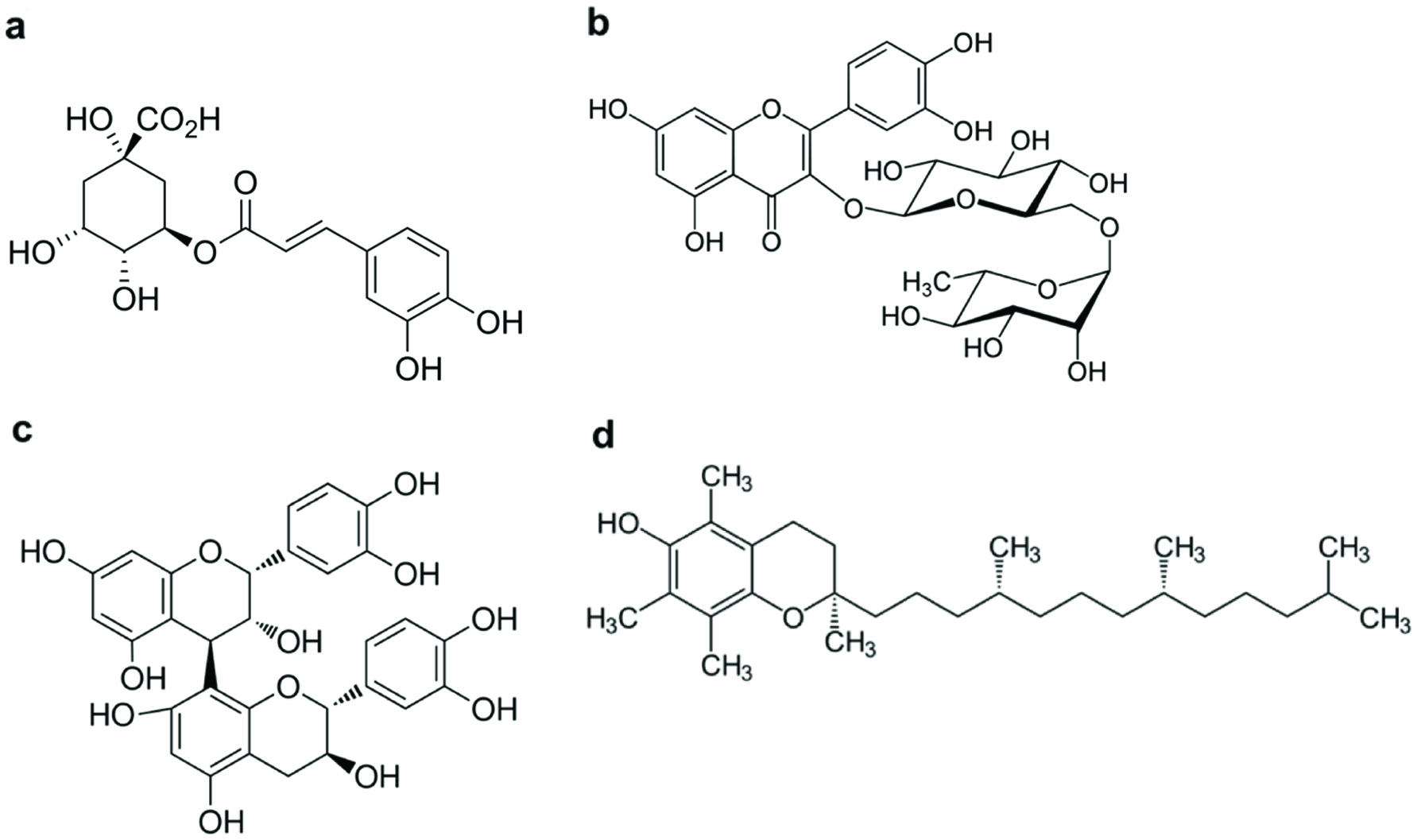
Figure 1. Chemical structures of phenolic compounds mentioned in the Brazilian Normative Instruction N° 28. (a) chlorogenic acid, (b) rutin, (c) procyanidin B2, and (d) α-tocopherol.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Perspective
Volume 7, September 2019, pages 7-17
A perspective on phenolic compounds, their potential health benefits, and international regulations: The revised Brazilian normative on food supplements
Figure

Tables
| Phenolic compound | 0-6 months | 7-11 months | 1-3 years old | 4-8 years old | 9-18 years old | +19 years old | Breastfeeding women | Pregnant women | Observations |
|---|---|---|---|---|---|---|---|---|---|
| Source: Adapted from Agência Nacional de Vigilância Sanitária (2019c). | |||||||||
| Chlorogenic acid | Not allowed | Not allowed | Not allowed | Not allowed | Not allowed | Min: Not established | Not allowed | Not allowed | – |
| Max: 0.12 mg | |||||||||
| Rutin | Not allowed | Not allowed | Not allowed | Not allowed | Not allowed | Min: Not established | Not allowed | Not allowed | – |
| Max: 0.6 mg | |||||||||
| Proanthocyanidins | Not allowed | Not allowed | Not allowed | Not allowed | Not allowed | Min: Not established | Not allowed | Not allowed | – |
| Max: 7.5 mg | |||||||||
| Vitamin E | Not allowed | Not allowed | Min: 0.9 mg | Min: 1.05 mg | Min: 2.25 mg | Min: 2.25 mg | Min: 2.85 mg | Min: 2.25 mg | As α-tocopherol. α-Tocopherol includes RRR-α-tocopherol, the only α-tocopherol form that naturally occurs in food., and the 2R-stereoisomers of α-tocopherol (RRR-, RSR-, RRS-, and RSS-α-tocopherol) that occur in fortified foods and supplements. Considering the commercially available synthetic form (rac-α-tocoferyl) with an activity of 0.67 x RRR-α-tocopherol, considering 1 IU of vitamin E as 1 mg of acetate of rac-α-tocoferyl. |
| Max: 200 mg | Max: 300 mg | Max: 600 mg | Max: 1,000 mg | Max: 800 mg | Max: 800 mg | ||||
| Authorized Component | CAS | Specifications | Function | Authorized claims and requirements to make them | Requirements for Complementary Labelling and other | Other information |
|---|---|---|---|---|---|---|
| Source: Agência Nacional de Vigilância Sanitária (2019c). | ||||||
| Tomato hydro soluble concentrate (Lycopersicon esculentum) | For this component, the approved specifications belong to the following manufacturers: C.A.S. S.p.A – Verona – Italy; Hans Zipperle AG/S.p.A – Merano – Italy; Indena S.A.S. – Tours – France | Source of chlorogenic acid | No claims | The warning “This product should not be consumed by pregnant and breastfeeding women and children” must be written on the label. | – | |
