Platelet aggregation inhibitory activity in collagen/epinephrine-induced thrombotic ICR mice, | – | – | Hyun et al. (2006) |
| Melanin | |||||
| Purified melanin-polysaccharide complex (<10 kDa) | Anti-hemolysis activity | Sheep erythrocytes | IC50∼4.9–8.4 µg/ml | – | Wold et al. (2020) |
| Anti-inflammatory activity | LPS + IFNγ-activated C57BL/6 primary macrophages | IC50∼24.1 ± 7.9 µg/ml | Reduced NO production | ||
| Antioxidant activity | DPPH radical scavenging assay | IC50∼61.4 μg/ml | – | ||
| Anti-proliferation activity | CI-H460 and HT29-MTX | IC50 > 50 μg/ml | – | ||
| Purified melanin (2–20 kDa or 90–100 kDa or more) | Antioxidant activity | Total antioxidant assay; DPPH, ABTS and hydroxyl radical assays; FRAP and Fe2+ chelation assays; β-carotene bleaching assay | – | – | Olennikov et al. (2012) |
| Crude melanin | Probiotic activity | Bifidobacterium bifidum 1 and Bifidobacterium animalis subsp. lactis | ED∼10−10, 10−7, 10−2 mg/cm3 | – | Burmasova et al. (2019) |
| Antioxidant activity | Total antioxidant assay (phosphomolybdate method) | – | – | ||
| Crude melanin | Antioxidant activity | Total antioxidant assay (phosphomolybdate method) and Ferric Ions reduction assay (phenanthroline method) | – | – | Parfenov et al. (2019) |
| Hepatoprotective activity | D-Galactosamine-treated normal human (Chang) Liver cell | – | – | ||
| Hepatoprotective effect | Tetrachloromethane-treated Sprague Dawley rats | ED∼100 mg/kg BW/day | Decreased steatosis, necrosis, fat accumulation, and normalized various indicators including the total and unconjugated bilirubin, total protein, serum cholinesterase, and γ-glutamyl transpeptidase levels | ||