| Tomato hydro soluble concentrate (Lycopersicon esculentum) | For this component, the approved specifications belong to the following manufacturers: C.A.S. S.p.A – Verona – Italy; Hans Zipperle AG/S.p.A – Merano – Italy; Indena S.A.S. – Tours – France | Source of rutin | No claims | The warning “This product should not be consumed by pregnant and breastfeeding women and children” must be written on the label. | – | |
| Powdered cranberry (Vaccinium macrocarpon) | For this component, the approved specifications belong to the following manufacturer: Naturex – DBS LLC – Sagamore – Massachusetts – USA | Source of proanthocyanidins | No claims | The warning “This product should not be consumed by pregnant and breastfeeding women and children” must be written on the label. | – | |
| Propolis extract | Normative Instruction N° 3, DE from January 19th, 2001 | Source of phenolic compounds | No claims | The warning “This product should not be consumed by pregnant and breastfeeding women and children” must be written on the label. | – | |
| Powdered guarana (Paulinia cupana) | These components must follow the specifications evaluated and approved by ANVISA. | Source of caffeine | 1) Claim: Caffeine enables an elevated state of alert and improves the concentration. Requirements: this claim is exclusive for the dietary supplements in which the caffeine content meets the minimum amount established by IN 28/2018–Appendix III. 2) Claim: Caffeine improves endurance and performance during endurance physical activities. Requirements: this claim is exclusive to the dietary supplements in which the caffeine content is 200 mg, consumed 1 h prior to the physical activity. | The warning “This product should not be consumed by pregnant and breastfeeding women and children” must be written on the label. | – | |
| Dextroalphatocopherol acetate/D-alpha-tocopherol acetate | 58-95-7 | FCC 6 FCC 10 ADOPTED: February 10th, 2016 PUBLISHED: March 2nd, 2016 doi: 10.2903/j.efsa.2016.4412 | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – |
| DL-alpha-tocopherol acetate/rac-alpha-tocopherol acetate/DL-alpha-tocopheryl acetate | 7695-91-2 | ADOPTED: February 10th, 2016 PUBLISHED: March 2nd, 2016 doi: 10.2903/j.efsa.2016.4412 European pharmacopoeia 9.0 | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – |
| Dextro-alpha-tocopherol/D-alpha-tocopherol | 59-02-9 | European pharmacopoeia 9.0 FCC 6 Prepared at the 55th JECFA (2000) and published in FNP 52 Add 8 (2000), superseding tentative specifications prepared at the 30th JECFA (1986) and published in FNP 37 (1986) and in FNP 52 (1992). A group ADI of 0.15-2 mg/kg bw for dl-alpha-tocopherol and d-alpha-tocopherol, concentrate, singly or in combination, was established at the 30th JECFA (1986). | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – |
| DL-alpha-tocopherol | 10191-41-0 | Prepared at the 30th JECFA (1986), published in FNP 37 (1986) and in FNP 52 (1992). Metals and arsenic specifications revised at the 61st JECFA (2003). A group ADI of 0.15-2 mg/kg bw for dl-alpha-tocopherol and d-alpha-tocopherol, concentrate, singly or in combination, was established at the 30th JECFA (1986). European pharmacopoeia 9.0 FCC 10 | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – |
| Mixture of tocopherols | FCC10 Prepared at the 30th JECFA (1986), published in FNP 37 (1986) and in FNP 52 (1992). Metals and arsenic specifications revised at the 61st JECFA (2003). A group ADI of 0.15-2 mg/kg bw for dl-alpha-tocopherol and d-alpha-tocopherol, concentrate, singly or in combination, was established at the 30th JECFA (1986). | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – | |
| Acidic succinate of D-alpha-tocopheryl | 893081 | European pharmacopoeia 9.0 FCC 10 | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – |
| Acidic succinate of DL-alpha-tocopheryl | 17407-37-3 | European pharmacopoeia 9.0 | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – |
| Acidic succinate of D-alpha-tocopheryl-polyethylene glycol-1000 | USP NF 34 | Source of Vitamin E | 1) Claim: Vitamin E is an antioxidant that helps protect against the damages caused by free radicals. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III 2) Claim: Source of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content meets the minimum amount established in IN 28/2018–Appendix III. 3) Claim: Rich in/High content of vitamin E. Requirements: this claim is exclusive to the dietary supplements in which the vitamin E content corresponds to the double of the minimum amount established in IN 28/2018–Appendix III, since it does not surpass the maximum amount established in the Appendix IV. | No additional requirements. | – | |
| Oil | Concentration (mg/100 g of oil) |
|---|---|
| Source: Adapted from Shahidi and de Camargo (2016) and de Camargo et al. (2019). nd, non-detected. | |
| Almonds | nd–34.9 |
| Barley | 14.2–20.1 |
| Brazil nuts | nd–2.2 |
| Camelina | 2.81–3.80 |
| Cashew | nd–7.84 |
| Coconut | 0.20–1.82 |
| Corn | 18.0–25.7 |
| Cottonseed | 30.5–57.3 |
| Grape | 11.8–18.8 |
| Hazelnut | 15.7–42.1 |
| Linseed | 0.54–1.20 |
| Macadamia | 0.08–0.11 |
| Olive | 11.0–17.0 |
| Palm | 6.05–42.0 |
| Peanut | 8.86–30.4 |
| Pecan | nd–1.82 |
| Pine | 2.2–16.6 |
| Pistachios | nd–32.8 |
| Rapeseed | 18.9–24.0 |
| Rice bran | 0.73–15.9 |
| Safflower | 36.7–47.7 |
| Sesame | 0.24–36.0 |
| Soybean | 9.53–12.0 |
| Sunflower | 32.7–59.0 |
| Walnut | nd–3.80 |
| Wheat germ | 151–192 |